Abstract
Microbially induced calcite precipitation (MICP) is an emerging ground improvement technique that uses microbes to induce cementation between soil particles. To date, the majority of research has focused on exploring MICP with silica-rich sands; however, the present study investigates the process and efficacy of MICP in a carbonate-rich natural soil, and a comparison is made with benchmark silica-rich sands. MICP column experiments were performed with a range of treatment formulations to optimize and understand the MICP process in carbonate-rich soil. Performance was quantified using chemical (pH, urea, and ammonium concentrations) and physical measurements (TGA and LOI tests). Micro-scale characterization of the cemented soils was performed with XRD, SEM, and EDS, while shear-wave velocity (Vs) and unconfined compressive strength tests were performed to evaluate the effect of precipitated calcite on macroscopic engineering properties. Natural carbonates were found to have a significant impact on the MICP process, resulting in an increase in MICP efficiency of 23% and increases in precipitated calcite contents by as much as 82% when compared to benchmark silica-rich soils receiving similar treatments. These results suggest that the presence of natural carbonate minerals within soils may lower the energy barrier and act as preferential sites for calcite precipitation during the MICP process. Furthermore, SEM images highlighted the association of bacterial cells with precipitated calcite crystals, differences in calcite morphologies and more widespread cementation bonds in carbonate-rich soil when compared to silica sand. Generated cementation also resulted in a linear increase in Vs with increases in precipitated calcite contents for MICP treated carbonate-rich soil, consistent with past results for silica sands. Lastly, differences in yeast extract concentrations applied in treatment solutions were also found to significantly impact the development of ureolytic microbial capacity and the efficiency of the MICP process in the considered soils.
Similar content being viewed by others
Introduction
Microbially induced calcite precipitation (MICP) is an emerging bio-mediated ground improvement technique that uses microbial activity to cement soil particles. Recently, MICP has gained attention as a more sustainable ground improvement technique as it does not involve the use of Portland cement and could potentially reduce carbon emissions in the construction sector, which accounted for 23% of global CO2 emissions in 20091.
Significant progress has been made in understanding and optimizing the MICP treatment process (see reviews in Ref.2,3,4,5,6,7,8,9,10,11,12,13,14,15). In MICP, the urea hydrolysis metabolic pathway has been commonly used, in which the urease enzyme hydrolyzes urea to produce ammonia and carbonate molecules2,5,6,7,8,16,17,18,19,20,21,22. Subsequently, a calcium salt is introduced to induce precipitation of calcium carbonate. The microbes can act as nucleation sites for calcite crystal formation thereby progressively binding the soil particles together2,5,16,23,24,25,26,27. The chemical reactions of the urea hydrolysis metabolic pathway are:
The MICP treatment methods can be divided into biostimulation and bioaugmentation6,7,20,28,29,30,31,32: in biostimulation, soils are treated with treatment solutions to allow the in-situ ureolytic bacterial community to develop and perform the MICP process, while in bioaugmentation, Sporosarcina pasteurii bacterium (documented with the highest ureolysis rate20,33,34,35,36) or other non-native strains, are introduced into the soil for MICP treatment. Additionally, biostimulation can also occur following bioaugmentation6, when nutrients are provided that can enrich native bacteria. Studies in microbiology have shown the convergence of microbial populations with time, regardless of the initial approach (stimulation vs. augmentation), indicating that the active population eventually reverts to the native microbial community20,28,32,37.
From a geotechnical standpoint, research has been performed to evaluate the engineering properties of MICP treated soil including evaluation of cohesive intercept and friction angle by triaxial testing, increases in soil strength by unconfined compression testing, increases in cyclic resistance by direct simple shear and cyclic triaxial testing, and changes in hydraulic conductivity, all of which have been well documented8,10,11,17,19,21,22,38,39,40,41,42,43,44,45,46. Clogging around injection ports/wells has posed an issue in the implementation of MICP; however, treatment formulations, rate of flow, and treatment resting time have been optimized to resolve and to achieve an uniform distribution of cementation throughout the soil column6,41,42,44,47,48. Most of these studies, however, have been performed on silica rich sands with quartz being the predominant mineral. Limited studies in the literature have explored the use of MICP with natural carbonate soils, which are a common and an important part of sedimentary rocks typically found in highest abundances along the coastal lines and reefs in the tropical and sub-tropical regions of the world between latitudes 30°N and latitude 30°S49,50,51. Xiao et al., (2018, 2019)52,53 performed undrained cyclic triaxial tests on MICP treated calcareous sand, consisting primarily of mollusk shells and shell fragments, which showed higher liquefaction resistance compared to untreated sands. Zhang et al.54 performed shake table tests on MICP treated calcareous sand with about 90% carbonate content and a similar improvement in liquefaction resistance and dynamic properties of calcareous soil was observed. While these results indicate that MICP process can be applied to calcareous soils, the mechanisms affording potential performance benefits in calcareous soils when compared to silica-rich soils remain unclear.
The objective of the present study was to explore the efficacy of the MICP process in naturally carbonate-rich soils and to compare performances with benchmark soils (silica sands) that have been well studied in the literature to date. To this end, MICP soil column experiments were performed with a naturally carbonate-rich soil and clean silica sands (F-110 and concrete sands) for different yeast extract enrichments, stimulation and cementation formulations. Chemical measurements were performed to monitor changes in pH, urea, and ammonium during the MICP treatment to assess the development of the ureolytic microbial community. Microscale characterization of cemented soil was performed to confirm the presence of precipitated calcite, obtain crystal characteristics such as shape, roughness, texture, and morphology, and provide insight into cementation bonds between soil particles. Macroscale tests were performed to quantify the strength of cemented samples, and bender element sensors were used to obtain shear-wave velocity data to monitor the evolution of soil stiffness with progression of cementation.
