Abstract
This study aims to evaluate the predictive capability of CRP-albumin-lymphocyte (CALLY) index in relation to hemorrhagic transformation (HT) and functional outcome in acute ischemic stroke (AIS). A total of 439 AIS patients were included in this analysis. Multivariate logistic regression was conducted to examine the relationship between the CALLY index, HT, and functional outcomes. To address its non-linear association, a restricted cubic spline (RCS) model identified an optimal threshold for the CALLY index. Subgroup analyses further explored the association between the CALLY index and HT. The receiver operating characteristic (ROC) curve, the net reclassification index (NRI), and the integrated discrimination index (IDI) were used to assess and compare the predictive performance of the CALLY index with established models for HT. Furthermore, mediation analysis was performed to elucidate the causal pathways linking the CALLY index, HT, and functional outcomes. Among the participants, 9.79% (43/439) experienced HT, and 49.32% (182/369) encountered adverse outcomes. A higher CALLY index was associated with a lower risk of developing HT (OR 0.449, 95% CI 0.283–0.713) and poor outcome (OR 0.691, 95% CI 0.558–0.855). RCS curves demonstrated an increased risk of HT when the CALLY index fell below 1.188. Compared to existing HT prediction models, the CALLY index demonstrates superior predictive performance, with an AUC of 0.746. Furthermore, the CALLY index exhibits improved reclassification ability, as indicated by enhanced NRI and IDI values. The CALLY index independently predicts HT and adverse outcomes in AIS, demonstrating superior accuracy to existing risk scores and offering a practical biomarker for clinical prognosis.
Similar content being viewed by others
Introduction
Stroke remains a leading cause of reduced life expectancy and Disability-Adjusted Life Years1, imposing a significant burden on patients and the public health system. In 2020, a nationwide survey involving 676,394 adults revealed an estimated 17.8 million cases of stroke in China, including 3.4 million new stroke events and 2.3 million stroke-related deaths2. Hemorrhagic transformation (HT) is a common and serious complication in acute ischemic stroke (AIS)3,4, exacerbating neurological deterioration and contributing to poor prognosis5. Therefore, the identification of HT is a critical component of managing AIS and remains a current research hotspot.
The exploration of effective predictive markers for HT and functional outcome is crucial for enhancing precision and timeliness in clinical decision-making. Consequently, several predictive models have been developed to assess the risk of intracranial HT and facilitate risk stratification, including the Hemorrhagic Transformation Index (HTI) score6, Hemorrhage Risk Stratification (HeRS) score7, Stroke Prognostication using Age and NIHSS (SPAN-100) score8, GWTG-Stroke sICH risk(GRASPS)score9, Hemorrhage After Thrombolysis (HAT) score10, Symptomatic Intracranial Hemorrhage after Stroke Thrombolysis (SEDAN) score11. However, existing predictive models have notable limitations, including the complexity due to an excessive number of components and difficulties in acquiring imaging data. Consequently, there is an urgent need for the development of more streamlined and accessible predictive markers that can be easily applied in clinical settings.
Numerous studies have demonstrated that the development and prognosis of ischemic stroke are influenced by multiple factors, including the inflammatory response, nutritional status, and immune function. In terms of inflammation, independent associations have been observed between high levels of inflammation with recurrent stroke occurrence and poor functional outcome12. Elevated levels of inflammatory markers were correlated with increased blood-brain barrier (BBB) permeability, thereby increasing the likelihood of HT13. Several studies have also shown that malnutrition impacts neurofunctional recovery and is associated with an increased long-term mortality rate in stroke patients14,15. Additionally, immune function plays a crucial role in stroke progression as stroke-induced immunosuppression increases the risk of infections among these patients leading to adverse clinical outcome16,17. Importantly, stroke progression is influenced by a combination of inflammation, nutritional status, and immunity rather than a single aspect. Therefore, to enhance predictive efficacy, efforts should be directed towards exploring biomarkers that comprehensively reflect the pathophysiological processes of stroke.
The CRP-albumin-lymphocyte (CALLY) index, comprising C-reactive protein (CRP), albumin, and lymphocytes, collectively reflects the inflammatory-nutrition-immune status of the body. In 2021, the CALLY index was first employed to predict the prognosis of hepatocellular carcinoma patients18,19, marking its potential as a promising prognostic biomarker. Given its low cost, clinical accessibility, and comprehensive nature, subsequent clinical studies have consistently demonstrated the robust predictive performance of CALLY in various malignancies20,21,22. However, the CALLY index is predominantly utilized in the field of oncology, with a paucity of research examining its clinical utility in the context of stroke. Consequently, this study seeks to elucidate the association between the CALLY index and both HT and functional outcomes in AIS, as well as to evaluate its predictive performance against established models.