Materials and methods
Soil
Samples of the carbonate-rich soil used in the study were obtained from the Blessington quarry (County Wicklow, Ireland). The carbonate-rich Blessington soil is a non-plastic silty sand (USCS classification: SM), with 70% coarse fraction in the fine sand particle size range (300—75 μm), and the 30% fines fraction consisting mainly of silt sized particles. The predominant minerals were quartz and calcite, while the minor mineral phases included dolomite, muscovite, and feldspar. The geotechnical index properties and mineralogical composition of the soil determined from X-ray diffraction are given in Table 1. Soil samples were shipped to each university for study, and were obtained from the same formation, but at slightly different depths at the quarry site, which resulted in small differences in the initial carbonate content (24% for the soil tested at Georgia Tech, and ~ 29% for the soil tested at UC Davis). Silica sand samples included concrete sand obtained from an alluvial deposit (Woodland, California)55,56,57,58 and Ottawa F-110 sand (US Silica, 99% pure silica sand). The particle size distribution of the three soils used in the study is shown in Figure S1.
Ottawa F-110 and concrete sands were chosen as benchmark soils to evaluate the MICP performance of carbonate-rich Blessington soil because Ottawa sands have been widely adopted for MICP testing in the literature, as these sands are almost 99% quartz and their geotechnical properties are well characterized8,19,21,22,44,48, and MICP performance of concrete sand has been extensively studied at UC Davis55,56,57,58. Furthermore, Ottawa F-110 sand was chosen in the present study because its particle size gradation is closer to that of Blessington soil (Figure S1).
Soil columns
The experimental setup consisted of soil columns (6 inch. height and 3 inch. diameter) in cylindrical polytetrafluoroethylene (PTFE) cylinders (Fig. 1). Prior to each test, the components were acid-washed, followed by rinse with deionized water and ethanol. Two bender elements were installed on either side of the column at mid-height to obtain shear-wave velocity (Vs) measurements, and a third sampling port was included to obtain aqueous samples for the measurement of pH, urea, and ammonium. The Blessington soil was placed in the column by moist deposition and tamped in 3 layers with 15 blows to obtain a relative density of 30–40%, and void ratio between 0.82 and 0.85. Porex filters (pore size = 125 to 195 μm size) were used at the top and bottom of the cylinder as porous boundaries to allow flow while minimizing soil loss (Figure S2). A bottom-up flow treatment was implemented to minimize air entry and a vertical stress of 100 kPa was applied at the top of the column using a spring loading system.
MICP experimental program
The experimental matrix included evaluating the effect of yeast extract and urea concentrations included in stimulation solutions, urea to calcium ratios included in cementation solutions, as well as the effects of commercial versus research grade chemicals, and the efficacy of post-treatment ammonium by-product removal (Table 2). Yeast extract concentrations of 0.02 g/L, 0.2 g/L, and 2 g/L were tested to examine differences in stimulated rates by modulating enriched ureolytic cell densities. A yeast extract concentration of 2 g/L represents a higher-end value that has been used in previous studies, with the lower values yeast extract concentrations targeting lower bulk ureolytic rates intended to achieve full hydrolysis of supplied 350 mM urea between 48 and 96 h. Lower ureolytic rates enriched using lower yeast extract concentrations have been shown to result in more uniform cementation along injection lengths in tests involving silica soils for specific flow conditions6,32,55,57,58,59. Two different concentrations of urea in stimulation solutions were also used (50 mM and 350 mM) to evaluate possible differences in ureolytic activity due to changes in generated ammonium concentrations and post-treatment pH values. All columns receiving a lower concentration of urea in stimulation solutions (50 mM urea) were treated with a 1:1 urea to calcium chloride ratio cementation solution (250 mM urea, 250 mM calcium chloride). In contrast, columns receiving a higher concentration of urea in stimulation solutions (350 mM urea) received cementation solutions with 1.4:1 urea to calcium chloride ratios (350 mM urea, 250 mM calcium chloride). These specific treatment schemes were adopted for the research program following past studies on silica sands, which used stimulation solutions with 350 mM urea and cementation solutions with higher 1.4:1 urea to calcium chloride ratios with success, as well as more recent studies, which suggested that stimulation solutions with a lower 50 mM urea could be used in combination with cementation solutions with lower 1:1 urea to calcium chloride ratios without impacting cementation efficacy6,32,55,57,58,59. The naming convention for soil columns is as follows: Soil type_Yeast extract concentration_Urea concentration during stimulation_Urea to Calcium chloride ratio_Choice of Chemicals used. For example, BS_0.2_50_1:1_COM represents the Blessington soil column that received 0.2 g/L yeast extract enrichment and 50 mM urea during stimulation, 1:1 ratio of urea to calcium chloride cementation solution, and commercial grade chemicals are used for testing.
Two column tests (BS_0.2_50_1:1_COM and CS_0.2_50_1:1_COM) were performed to evaluate the effect of commercial grade chemicals compared to tests performed with laboratory grade chemicals (BS_0.2_50_1:1 and CS_0.2_50_1:1). Additionally, the columns treated with commercial grade chemicals were also used to test the effect of using rinsing solution to remove aqueous and sorbed ammonium from soil. Soil samples were obtained from counterpart columns BS_0.2_50_1:1 and CS_0.2_50_1:1 after completing cementation treatment to measure sorbed ammonium in soil columns that have not undergone rinsing, and compared to sorbed ammonium concentrations in soil samples from BS_0.2_50_1:1_COM and CS_0.2_50_1:1_COM which underwent rinsing for ammonia removal.
MICP testing on columns BS_0.2_350_1:1, BS_2_350_1:1, OS_0.2_350_1:1, and OS_2_350_1:1 was performed at Georgia Tech, and the rest of the column tests were performed at UC Davis.