Materials and methods
Study design
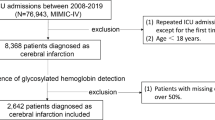
This study included consecutive patients with AIS who were admitted to Xiangya Hospital between December 2020 and June 2023. AIS diagnosis was confirmed using diffusion-weighted imaging (DWI). The inclusion criteria were defined as follows: (1) age ≥ 18 years, (2) disease onset within 7 days, and (3) undergoing computed tomography (CT) and magnetic resonance imaging (MRI) scans of the head. Exclusion criteria comprised incomplete medical records, lack of imaging data, presence of malignant tumors or severe hepatic or renal insufficiency, as well as autoimmune diseases. The severity of liver function impairment is assessed using Child-Pugh score23, when the Child-Pugh score is class C, it indicates severe liver dysfunction. Severe renal insufficiency is defined as having a significantly reduced estimated glomerular filtration rate (eGFR < 30 mL/min/1.73 m2), which was calculated upon admission using Modification of Diet in Renal Disease (MDRD) Equation[24].A comprehensive eligibility screening was conducted on a cohort of 2322 hospitalized patients diagnosed with ischemic stroke. Figure 1 illustrates the flowchart for patient selection. Ethical approval for this study involving human participants was obtained from the Xiangya Hospital Ethics Committee and conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Written informed consent was acquired from all participants.
Patient characteristics and outcome
Demographic data, including age, sex, and vascular risk factors such as current smoking, alcohol consumption, hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, and prior stroke were extracted from patients’ medical records. Smoking was described as the regular or accumulated use of tobacco for a duration exceeding six months. Alcohol consumption was characterized as consuming alcoholic beverages at a frequency of three or more times weekly, with each session involving at least 100 mL25. Hypertension was determined by a blood pressure measurement of ≥ 140/90 mmHg, a recorded history of hypertension, or the current use of antihypertensive drugs26. Dyslipidemia was categorized as having a serum total cholesterol (TC) level of ≥ 6.22 mmol/L (240 mg/dL), low-density lipoprotein cholesterol (LDL-C) of ≥ 4.14 mmol/L (160 mg/dL), triglycerides (TG) of ≥ 2.26 mmol/L (200 mg/dL), or high-density lipoprotein cholesterol (HDL-C) of < 1.04 mmol/L (40 mg/dL), or a documented history of hyperlipidemia accompanied by the use of lipid-lowering medications27. Diabetes was identified as a fasting plasma glucose level of ≥ 7.0 mmol/L (126 mg/dL), a previous diagnosis of diabetes, or the use of antidiabetic drugs or insulin28. Atrial fibrillation was verified through electrocardiographic findings or active treatment for the condition29. Simultaneously recorded was the detailed treatment status of patients within 24 h after admission; this encompassed the administration of intravenous thrombolysis as well as documentation of NIHSS score on admission and baseline systolic blood pressure (SBP) and diastolic blood pressure (DBP). Moreover, Stroke etiology was determined according to the criteria of the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification30.
Fasting venous blood samples were collected from patients within the first 24 h after hospital admission for analysis of white blood cell count (WBC), neutrophil count, lymphocyte count, triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL) level, high-density lipoprotein (HDL) level, fasting blood glucose (FBG), C-reactive protein (CRP) level, and albumin level. All patients received a baseline brain CT/MRI scan within 24 h of the onset of symptoms. The HT was determined through follow-up CT scans or susceptibility-weighted imaging (SWI), conducted 4 days (± 2) after the initiation of stroke, or immediately if there was a deterioration in condition. HT was categorized into four groups based on the criteria established by the European Cooperative Acute Stroke Study (ECASS-II)31: Hemorrhagic Infarction type 1 and 2 (HI-1, HI-2), Parenchymal Hemorrhage type 1 and 2 (PH-1, PH-2). The infarct volume was estimated using a well-established equation: (length × width × slice thickness × number of slices) / 2. High-Density Middle Cerebral Artery (HMCA) Sign and Alberta Stroke Program Early CT Score (ASPECTS) are both assessed using non-enhanced head CT scans. The HMCA sign is defined as a significant enhancement of the middle cerebral artery on the CT image relative to the contralateral vessel, and the scoring criteria for the ASPECTS have been described in previous studies32. All imaging assessments were performed independently by two neuroradiologists, and any disagreements were resolved through third-party arbitration.
In this study, HT was the primary outcome, and functional outcome was the secondary outcome. Functional outcome were evaluated at three months post-symptom onset using the modified Rankin Scale (mRS)33. A favorable outcome was defined as an mRS score of 0–1, while a poor outcome was indicated by an mRS score of 2–6.
Measurements
The CALLY index was calculated as Albumin × Lymphocyte/(CRP×10). The measurement methods for the HTI score, HeRS score, SPAN-100 score, GRASPS score, HAT score, and SEDAN score were based on previous research reports6,7,8,9,10,11.
Statistical analysis
The distributions of all continuous variables were found to deviate from normality, and thus they were reported as medians accompanied by interquartile ranges (IQR). Categorical variables were presented in the form of percentages. We conducted a comparative analysis of the baseline characteristics between participants without HT and those with HT, employing the Mann-Whitney test for continuous variables and the chi-square test for categorical variables. Multivariate logistic regression analysis was conducted to investigate the association between the CALLY index and the risk of HT and functional prognosis in AIS. Furthermore, restricted cubic spline (RCS) analysis was utilized to explore potential non-linear relationships and identify threshold effects between the CALLY index and HT. Subgroup analyses were conducted to investigate the association between CALLY index and HT in various subgroups based on factors including age, gender, hypertension, diabetes mellitus, atrial fibrillation, NIHSS score upon admission, and IV thrombolysis therapy. Additionally, the Receiver Operating Characteristic (ROC) curve analysis, the net reclassification index (NRI), and the integrated discrimination index (IDI) were used to assess and compare the predictive performance of the CALLY index with established models, namely the HTI score, HeRS score, SPAN–100 score, GRASPS, HAT, SEDAN score, for the purpose of identifying patients with HT and poor outcome. The NRI and IDI quantify the capacity of a novel model to accurately reclassify individuals across distinct risk strata34.
The statistical analyses were performed using SPSS version 25.0 (IBM SPSS, Armonk, NY, USA) and R version 4.2.3 (R Development Core Team, Vienna, Austria). Statistical significance was determined at a two-tailed P-value below 0.05.
Results
Baseline characteristics of the study participants
From December 2020 to June 2023, a total of 439 patients diagnosed with AIS were included in our analysis. The median age of the participants was 64 years, ranging from 55 to 71 years. Among them, 311 patients (70.8%) were male. For a detailed description of the clinical characteristics of our study population, please refer to Table 1.
In our analysis, we observed an incidence rate of approximately 9.79% (43/439) for HT occurrence and around 49.32% (182/369) experienced adverse outcome (mRS ≥ 2). Among the 43 patients with HT, 10 (23.26%) presented with hemorrhagic infarction type 1 (HI1), while HI2 was exhibited by 20 patients (46.52%). Additionally, parenchymal hematoma type 1 (PH1) was observed in 5 patients (11.63%), and PH2 was displayed in 8 patients (18.60%). The average age for those who developed HT was approximately 65 years old and predominantly male (76.7%). When comparing patients with and without HT, we found that those with HT had higher admission NIHSS scores and a greater proportion received intravenous thrombolysis treatment as well as had comorbidities such as atrial fibrillation and infectious diseases. Additionally, elevated levels of white blood cell count, neutrophil count, and CRP were observed upon admission for HT patients compared to non-HT individuals (p < 0.05). However, lymphocyte count upon admission show lower values among HT patients (p < 0.05). Furthermore, relative to the Non-HT group, HT patients exhibited higher scores in the HTI, HeRS, HAT, and SEDAN models (Table 1, Fig. S1).
Association between CALLY and HT
The logistic regression analysis in Table 2 demonstrates the association between CALLY index and the risk of HT. Univariate logistic regression analysis revealed a significant negative correlation between CALLY and HT when considering it as a continuous variable. This relationship remained statistically significant even after adjusting for age, sex, hypertension, diabetes mellitus, atrial fibrillation, platelets, NIHSS score at admission, and IV thrombolysis therapy (OR 0.449, 95% CI 0.283–0.713, p = 0.001). When the CALLY index was divided into 4 quartiles, a comparison with quartile 1 revealed lower prevalence of HT in the other three quartiles (quartile3: OR 0.197, 95% CI 0.066–0.783, P = 0.004; and for quartile 4: OR 0.038, 95% CI 0.005–0.305, P = 0.002).
Nonlinear correlation and subgroup analysis for the HT
Further evidence of a dose-response relationship between reduction in the CALLY index and HT is provided by RCS regression analysis (Fig. 2). The result indicates that lower levels of CALLY is significantly associated with a higher prevalence of HT, particularly in AIS patients with CALLY levels below 1.188. Subgroup analyses also confirm a robust association between CALLY and the prevalence of HT. Fig. S2 presents the results of subgroup analyses stratified by age (< 60 or ≥ 60 years), sex (male or female), NIHSS score at admission (< 5 or ≥ 5), history of atrial fibrillation, hypertension, and diabetes mellitus (DM), as well as intravenous thrombolytic therapy. Result indicates that a higher CALLY index is generally associated with a reduced risk of HT across most subgroups. Notably, the effect was significantly modified by age and DM status, with older patients(age ≥ 60) and those without DM showing stronger associations.
The RCS curve of the association between CALLY and HT among AIS population.
The analyses have been adjusted potential confounders, including age, sex, hypertension, diabetes mellitus, atrial fibrillation, platelets, NIHSS score on admission, and IVT therapy. The red solid line represents the adjusted ORs, while the shaded light pink areas indicate the corresponding 95% CI. CALLY index could increase the risk of HT when lower than 1.188.
RCS, restricted cubic spline; CALLY, C-reactive protein–albumin–lymphocyte; HT, hemorrhagic transformation. OR, odds ratio; CI, confidence interval.