MICP treatment procedure
The treatment solutions were prepared in artificial groundwater (0.45 mM magnesium sulfate, 0.04 mM sodium nitrate, 0.04 mM potassium nitrate, 1.75 mM calcium chloride, 1.10 mM sodium bicarbonate, and 0.06 mM potassium bicarbonate), after60. All soil columns were initially flushed with a minimum of 1 L (~ 3 to 3.2 times the pore volume) of artificial ground water solution. Treatment solutions of 500 ml volume (~ 1.5 to 1.6 times the pore volume) were pumped at a flow rate of 20 ml/min from bottom to top of the soil columns to ensure that the soil sample remains saturated (Table 3). For biostimulation columns, the stimulation solutions were prepared at approximately pH 9 and given 48 h to develop the ureolytic bacterial community before the second stimulation treatment6,32,57,58,59. Thereafter, the stimulation treatments were applied every 24 h for 5 additional days, resulting in a total stimulation treatment period of 7 days. Before starting with the cementation treatment, a flush solution was pumped through the columns to prevent abiotic calcite precipitation due to high residual carbonate species. Cementation treatments were provided every 24 h for a period of 10 days except in the case of slow stimulation columns (BS_0.02_50_1:1 and BS_0.02_350_1.4:1) that had a 48-h treatment interval for both the stimulation and cementation phase to enable complete hydrolysis of urea (Figure S3). During each treatment cycle, aqueous samples were collected for measurement of pH, urea, and ammonium, and shear-wave velocity measurements were obtained before the start of a new treatment cycle. After completion of 10 cementation treatments, the columns were disassembled, and samples from specific sections were obtained for calcite measurement, except in the case of BS_0.2_50_1:1_COM and CS_0.2_50_1:1_COM. These columns received staged injections of a potassium-rich rinse solution (a total of 12 pore volumes applied in 1 pore volume injections once every 24-h) for removal of ammonium species6,57,58,61,62. This specific rinse solution strategy has been found in previous studies to improve removal of both aqueous and sorbed ammonium concentrations when compared to other cation-enriched solutions and deionized water61.
Aqueous sampling
Aqueous samples were collected in two 2 mL samples daily before and after pumping of the treatment solution via needle and syringe from mid-height sampling ports to monitor changes in solution pH, urea, and ammonium concentrations. Additional 2 mL samples were collected for urea time course mapping during stimulation treatments 2nd, 4th, and 6th as well as during cementation treatments 2nd, 6th, and 10th. Time course samples were collected at 1, 2, 4, and 8 h after treatment injections for the fast stimulation columns, i.e., columns receiving yeast extract concentration of 0.2 g/L, and at 4, 8, 24, and 32 h after injections for the slow stimulation columns receiving yeast extract concentration of 0.02 g/L. Samples were measured for pH and then frozen at -20 °C for urea and ammonium measurements.
Chemical measurements
Urea measurements were performed using a colorimetric assay similar to63. Aqueous samples were diluted and reacted with a colorimetric reagent, consisting of absolute ethanol with 4% (w/v) p-dimethyaminobenzaldehyde and 4% (v/v) 12 M hydrochloric acid. Sample absorbances were measured at 422 nm using a microplate spectrophotometer.
Samples for ammonium analyses were collected in 2 mL tubes before and after each injection of the potassium chloride rinse from the sampling port at mid-height. An additional 2 mL sample was collected from the homogenized effluent solution. Total NH4+ measurements were done using a salicylate reaction method similar to62,64. Two reagents were used to dilute the samples and then measure absorbance values with a microplate spectrophotometer at 650 nm. The first reagent contained (weight/volume) 0.05% sodium nitroprusside, 13% sodium salicylate, 10% sodium citrate, and 10% sodium tartrate in water in weight per volume. The second reagent contained 5% sodium hypochlorite (volume/volume) and 6% sodium hydroxide (weight/volume) in water.
Sorbed ammonium measurements
Soil samples were obtained from top, middle, and bottom sections of soil columns BS_0.2_50_1:1, BS_0.2_50_1:1_COM, CS_0.2_50_1:1, and CS_0.2_50_1:1_COM after extrusion and frozen at -20 °C with residual moisture for later extraction to quantify the NH4+ concentration remaining sorbed on the soil particle surface. Sorbed ammonium measurement was performed following similar protocol outlined by62. Moist soil samples were thawed and homogenized, and then sample water contents and masses were obtained. Approximately 30 g of moist soil sample was filtered with a with 0.45 micron nylon filter basket and centrifuged at 3800 rpm for 1 h to extract solutions. Approximately 2 mL of solution was collected and analyzed for aqueous NH4+ concentrations. Sorbed NH4+ concentrations were measured using the KCl extraction process outlined by65. A 20 mL solution of a 2 M KCl was mixed with 10 g of the centrifuged soil and allowed to equilibrate to remove NH4+ ions from soil particle surface. Then, 2 mL of the solution was collected and measured for NH4+ concentrations. Sorbed NH4+ masses were determined by subtracting the NH4+ masses existing in free solution from the NH4+ masses measured after the KCl extraction.
Carbonate measurement tests
The quantity of carbonate minerals in the sample was determined in accordance with66 using a rapid carbonate analyzer (HM-4501, Humboldt). Calibration was performed with 99.9% pure ACS grade calcium carbonate. 1 M hydrochloric acid was used for dissolving the carbonates, and the pressure generated by CO2 was taken after a reaction time of 10 min. Thermogravimetric analysis (TGA) tests were performed using an Exstar TG/DTA 7300 analyzer. Soil samples weighing about 30–50 mg were heated to 105 °C in a nitrogen rich atmosphere to measure the mass loss due to reduction in free water content. The heating was increased to 950 °C in the same nitrogen rich atmosphere allowing for the decomposition of different mineral phases present in the soil sample. Then, the nitrogen was switched to an oxygen-rich atmosphere to allow for the combustion of residual carbon phases. The carbonate content was estimated from the mass loss curves. The loss on ignition test was performed using Nabertherm P300 in accordance with67 Method B. The samples were initially heated to 500 °C and held at that temperature for 30 min, and then the heating was continued to 950 °C. The samples were combusted at 950 °C for two hours. The carbonate content was estimated from the mass loss at the end of test.
Microscale characterization tests
X-ray diffraction (XRD) was performed to determine the mineralogy of soil samples (Malvern PANalytical Alpha-1). Cu-Kα radiation (λ = 1.5406 Å) of 45 kV and an electrical input of 40 mA were used with an incident-beam monochromator (Inc. beam Johansson 1xGe111 Cu/Co) and a X’Celerator detector. The diffraction pattern was recorded over a 2θ range of 4˚ to 80°. Anti-scatter slit of ¼˚ and divergence slit of 1/8°, mask of 15 mm, and diffracted beam anti-scatter slit of 5˚ were used. The diffraction pattern was analyzed using the X’Pert HighScore software to identify minerals present within samples.
Field Emission—Scanning electron microscopy (FE-SEM) was performed under low voltage conditions of 3 kV (Hitachi SU8230). For samples that showed specimen charging, gold coating was used (Hummer-6 sputter gold coater). Scanning electron microscopy—Energy Dispersive X-ray Spectroscopy (SEM–EDS) was performed on the same instrument with an Oxford EDS detector. The Aztec software suite was used to record and analyze the EDS data. EDS maps were performed at 1024 resolution, 5 frames per map, pixel dwell time of 50 µs, and process time between 4 and 5 to obtain sufficient counts and detector dead time for EDS analysis.
Biological sample preparation for SEM was performed to obtain SEM images of bacteria during the MICP process. After the final cementation treatment, the columns were disassembled, and small cemented samples were carefully immersed in a fixating agent (glutaraldehyde 2%, paraformaldehyde 2% in phosphate buffer of pH 7.4) to stabilize and prevent the biological structures from decomposition. The sample was immersed in the fixation agent for 1 h in the fume hood and then moved to 4° C in the refrigerator. After 24 h, the excess fixation agent was removed by rinsing with deionized water and the sample was immersed in phosphate-buffered saline (PBS). It was then immersed in deionized water to dissolve any remaining salts followed by drying with ethanol in sequential concentrations of 20%, 35%, 50%, 75%, 90%, and 100% for 30 min each. This was followed by drying in Hexamethyldisilazane (HMDS). Finally, the specimen was coated with gold sputter for SEM imaging.
Shear-wave velocity measurements
At Georgia Tech, parallel type piezoelectric bender elements were fabricated for the measurement of shear-wave velocity. The dimensions of the bender elements were approximately 12 × 7 × 1 mm (length x width x thickness). Thin polyurethane coats were applied for waterproofing, and grounding of the experimental setup was ensured to reduce crosstalk. The bender elements were fixed at mid-height of the column and oriented horizontally. A function generator was used to produce a 10 V, 20 Hz square wave as the input wave for the transmitter bender element (Agilent 33210A). The signal from the receiver bender element was filtered and amplified through a filter (Krohn-Hite Model 3364), using a high pass cutoff frequency of 100 Hz and low pass frequency of 30 kHz. The signal was viewed and acquired using a digital oscilloscope with a sampling frequency of 100 MHz (Agilent DSO-X 3014A). A total of 1024 signals were stacked to reduce the noise in the output signal, and saved for further analysis and determination of first arrival of shear wave.
At UC Davis, shear wave velocity (Vs) measurements were taken using bender element pairs at the mid-height of each column, oriented horizontally. Shear waves propagated horizontally and were polarized in the vertical direction. Bender elements were fabricated and waterproofed with epoxy, electronics wax, and an insulation coating55,68,69,70. A 24V, 100 Hz square wave was used as the input wave for the transmitter bender element and the received signal from the receiver bender element was viewed using an oscilloscope with a sampling frequency of 1 MHz (National Instruments DAQ system). Vs measurements were visually interpreted based on the arrival time at the first peak of the shear wave and measured sensor spacings. Measurements were filtered using a Python 3.7 script with a 30 kHz high frequency corner and 200 Hz low frequency corner.
Unconfined compression testing
Unconfined compressive strength (UCS) testing was performed on soil specimens obtained from three columns: Columns BS_0.02_50_1:1, BS_0.02_350_1.4:1 and BS_0.2_350_1.4:1. The bio-cemented soil columns were extruded using a hydraulic jack (maximum pressure of 3500 kPa to limit sample disturbance). Extruded specimens were oven dried at 60 °C for at least 72 h prior to testing. UCS testing was performed using a Geo-Tac instrument subjecting the bio-cemented soil specimens to 1% strain per minute until failure.
Results and discussion
Comparison of MICP treatment of carbonate-rich Blessington soil with silica-rich benchmark soils
The calcite profiles of MICP treated Blessington, concrete, and Ottawa F-110 sands for 0.2 g/L yeast extract enrichment are shown in Fig. 2. In the case of Blessington sand, the precipitated calcite profile is calculated as the difference between total calcite measured after MICP treatment and the initial calcite content of the untreated sample. The Blessington calcite profile followed a non-uniform trend, with more cementation observed near the injection port and progressively less calcite content away from it, whereas, the calcite profiles of Ottawa F-110 and concrete sands were relatively more uniform when compared to Blessington sand. This non-uniform cementation trend is well-documented in the MICP literature17,18,48 and is normally attributed to a non-uniform reaction front that develops because of immediate urea hydrolysis by microbes near the injection port as nutrients are provided71. A localized, non-uniform distribution of calcite precipitation develops in the soil column which intensifies with each cementation treatment. Additionally, although the MICP experiments were performed at the same flow rate conditions (20 mL/min) for the soils tested, it is possible that the intrinsic pore structure of Blessington soil may have played a role in the non-uniformity of cementation observed to a greater degree compared to Ottawa and concrete sands.
Furthermore, from Fig. 2, the precipitated calcite content was higher in Blessington sand for bottom half of the column compared to F-110 sand (as high as 3% near the injection port), and it was slightly higher in Blessington than in concrete sand (~ 1%), having adopted the same MICP treatment formulation, flow rate, and treatment scheme between the two sets of carbonate-rich and silica-rich sands. This observed increase in calcite precipitation in Blessington soil suggests that the presence of natural carbonates has an impact on the MICP process, which could provide preferential sites for calcite crystal formation because of a reduction in energy barrier for crystal nucleation72 and therefore result in higher calcite precipitation; more details with respect to this aspect are discussed in the following section. Additionally, the MICP performance of Blessington soil was similar between the two laboratories, Georgia Tech – BS_0.2_350_1:1 and UC Davis – BS_0.2_50_1:1, and the small differences in calcite content could be due to inherent variability of natural carbonates in Blessington soil and different concentrations of stimulation solution adopted.