Association between CALLY and 3-month functional outcome
Out of the AIS patients, 70 were lost to follow-up during the 3-month post-discharge assessment, resulting in a total of 369 patients whose final mRS scores were analyzed. Figure 3 illustrates the distribution of 3-month mRS scores across different quartiles of CALLY in individuals with AIS. A progressive increase in the proportion of adverse outcome(mRS ≥ 2) is observed as CALLY levels decrease. In the fourth quartile of CALLY ( > 2.414), the proportion of adverse outcome is 25.0%. However, in the first quartile of CALLY (< 0.527), accounting for 81.7% experience adverse outcome. Multivariable logistic regression analysis in Table 3 indicating a significant association between the CALLY index and adverse functional outcome (aOR: 0.691, 95% CI 0.558–0.855, p = 0.001) after adjusting for admission NIHSS score, age, sex,TOAST classification, diabetes, HT and intravenous thrombolysis. Furthermore, to further investigate the relationship among CALLY, HT, and functional outcomes, we performed a mediation analysis. As illustrated in Fig. S3, the findings suggest that the association between the CALLY index and prognosis is not mediated by HT and remains significant even after adjusting for the effects of HT. This indicates that the CALLY index may have an independent role in predicting prognosis, beyond its association with HT.
Predictive values of CALLY for the HT and prognosis
The HTI score, HeRS score, SPAN-100 score, HAT score, SEDAN score, and GRASPS score based on laboratory test indicators and/or imaging parameters have been utilized for assessing the risk of HT in AIS. To evaluate the predictive efficacy of CALLY index, we compared it with the above prediction models. The ROC curve analysis (Fig. 4) revealed that the area under the curve (AUC) for the CALLY index was 0.746 (95% CI 0.677–0.815) for predicting HT in AIS patients. At the optimal threshold of 1.081, sensitivity was 86% and specificity was 59%.Notably, the predictive performance of the CALLY index surpassed existing other scores, including HeRS score (AUC: 0.713, 95% CI 0.630–0.797), HTI score (AUC: 0.649, 95% CI 0.563–0.735), SPAN-100 score (AUC: 0.583, 95%CI 0.495–0.672), HAT score (AUC: 0.661, 95% CI 0.598–0.723), SEDAN score (AUC: 0.634, 95% CI 0.563–0.705), and GRASPS Score (AUC: 0.522, 95%CI 0.429–0.616). Furthermore, the CALLY index demonstrated superior reclassification performance compared to the HeRS score, HTI score, SPAN-100 score, GRASPS score, HAT score and SEDAN score with NRI values of 0.439 (p < 0.001), 0.353 (p < 0.006), 0.441 (p < 0.001), 0.383 (p < 0.001), 0.439 (p < 0.001), and 0.169 (p < 0.05), respectively, while the corresponding IDI values were 0.120 (p < 0.001), 0.128 (p < 0.001), 0.129 (p < 0.001), 0.136 (p < 0.001), 0.131 (p < 0.001), and 0.055 (p < 0.05) (supplementary Table 1). These results indicate that the CALLY index significantly improves predictive accuracy compared to previous models. Particularly when combined with HeRS Score, CALLY index exhibited even more significant predictive efficacy (AUC: 0.752, 95%CI 0.684–0.820) (Fig. S4). Furthermore, the CALLY index exhibited superior predictive performance for functional outcome in AIS compared to other prediction scores, with an AUC of 0.757(95% CI 0.708–0.806) (Fig. 5).
The ROC value of CALLY index, HTI score, HeRS score, HAT score, SEDAN score, SPAN–100 score, GRASPS score in predicting HT in AIS patients. ROC, the receiver operating characteristic; AUC, area under curve; CALLY, C-reactive protein–albumin–lymphocyte; HTI, the Hemorrhagic Transformation Index score; HeRS, Hemorrhage Risk Stratification score; SPAN-100, Stroke Prognostication using Age and NIHSS score; GRASPS, GWTG-Stroke sICH risk score; HAT, Hemorrhage After Thrombolysis score; SEDAN, Symptomatic Intracranial Hemorrhage after Stroke Thrombolysis score.
The ROC value of CALLY, HTI score, HeRS score, HAT score, SEDAN score, SPAN–100 score, GRASPS score in predicting functional outcome in AIS patients. ROC, the receiver operating characteristic; AUC, area under curve; CALLY, C-reactive protein–albumin–lymphocyte; HTI, the Hemorrhagic Transformation Index score; HeRS, Hemorrhage Risk Stratification score; SPAN-100, Stroke Prognostication using Age and NIHSS score; GRASPS, GWTG-Stroke sICH risk score; HAT, Hemorrhage After Thrombolysis score; SEDAN, Symptomatic Intracranial Hemorrhage after Stroke Thrombolysis score.
Discussion
Our study consistently demonstrated a significant negative correlation between the CALLY index and the risk of HT as well as poor prognosis in patients with AIS. The ROC, NRI and IDI analysis indicated that the predictive performance of the CALLY index exhibited notable advantages. In conclusion, this study addresses the research gap regarding the CALLY index in AIS, suggesting that it may serve as a valuable indicator for predicting HT and functional outcome in AIS individuals.