The corresponding shear-wave velocity (Vs) profiles, which provides a measure of soil stiffness and insight into degree of cementation bonds formed between soil particles, for the MICP treated Blessington, concrete, and F-110 sands are shown in Fig. 3, where the improvement of shear wave velocity with every cementation treatment was observed. Initial shear-wave velocity of concrete sand was the highest (~ 190 m/s) among the three sands, which steadily increased and remained higher than Blessington soil (BS_0.2_50_1:1) for the entire cementation treatment (maximum Vs of 580 m/s). The higher Vs of concrete sand could be because of fewer contact points between sand particles, owing to its larger particle size, as compared to Blessington sand with a greater number of particle contacts because of small particle size and 30% fines content. Therefore, even though the precipitated calcite profiles between Blessington and concrete sands were similar, the shear-wave velocity profile of concrete sand was on average ~ 130 m/s higher than Blessington sand. The initial Vs of F-110 sand was low (~ 82 m/s), however, its final Vs of 580 m/s was similar to that of concrete sand which implies a higher rate of increase in shear-wave velocity (ΔVs). This suggests that comparatively, more of the calcite precipitation in F-110 sand resulted in inter-particle contact bonds resulting in higher ΔVs, whereas in concrete sand some of the calcite precipitation may have also resulted in particle coating as the particle size of concrete sand was larger. Furthermore, the Vs profile of F-110 sand was also higher than Blessington sand, indicating that the presence of fines in Blessington sand resulted in a greater number of inter-particle contacts for the shear-wave to pass through and may have resulted in lower Vs values of Blessington sand. The Vs profiles of Blessington sand from both the labs were comparable and agreed well with each other; the slightly higher Vs values of BS_0.2_350_1:1 (Georgia Tech) compared to BS_0.2_50_1:1 (UC Davis) could be due to higher precipitated calcite content in BS_0.2_350_1:1 (Georgia Tech) sample which likely resulted in more inter-particle bonds.
Effect of natural carbonates, yeast extract concentration, and treatment formulations on MICP process in carbonate-rich Blessington soil
Calcite profiles of Blessington and sands treated with different treatment formulations adopted in the present study are shown in Fig. 4. Among the different MICP treatment variables considered in the experimental matrix (Table 2), yeast extract concentration had a significant impact on calcite precipitation in Blessington sand. For the same flow rate conditions, increasing yeast extract concentration from 0.2 g/L to 2 g/L resulted in a high increase in MICP efficiency [calculated as (Mass of Ca2+ in precipitated CaCO3)/Mass of input Ca2+ in cementation solution)] in carbonate-rich Blessington soil from 48 to 72% (BS_0.2_350_1:1 and BS_2_350_1:1) compared to 44% to 49% in silica-rich F-110 sand (OS_0.2_350_1:1 and OS_2_350_1:1); and, the increase in calcite precipitation was as high as 80% in Blessington sand (BS_0.2_350_1:1 and BS_2_350_1:1) compared to 27% in Ottawa F-110 sand (OS_0.2_350_1:1 and OS_2_350_1:1). Comparing MICP efficiency between carbonate-rich and silica-rich soils for the same flow rate, treatment formulation, and yeast extract concentration of 2g/L, it increased from 49% in silica-rich F-110 sand to ~ 72% in carbonate-rich Blessington soil.
The observed increase in MICP efficiency of Blessington soil suggests that the use of higher yeast extract concentration for MICP treatment resulted in increased microbial growth and activity, which increased urea hydrolysis, and subsequently the pH of and total carbonate within pore fluids. The increase in pH resulted in higher carbonate ion concentration, thus increasing the saturation state of the system to greater than 1, S > 1,
where \(\left({{\varvec{a}}}_{{{\varvec{C}}{\varvec{a}}}^{2+}}\right).\left({{\varvec{a}}}_{{{\varvec{C}}{\varvec{O}}}_{3}^{2-}}\right)\) is the ion activity product of calcium and carbonate ions in the solution, and Ksp is the solubility constant of CaCO3. Supersaturation is critical for calcite nucleation and subsequent crystal growth60; consequently, by providing higher yeast extract formulation, supersaturation conditions were initiated faster compared to lower yeast extract formulations which led to more calcite precipitation.
Furthermore, the observed increase in precipitated calcite content with higher yeast extract formulation in Blessington soil compared to Ottawa F-110 sand suggests a compounding effect of lower energy barrier offered by the natural carbonates acting as preferential sites for calcite precipitation in the Blessington soil. In the absence of natural carbonates (Ottawa F-110 sand), it is highly likely that the bacteria serve as the nucleation sites due to increase in localized pH and supersaturation around the cell wall16,24,73,74. However, the presence of natural carbonates in the system offers another reduced energy path for calcite precipitation72,75,76,77,78. Zehner et al.72 have observed this mechanism at a micro-scale by adding calcite seeds, about 8 μm in size, to bacterial cultures and performing MICP experiments in a 96-well plate. The preferential growth of calcite crystals on calcite seeds was observed through optical microscopy, scanning electron microscopy, and quantified through absorption spectroscopy and optical density measurements. The results were compared with unseeded MICP experiments to provide evidence that calcite seeds were indeed serving as preferential sites for calcite precipitation. Furthermore, the amount of calcite precipitation was also observed to increase for two bacterial cultures with higher cell concentrations as compared to bacterial cultures with lower cell concentrations. These micro-scale results from Zehner et al., (2021) agree well with the macro-scale results obtained in the present study.
According to classical nucleation theory, the nucleation rate is given by:
Where, \({J}_{n}\) – nucleation rate,\({J}_{0}\)—kinetic factor related to the frequency and efficiency of collision, \({\nu }_{m}\) – molecular volume of the forming phase (cm3/molecule, for calcite: 6.13 × 10–23 cm3), \({\alpha }{\prime}\)—effective interfacial energy (mJ/m2), \({k}_{B}\)—Boltzmann constant (1.38 × 10−23 J K−1), T—Temperature (K), and—\({\sigma }\)Supersaturation, ln(IAP/Ksp) [IAP—Ion activity product of (Ca2+)(CO32)].
Between carbonate and silica quartz substrates, the interfacial energy term controls the nucleation rate of calcite on the two substrates. For spherical nucleating phase particles, the effective interfacial energy term is given by79,80:
where, \({\alpha }_{lc}\)—interfacial energy between liquid and crystal, \({\alpha }_{ls}\)—interfacial energy between liquid and substrate, \({\alpha }_{sc}\)—interfacial energy between substrate and crystal, and \(\theta\) – contact angle between the nucleating phase and substrate.
From in situ grazing incidence small-angle X-ray scattering (GISAXS) experiments79,80, the interfacial energy for nucleating calcite phase on quartz substrate has been found to be 36 and 47 mJ/m2 by Fernandez-Martinez et al. (2013) and Li et al. (2014), respectively, and Lioliou et al. (2007) have reported 31.1 mJ/m2 as the interfacial energy for calcite on quartz seeds based on bulk rates of precipitation, which is at least half the homogeneous nucleation interfacial energy value of calcite (Tables 4 and 5). Based on Lioliou et al., (2007) data for bulk rate of precipitation of calcite crystals on calcite seeds, the interfacial energy was estimated to be ~ 10.14 mJ/m2 (a conservative estimate), and is at least one-third the interfacial energy between calcite and quartz. Furthermore, Freeman & Harding, (2023) report interfacial energy in the range of 0.03–0.5 mJ/m2 for amorphous calcium carbonate (ACC) on calcite, based on molecular dynamics simulations. The possibility of calcite nucleation based on ACC precursor phase cannot be ignored as well82,83,84,85,86,87,88,89,90,91,92. Based on the reported literature and estimated interfacial energy values (Tables 4 and 5), it can be stated that the carbonate substrate offers a lower energy barrier compared to quartz, and calcite precipitation on carbonate substrate is, in fact, a continuation of crystal growth of pre-existing calcite crystals77,78.
From Fig. 4, calcite profiles of BS_0.02_50_1:1 and BS_0.2_50_1:1 columns were similar which suggested that yeast extract concentrations of 0.02 g/L and 0.2 g/L were not sufficient to induce a high saturation state immediately, and hence calcite nucleation and precipitation took a longer time. In contrast, by increasing the yeast extract concentration to 2 g/L, sufficient nutrients were provided for microbial growth and activity to induce a faster saturation state because of greater amount of urea hydrolysis (chemical measurements discussed in the following sections). Once calcite nucleates, a lower saturation state is sufficient for crystal growth, and therefore, a higher amount of calcite precipitation was observed (BS_0.2_350_1:1 and BS_2_350_1:1). It should be noted that recent experimental results have shown lower yeast extract concentrations have served well to produce more uniform cementation profiles in the soil columns without sacrificing MICP efficiency greatly32,57; however, in the case of Blessington soil, the presence of natural carbonates added complexity to the process by changing the MICP efficiency with change in yeast extract concentration.
Comparing the precipitated calcite profiles of Blessington sand treated with lower and higher concentration of stimulation solutions during MICP treatment (BS_0.02_50_1:1 and BS_0.02_350_1.4:1, and BS_0.2_50_1:1 and BS_0.2_350_1.4:1), similar level of cementation across these profiles was observed. Hence, it is possible to reduce concentration of urea from 350 to 50 mM during stimulation phase, and reduce urea to calcium ratio from 1.4:1 to 1:1 during cementation phase, and still achieve similar level of cementation while reducing the ammonium concentrations. Further, the use of commercial chemicals over laboratory grade chemicals did not significantly impact MICP efficiency (BS_0.2_50_1:1 and BS_0.2_50_1:1_COM, and CS_0.2_50_1:1 and CS_0.2_50_1:1_COM).
Chemical measurements
pH
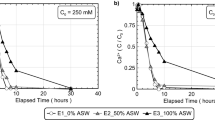
The pH measurements taken before and after treatment solution injection for different MICP columns are shown in Fig. 5. The effect of using higher and lower concentration of stimulation solution, with yeast extract concentration of 0.02 g/L, on pH values showed that: during the cementation phase (Fig. 5(a) columns BS_0.02_50_1:1 and BS_0.02_350_1.4:1), the column receiving 1:1 (250 mM urea to 250 mM CaCl2) treatment formulation did not show an increase in pH until the 6th treatment which signified that ureolytic bacterial community was not fully developed in this column, whereas the column receiving 1.4:1 treatment formulation (350 mM urea to 250 mM CaCl2) showed an increase in pH with every cementation treatment from 7.2 to 8.5–9.0, which signified urea hydrolysis and the effect of additional 100 mM of urea, which continued to be hydrolyzed resulting in higher pH indicating that an active ureolytic microbial community had indeed developed and was present in this column. Both the columns showed a pH reduction to 7.2–7.3 after calcite precipitation, which signified the completion of reactions.
pH measurements before and after pumping of each treatment for different MICP column experiments: (A) BS_0.02_50_1:1 and BS_0.02_350_1.4:1, (B) BS_0.2_50_1:1, BS_0.2_350_1.4:1, BS_0.2_350_1:1, and BS_2_350_1:1, (C) BS_0.2_50_1:1 and BS_0.2_50_1:1_COM, and (D) CS_0.2_50_1:1, CS_0.2_50_1:1_COM, OS_0.2_350_1:1, and OS_2_350_1:1.
Comparing Fig. 5(a) and (b), the effect of increasing yeast extract concentration on pH values was observed (columns BS_0.02_50_1:1, BS_0.2_50_1:1, and BS_2_350_1:1). During the cementation phase, it was observed that for columns receiving lower concentration of stimulation solution (50 mM), providing a yeast extract concentration of 0.2 g/L successfully stimulated the ureolytic bacteria and resulted in pH increase from 7.3 to 8.1, which was not the case with yeast extract concentration of 0.02 g/L. Increasing the yeast extract concentration from 0.2 to 2 g/L resulted in a slightly higher pH values to 8.3, which can be attributed to faster rate of urea hydrolysis during pumping of treatment solutions. After calcite precipitation, pH decreased to similar values (~ 7.3) signifying completion of reactions. However, in the case of columns receiving a higher concentration of stimulation solution (350 mM), the pH increased from 7.3 to around 8.8 to 9.1 due to hydrolysis of additional 100 mM urea, which was similar to the observations made in Fig. 5(a).
The effect of using commercial grade chemicals during MICP treatment is compared in Fig. 5(c) for the columns BS_0.2_50_1:1 and BS_0.2_50_1:1_COM. It can be seen that the pH values in both the columns followed a similar trend, and the use of commercial grade chemicals did not change the geochemical dynamics of the MICP process significantly. Furthermore, similar observations were made for the commercial grade chemicals used in the concrete sand columns, CS_0.2_50_1:1 and CS_0.2_50_1:1_COM from Fig. 5(d).
Comparing Fig. 5(b), (c), and (d), the difference in pH values was observed between Blessington and silica sands (BS_0.2_50_1:1, BS_2_350_1:1, CS_0.2_50_1:1, OS_0.2_350_1:1, and OS_2_350_1:1). During cementation phase, for similar yeast extract concentration and rate of stimulation, the Blessington, Ottawa F-110, and concrete sands reached similar pH values of 8.1, however, after calcite precipitation and at the end of 24 h, Blessington soil reached a pH around 7.3 while concrete and Ottawa F-110 sands reached a pH value around 7.5 to 7.7. This suggests that higher calcite precipitation observed in Blessington soil might be responsible for driving pH further down compared to silica sands, which did not show this behavior.