In recent years, the role of inflammation immune in blood-brain barrier (BBB) disruption has gained increasing attention, leading to a shift in the conceptual framework from the “neurovascular unit” to the “neurovascular-immune network”35. The CALLY index (albumin × lymphocytes / CRP), which integrates CRP, lymphocytes, and albumin, reflects systemic inflammation and immune status, suggesting a network imbalance driven by inflammation and immunity in the pathogenesis of HT. First, CRP is a well-known inflammatory marker, while neuroinflammation-induced BBB disruption is a key driver of HT36. After AIS, ischemia and hypoxia activate central and peripheral immune cells, releasing inflammatory mediators that trigger a neuroinflammatory storm, exacerbate BBB damage, and increase HT risk37,38. Preclinical and clinical studies have explored targeting neurovascular inflammation to reduce HT39. Fan et al. showed that minocycline inhibits microglial activation, neutrophil infiltration, and apoptosis, reducing HT in a rat stroke model40. The MARVEL clinical study found that methylprednisolone treatment significantly lowered symptomatic intracranial hemorrhage rates35. Second, immune system plays a critical role in HT. Lymphocytes act as neuroprotective agents, promoting functional recovery41. Ischemic stroke induces immune suppression through multifaceted mechanisms, leading to lymphocytopenia42. Planas et al. used a Rag2−/− mouse model to demonstrate that lymphocytopenia exacerbates post-stroke HT risk43. Regulatory T cells (Tregs) modulate neuroinflammation and exert neuroprotective effects44. In stroke models, depletion of Tregs exacerbates neuroinflammatory pathology45. Preclinical studies by Liston and colleagues further elucidated that IL-2-mediated Treg expansion suppresses excessive neuroinflammation and reduces HT risk46. Finally, albumin is also closely associated with HT47. Low serum albumin during AIS correlates with elevated S100B (a BBB disruption marker) and larger lesion volumes48. In a middle cerebral artery occlusion rat model, elevated albumin levels during early stroke phases suppressed vascular endothelial growth factor expression, reduce BBB damage, and lower vascular permeability49. Additionally, albumin enhances Treg-mediated immunomodulation, contributing to neuroprotection50. Moreover, albumin enhances Treg-mediated immunomodulation, contributing to neuroprotection51. In our study, multivariate logistic regression analysis identified low CALLY index levels as an independent predictor of HT in AIS patients. RCS analysis revealed a significant reduction in HT risk when the CALLY index exceeded 1.188. This protective association may be attributed to the synergistic effects of its components: elevated albumin and lymphocyte levels (numerator) combined with reduced CRP concentrations (denominator), collectively positioning the CALLY index as a protective marker.
To the best of our knowledge, this study is the first to investigate the relationship between the CALLY index and HT. Currently, there is a noticeable lack of predictive markers for HT. Existing predictive models, such as the HTI score and HAT score, encompass various indicators and incorporating comprehensive imaging assessments in primary healthcare settings remains challenging. Furthermore, certain serum molecules have been explored as potential biomarkers for HT52,53; nevertheless, their expensive testing costs present significant obstacles to widespread clinical application. Therefore, it is crucial to identify more accessible and reliable predictive indicators.
In clinical practice, the CALLY index emerges as a promising predictive marker due to its exceptional accessibility and reliability. Its calculation relies solely on routine laboratory parameters—albumin, lymphocyte count, and CRP, without the need for additional assays, including NIHSS scores or imaging data. Furthermore, the CALLY index has been validated as a robust prognostic tool in malignancies, including hepatocellular carcinoma, oral squamous cell carcinoma, and esophageal cancer18,54,55, etc. The findings of this study also demonstrate the superiority of the CALLY index in predicting HT and functional outcomes in AIS patients. Analyses using the ROC curve, NRI, and IDI indicate that the CALLY index significantly improves predictive accuracy for HT compared to previous models. These findings hold significant clinical application, as early and precise risk stratification enables the implementation of personalized therapeutic strategies. For patients with low CALLY index levels—indicating elevated HT risk and unfavorable outcomes—enhanced focus on diagnosis, treatment, and follow-up is warranted. The CALLY index could be seamlessly incorporated into existing acute stroke management protocols, including pre- and post-recanalization monitoring, to optimize treatment decisions and prognostic assessments. Given these promising results, further prospective studies are warranted to validate the application of the CALLY index in AIS patient populations.
In the subgroup analysis, it was found that the decreased CALLY level is strongly associated with an increased risk of HT in patients characterized by advanced age and non-diabetes status. This finding suggests that elderly patients without diabetes may be particularly vulnerable to HT when presenting with low CALLY levels. Therefore, it is of paramount importance to thoroughly evaluate the CALLY index in these specific patient cohorts. Furthermore, mediation analysis reveals that the impact of the CALLY index on functional outcomes is not solely confined to its relationship with HT. This observation suggests that additional, potentially independent mechanisms may be at play, which extend the influence of CALLY levels beyond merely the increased risk of hemorrhagic complications. These findings highlight the complexity of the underlying pathophysiological processes and provide novel insights that warrant further in-depth research.
The study’s most notable strength lies in its pioneering investigation of the role of the CALLY index in predicting HT and outcome among AIS patients. However, there are still some limitations to consider: firstly, due to the limited sample size, our analysis focused solely on examining the association between CALLY index and any HT, without considering specific HT subtypes or conducting a separate analysis for symptomatic HT. Secondly, this study was conducted at a single center. To enhance the reliability of the conclusions, future studies should include multiple centers across diverse stroke populations or geographic regions for external validation. Additionally, the current study only utilized baseline data, and incorporating dynamic monitoring of the study indicators may provide more accurate assessment of the relationship between CALLY index and AIS prognosis.
Conclusion
The CALLY index is simple and rapid to calculate, requiring no additional NIHSS scores or imaging parameters, which highlights its significant clinical utility. A decrease in the CALLY index is associated with an increased risk of HT and poor functional outcomes in AIS. Specifically, when the CALLY index drops below 1.188, the risk of HT rises. In comparison to existing predictive models, such as HeRS, HTI, SPAN-100, GRASPS, HAT, and SEDAN scores, the CALLY index demonstrates superior predictive performance for both HT and functional outcomes.
Data availability
All data can be provided upon request. For any additional queries, please contact the corresponding author.
References
Ma, Q. et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: An analysis for the global burden of disease study 2019. Lancet Public. Health. 6, e897–e906. https://doi.org/10.1016/s2468-2667(21)00228-0 (2021).
Tu, W. J. et al. Estimated burden of stroke in China in 2020. JAMA Netw. Open. 6, e231455. https://doi.org/10.1001/jamanetworkopen.2023.1455 (2023).
Honig, A. et al. Hemorrhagic transformation in acute ischemic stroke: A quantitative systematic review. J. Clin. Med. 11 https://doi.org/10.3390/jcm11051162 (2022).
Zubair, A. S. & Sheth, K. N. Hemorrhagic conversion of acute ischemic stroke. Neurotherapeutics 20, 705–711. https://doi.org/10.1007/s13311-023-01377-1 (2023).
He, J., Fu, F., Zhang, W., Zhan, Z. & Cheng, Z. Prognostic significance of the clinical and radiological haemorrhagic transformation subtypes in acute ischaemic stroke: A systematic review and meta-analysis. Eur. J. Neurol. 29, 3449–3459. https://doi.org/10.1111/ene.15482 (2022).
Kalinin, M. N., Khasanova, D. R. & Ibatullin, M. M. The hemorrhagic transformation index score: A prediction tool in middle cerebral artery ischemic stroke. BMC Neurol. 17, 177. https://doi.org/10.1186/s12883-017-0958-3 (2017).
Marsh, E. B. et al. Predicting hemorrhagic transformation of acute ischemic stroke: Prospective validation of the hers score. Med. (Baltim). 95, e2430. https://doi.org/10.1097/md.0000000000002430 (2016).
Yufe, R. Stroke prognostication using age and NIH stroke scale: SPAN-100. Neurology 81, 603. https://doi.org/10.1212/01.wnl.0000433418.06773.33 (2013).
Menon, B. K. et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke 43, 2293–2299. https://doi.org/10.1161/strokeaha.112.660415 (2012).
Lou, M. et al. The HAT score: A simple grading scale for predicting hemorrhage after thrombolysis. Neurology 71, 1417–1423. https://doi.org/10.1212/01.wnl.0000330297.58334.dd (2008).
Strbian, D. et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: The SEDAN score. Ann. Neurol. 71, 634–641. https://doi.org/10.1002/ana.23546 (2012).
Li, J. et al. Interleukin-6 and YKL-40 predicted recurrent stroke after ischemic stroke or TIA: Analysis of 6 inflammation biomarkers in a prospective cohort study. J. Neuroinflamm. 19, 131. https://doi.org/10.1186/s12974-022-02467-1 (2022).
Bani-Sadr, A. et al. Blood-Brain barrier permeability and kinetics of inflammatory markers in acute stroke patients treated with thrombectomy. Neurology 101, e502–e511. https://doi.org/10.1212/wnl.0000000000207460 (2023).
Zhang, G. et al. Prevalence and prognostic significance of malnutrition risk in patients with acute ischemic stroke: Results from the third China National stroke registry. Stroke 53, 111–119. https://doi.org/10.1161/strokeaha.121.034366 (2022).
Yuan, K. et al. Association between malnutrition and long-term mortality in older adults with ischemic stroke. Clin. Nutr. 40, 2535–2542. https://doi.org/10.1016/j.clnu.2021.04.018 (2021).
Elkind, M. S. V., Boehme, A. K., Smith, C. J., Meisel, A. & Buckwalter, M. S. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke 51, 3156–3168. https://doi.org/10.1161/strokeaha.120.030429 (2020).