Urea measurements before and after treatment
The urea measurements performed before and after treatment solution injection for different MICP columns are shown in Fig. 6. For the columns treated with lower yeast extract concentration (0.02 g/L), it can be observed that the urea, 50 mM and 350 mM, provided during stimulation phase was not hydrolyzed completely (Fig. 6(a)). For the column BS_0.02_50_1:1, it required about 6 cementation treatments for complete hydrolysis to occur, which suggests that the ureolytic microbial community had not fully developed in the initial stages of the MICP experiment for this column, and a yeast extract concentration of 0.02 g/L may not be sufficient. This observation was also reflected in pH measurements discussed in the previous section. Hence, reducing the yeast extract concentration to 0.02 g/L and adopting a lower concentration of stimulation solution can slow the development of ureolytic microbial community and decrease the rate of urea hydrolysis. In the case of BS_0.02_350_1.4:1, the column with higher concentration of stimulation, complete urea hydrolysis of 350 mM occurs in the first cementation treatment, and the trend continued throughout the rest of the experiment. This was also reflected in the pH measurements which increased to 8.5–9.0, indicating that even for a low yeast extract concentration of 0.02 g/L, providing a higher concentration of stimulation solution can establish a ureolytic microbial community that completely hydrolyzes the urea. The effect of these variables was also reflected in the precipitated calcite profiles (Fig. 4), wherein 0.5–1% increase in precipitation calcite was observed for BS_0.02_350_1.4:1 compared to BS_0.02_50_1:1 column.
Increasing the yeast extract concentration to 0.2 g/L, demonstrated that urea hydrolysis was fully complete by the third stimulation cycle for both the columns (BS_0.2_50_1:1 and BS_0.2_350_1.4:1, Fig. 6(b)). This trend continued into the cementation phase wherein complete urea hydrolysis occurred in both the columns from the first cementation treatment cycle. Consequently, for a yeast extract concentration of 0.2 g/L, the lower stimulation concentration of 50 mM urea with 1:1 cementation formulation was sufficient for MICP treatment without reducing its efficiency.
Urea measurement profiles for the column experiments (BS_0.2_50_1:1_COM and CS_0.2_50_1:1_COM) were performed with commercial grade chemicals and compared with their respective counterpart columns (BS_0.2_50_1:1 and CS_0.2_50_1:1) (Figs. 6(c) and (d)). The use of commercial grade chemicals during the MICP treatment did not result in significant changes to urea hydrolysis for both Blessington and concrete sands, indicating that that commercial chemicals will likely perform as well as laboratory grade chemicals. Furthermore, urea measurements in the concrete sand were similar to that of Blessington sand indicating that complete urea hydrolysis also occurred in the concrete sand.
Urea time-course measurements
Urea time-course sampling measurements were performed during stimulation treatments 2, 4, and 6 (Fig. 7). Urea hydrolysis was not significant for the column BS_0.02_50_1:1 with low yeast extract of 0.02 g/L and low concentration of stimulation solution (50 mM) across all the stimulation treatments of 24-h duration, which is in agreement with the urea measurements from the previous section. The column BS_0.02_350_1.4:1 achieved nearly 40% urea hydrolysis by the end of the sixth stimulation cycle; this was also reflected in final urea measurements (Fig. 6(a)) and the increase in pH to 9.3. Additional urea during the stimulation phase did help establish an ureolytic microbial community even when a low yeast extract concentration of 0.02 g/L was used. For the second stimulation treatment, the Blessington soil columns (BS_0.2_50_1:1, BS_0.2_350_1.4:1 and BS_0.2_50_1:1_COM) achieved only partial urea hydrolysis; however, the concrete sand columns (CS_0.2_50_1:1 and CS_0.2_50_1:1_COM) achieved full urea hydrolysis by end of 24 h (Fig. 7(a)). However, by the fourth stimulation treatment, all the columns with higher yeast extract concentration (0.2 g/L) achieved full urea hydrolysis within 8 h after injections, with similar results produced for the sixth stimulation treatment. Providing a yeast extract of 0.2 g/L, however, resulted in significant ureolytic activity after 4 stimulation treatments. These results agree and match well with previous pH and urea measurements (Figs. 5(b) and 6(b)) where the increase in pH and complete urea hydrolysis was observed by the end of the third stimulation treatment for columns treated with yeast extract concentration of 0.2 g/L.
Concentrations of aqueous urea during time course sampling at different stimulation and cementation treatment cycles for different MICP treatment formulations: (A) 2nd stimulation treatment, (B) 4th stimulation treatment, (C) 6th stimulation treatment, (D) 2nd cementation treatment – YE 0.2 g/L, (E) 6th cementation treatment – YE 0.2 g/L, (F) 10th cementation treatment – YE 0.2 g/L, (G) 2nd cementation treatment – YE 0.02 g/L, (H) 6th cementation treatment – YE 0.02 g/L, and (I) 10th cementation treatment – YE 0.02 g/L.
For columns treated with yeast extract concentration of 0.2 g/L and 250 mM urea (1:1 cementation treatment) 80% of urea hydrolysis (reduction in urea concentration from 250 to 50 mM), occurred within 4 h for the second cementation treatment and within 2 h for the sixth and tenth cementation treatments, and 100% urea hydrolysis within 6 h for all cementation treatments (Fig. 7(d) - (f)). For columns treated with 350 mM urea (1.4:1 cementation treatment) 85% of urea hydrolysis (reduction in urea concentration from 350 to 50 mM) occurred within 4 h for the second cementation treatment and within 2 h for the sixth treatment, and 100% urea hydrolysis occurred within 8 h for all cementation treatments. Comparing urea hydrolysis profiles between Blessington and concrete sands, the urea degradation curves were similar and followed the same trend. Furthermore, the use of commercial grade chemicals did not affect the rate of urea hydrolysis. These results agree with the final urea measurements from Fig. 6(b), (c), and (d) performed after each cementation treatment, which showed complete urea utilization for the columns treated with yeast extract of 0.2 g/L.