Faura, J., Bustamante, A., Miro-Mur, F. & Montaner, J. Stroke-induced immunosuppression: Implications for the prevention and prediction of post-stroke infections. J. Neuroinflammation. 18, 127. https://doi.org/10.1186/s12974-021-02177-0 (2021).
Iida, H. et al. Superiority of CRP-albumin-lymphocyte index (CALLY index) as a non-invasive prognostic biomarker after hepatectomy for hepatocellular carcinoma. HPB (Oxford). 24, 101–115. https://doi.org/10.1016/j.hpb.2021.06.414 (2022).
Müller, L. et al. Immunonutritive scoring for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: evaluation of the CALLY index. Cancers (Basel). 13. https://doi.org/10.3390/cancers13195018 (2021).
Zhang, H. et al. Superiority of CRP-albumin-lymphocyte index as a prognostic biomarker for patients with gastric cancer. Nutrition 116, 112191. https://doi.org/10.1016/j.nut.2023.112191 (2023).
Yang, M. et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: From the investigation on nutrition status and clinical outcome of common cancers study. Front. Immunol. 14, 1131496. https://doi.org/10.3389/fimmu.2023.1131496 (2023).
Wang, W. et al. Pre-Treatment CRP-Albumin-Lymphocyte index (CALLY index) as a prognostic biomarker of survival in patients with epithelial ovarian cancer. Cancer Manag. Res. 14, 2803–2812. https://doi.org/10.2147/cmar.S359968 (2022).
Romano, F. et al. Rethinking the Barcelona clinic liver cancer guidelines: Intermediate stage and Child-Pugh B patients are suitable for surgery? World J. Gastroenterol. 27, 2784–2794. https://doi.org/10.3748/wjg.v27.i21.2784 (2021).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann. Intern. Med. 130, 461–470. https://doi.org/10.7326/0003-4819-130-6-199903160-00002 (1999).
Tu, W. J. et al. Prevalence of stroke in China, 2013–2019: A population-based study. Lancet Reg. Health West. Pac. 28, 100550. https://doi.org/10.1016/j.lanwpc.2022.100550 (2022).
Gidding, S. S., Whelton, P. K., Carey, R. M. & Levine, G. N. Writing a trustworthy hypertension guideline. J. Am. Coll. Cardiol. 74, 2424–2427. https://doi.org/10.1016/j.jacc.2019.09.008 (2019).
Zhang, H. et al. Predictors of the prevalence of dyslipidemia and influencing factors for young health examination cohort: A cross-sectional survey. Front. Public. Health. 8, 400. https://doi.org/10.3389/fpubh.2020.00400 (2020).
Zhang, W. et al. Association between Gamma-Glutamyl transferase, total bilirubin and systemic lupus erythematosus in Chinese women. Front. Immunol. 12, 682400. https://doi.org/10.3389/fimmu.2021.682400 (2021).
Liu, Z. et al. Impact of Off-Hour admission on In-Hospital outcomes for patients with stroke receiving reperfusion therapy in China. Stroke 55, 1359–1369. https://doi.org/10.1161/strokeaha.123.046096 (2024).
Landau, W. M. & Nassief, A. Editorial comment–time to burn the TOAST. Stroke 36, 902–904 (2005).
Larrue, V., von Kummer, R. R., Müller, A. & Bluhmki, E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with Recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian acute stroke study (ECASS II). Stroke 32, 438–441. https://doi.org/10.1161/01.str.32.2.438 (2001).
Barber, P. A., Demchuk, A. M., Zhang, J. & Buchan, A. M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group. Alberta stroke programme early CT score. Lancet 355, 1670–1674. https://doi.org/10.1016/s0140-6736(00)02237-6 (2000).
Banks, J. L. & Marotta, C. A. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke 38, 1091–1096. https://doi.org/10.1161/01.STR.0000258355.23810.c6 (2007).
Lin, J. S. et al. Nontraditional risk factors in cardiovascular disease risk assessment: Updated evidence report and systematic review for the US preventive services task force. Jama 320, 281–297. https://doi.org/10.1001/jama.2018.4242 (2018).
Akassoglou, K. et al. Pioneering discovery and therapeutics at the brain-vascular-immune interface. Cell 187, 5871–5876. https://doi.org/10.1016/j.cell.2024.09.018 (2024).
Ma, G., Pan, Z., Kong, L. & Du, G. Neuroinflammation in hemorrhagic transformation after tissue plasminogen activator thrombolysis: Potential mechanisms, targets, therapeutic drugs and biomarkers. Int. Immunopharmacol. 90, 107216. https://doi.org/10.1016/j.intimp.2020.107216 (2021).
Li, Q. Q., Ding, D. H., Wang, X. Y., Sun, Y. Y. & Wu, J. Lipoxin A4 regulates microglial M1/M2 polarization after cerebral ischemia-reperfusion injury via the Notch signaling pathway. Exp. Neurol. 339, 113645. https://doi.org/10.1016/j.expneurol.2021.113645 (2021).
Iadecola, C., Buckwalter, M. S. & Anrather, J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Invest. 130, 2777–2788. https://doi.org/10.1172/jci135530 (2020).
Liu, Q., Shi, K., Wang, Y. & Shi, F. D. Neurovascular inflammation and complications of thrombolysis therapy in stroke. Stroke 54, 2688–2697. https://doi.org/10.1161/strokeaha.123.044123 (2023).
Fan, X., Lo, E. H. & Wang, X. Effects of Minocycline plus tissue plasminogen activator combination therapy after focal embolic stroke in type 1 diabetic rats. Stroke 44, 745–752. https://doi.org/10.1161/strokeaha.111.000309 (2013).
Macrez, R. et al. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 10, 471–480. https://doi.org/10.1016/s1474-4422(11)70066-7 (2011).
Mracsko, E. et al. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav. Immun. 41, 200–209. https://doi.org/10.1016/j.bbi.2014.05.015 (2014).
Salas-Perdomo, A. et al. T cells prevent hemorrhagic transformation in ischemic stroke by P-Selectin binding. Arterioscler. Thromb. Vasc Biol. 38, 1761–1771. https://doi.org/10.1161/atvbaha.118.311284 (2018).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199. https://doi.org/10.1038/nm.1927 (2009).
Krämer, T. J. et al. Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-γ gene expression in acute experimental traumatic brain injury. J. Neuroinflamm. 16, 163. https://doi.org/10.1186/s12974-019-1550-0 (2019).
Yshii, L. et al. Astrocyte-targeted gene delivery of Interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation. Nat. Immunol. 23, 878–891. https://doi.org/10.1038/s41590-022-01208-z (2022).
Yuan, C. X., Zhang, Y. N., Chen, X. Y. & Hu, B. L. Association between malnutrition risk and hemorrhagic transformation in patients with acute ischemic stroke. Front. Nutr. 9, 993407. https://doi.org/10.3389/fnut.2022.993407 (2022).
Bielewicz, J. et al. Worse neurological state during acute ischemic stroke is associated with a decrease in serum albumin levels. J. Mol. Neurosci. 58, 493–496. https://doi.org/10.1007/s12031-015-0705-4 (2016).
Yao, X. et al. Protective effect of albumin on VEGF and brain edema in acute ischemia in rats. Neurosci. Lett. 472, 179–183. https://doi.org/10.1016/j.neulet.2010.02.002 (2010).
Bilbao, D., Luciani, L., Johannesson, B., Piszczek, A. & Rosenthal, N. Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Mol. Med. 6, 1423–1435. https://doi.org/10.15252/emmm.201303376 (2014).
Wang, M. et al. Albumin induces neuroprotection against ischemic stroke by altering Toll-like receptor 4 and regulatory T cells in mice. CNS Neurol. Disord. Drug. Targets. 12, 220–227. https://doi.org/10.2174/18715273113129990058 (2013).
Li, W. et al. Serum occludin as a biomarker to predict the severity of acute ischemic stroke, hemorrhagic transformation, and patient prognosis. Aging Dis. 11, 1395–1406. https://doi.org/10.14336/ad.2020.0119 (2020).
Licari, C. et al. Nuclear magnetic resonance-based metabolomics to predict early and late adverse outcomes in ischemic stroke treated with intravenous thrombolysis. J. Proteome Res. 22, 16–25. https://doi.org/10.1021/acs.jproteome.2c00333 (2023).
Tsai, Y. T. et al. Prognostic value of CRP-Albumin-Lymphocyte (CALLY) index in patients undergoing surgery for oral cavity cancer. J. Cancer. 13, 3000–3012. https://doi.org/10.7150/jca.74930 (2022).
Feng, J., Wang, L., Yang, X. & Chen, Q. Clinical significance of preoperative CALLY index for prognostication in patients with esophageal squamous cell carcinoma undergoing surgery. Sci. Rep. 14, 713. https://doi.org/10.1038/s41598-023-51109-w (2024).
Funding
The study was funded by the National Key Research and Development Projects (2022YFC3602400, 2022YFC3602401), the National Natural Science Foundation of China (82271369, 82471365).
Author information
Authors and Affiliations
Contributions
Yinghuan Pan: Data curation, Formal analysis, Software, Visualization, Writing-original draft. Zeyu Liu: Conceptualization, Method ology, Supervision, Validation, Writing -review & editing. Ruxin Tu, Xianjing Feng, Fang Yu, Minping Wei, Weijia Xie and Bi Deng: Data curation, Software, Supervision, Validation. Jian Xia: Conceptualization, Project administration. Jun Yin: Supervision, Writing-review & editing.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pan, Y., Liu, Z., Tu, R. et al. The value of the CRP-albumin-lymphocyte index (CALLY index) as a prognostic biomarker in acute ischemic stroke. Sci Rep 15, 13672 (2025). https://doi.org/10.1038/s41598-025-97538-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97538-7