The effect of low yeast extract concentration (0.02 g/L) on the extent of urea hydrolysis during cementation treatments can be seen in Fig. 7(g), (h), and (i). The column BS_0.02_50_1:1 achieved 40% urea hydrolysis from 250 to 150 mM in 48 h for the second cementation treatment, and achieved 100% urea hydrolysis for the sixth and tenth cementation treatments. Comparing this result with final urea measurements from Fig. 6(a), it is likely that complete urea hydrolysis occurred in this column from the sixth cementation treatment onward. In contrast, the column with higher concentration of stimulation solution (BS_0.02_350_1.4:1) achieved 100% urea hydrolysis from the first cementation treatment onwards, and was consistent with final urea measurements from Fig. 6(a). Column BS_0.02_350_1.4:1 achieved 90% urea hydrolysis in 30- and 24-h (second and sixth cementation treatments respectively), whereas column BS_0.02_50_1:1 achieved 90% urea hydrolysis in 46 h during the sixth treatment but did not achieve 90% urea hydrolysis during the second treatment. Furthermore, it can be observed that the rate of hydrolysis in these columns increased with the progression of cementation treatments although it remained less than the rate of urea hydrolysis of columns treated with the higher yeast extract concentration of 0.2 g/L (Fig. 7(d) - (f)). The columns treated with yeast extract concentration of 0.2 g/L required at least 6 h to reach 90% urea hydrolysis whereas BS_0.02_350_1.4:1 required at least 5 times more time (i.e., 30 h). Similar to results by Lee et al. 2023, differences in yeast extract concentration were shown to alter ureolytic enrichment and bulk urea hydrolysis rates, with other differences such as differences in urea concentration during stimulation and cementation treatment recipes, having only minor effects.
Sorbed ammonium measurements
Aqueous and sorbed ammonium concentrations are shown in Fig. 8. BS_0.2_50_1:1_COM and CS_0.2_50_1:1_COM were used to test the efficacy of using KCl rinsing solution for removal of sorbed ammonium from soil; these columns received 12 treatments of rinsing solution similar to past studies58,61. The results are compared with BS_0.2_50_1:1 and CS_0.2_50_1:1 columns, which did not undergo rinsing, and soil samples were obtained from these columns to measure sorbed ammonium concentrations. The reduction of aqueous ammonium concentration in BS_0.2_50_1:1_COM and CS_0.2_50_1:1_COM columns with respect to rinse treatment and pore volume is shown in Fig. 8(a) and (b), respectively. The reduction in aqueous ammonium concentration from 455 mM to 0.03 mM for column BS_0.2_50_1:1_COM, and from 468 mM to 0.09 mM for column CS_0.2_50_1:1_COM can be observed in Fig. 8(a). These initial ammonium concentrations of 455 mM and 468 mM suggest that the 250 mM of urea provided during cementation treatment was almost completely hydrolyzed. During rinse treatments, the column BS_0.2_50_1:1_COM received approximately 12.6 pore volume of KCl rinse solution and CS_0.2_50_1:1_COM received approximately 10.6 pore volumes.
Changes in aqueous ammonium concentrations from the pore fluid of BS_0.2_50_1:1_COM and CS_0.2_50_1:1_COM versus (A) rinse treatment and (B) number of pore volumes. Ammonium removal versus rinse treatment for rinsed columns (BS_0.2_50_1:1_COM and CS_0.2_50_1:1_COM) in (C) cumulative ammonium concentration and (D) percent ammonium removal with respect to sorption concentrations obtained from unrinsed columns. Ammonium concentrations in unrinsed columns (BS_0.2_50_1:1 and CS_0.2_50_1:1) along the column length from (E) soil pore fluid and (F) sorbed ammonium in soil samples.
When compared to unrinsed columns, the applied KCl solution decreased pore fluid ammonium concentrations near 4 orders of magnitude after 12 treatments. Cumulative ammonium removal with respect to the total aqueous ammonium removed is also provided in Fig. 8(c) with ammonium removal percentages as a function of the total ammonium determined from data from the unrinsed columns BS_0.2_50_1:1 and CS_0.2_50_1:1 shown in Fig. 8(d). As shown, nearly 99% cumulative ammonium removal occurred after 4 rinse treatments with less dramatic reductions in ammonium concentrations with further rinse treatments. The efficacy of ammonium removal using K+ enriched solutions has been more extensively studied in other studies61 and can likely be attributed to both cation exchange between sorbed ammonium and potassium on soil surfaces and the high pH of the rinse solution, which may promote speciation of NH4+ ions to charge-neutral NH3, thereby enabling greater removal by limiting molecule and soil surface interactions58.
The aqueous and sorbed ammonium concentrations for unrinsed columns BS_0.2_50_1:1 and CS_0.2_50_1:1 are shown in Fig. 8(e) and (f), respectively. The average aqueous ammonium concentrations from soil samples taken from BS_0.2_50_1:1 and CS_0.2_50_1:1 were found to be 442 mM and 518 mM respectively. Furthermore, the average sorbed ammonium concentrations were 58 μmol/g of dry soil for Blessington sand, and 53 μmol/g of dry soil for concrete sand. Sorbed ammonium concentrations may remain in MICP treated soils after rinsing treatments, but could be addressed through other processes and/or remain largely sorbed depending on surrounding soil and pore fluid chemistry. Presently, the Environmental Protection Agency (EPA) has no maximum limit on the NH4+ concentration in drinking water; however, a maximum total NH4+ concentration of 17 mg/L (approximately 1 mM) for aquatic life, and 1.9 mg/L (approximately 0.1 mM) for acute and chronic exposure have been recommended101.
TGA and LOI measurements
Thermogravimetric analysis (TGA) results of untreated and MICP treated samples are shown in Fig. 9 with the normalized mass (TG) loss curves (Fig. 9(a) and (b) and the first differential of mass loss (DTG) with respect to change in temperature (Fig. 9(c) and (d)). A 99.9% ACS grade pure calcium carbonate sample (Sigma-Aldrich) was tested to obtain a reference mass loss curve (shown as red dashed line). During TGA, essentially the entire loss in mass of the calcium carbonate sample (~ 46%), occurred in the temperature range of 600–800 °C. The loss in mass was due to the decomposition of calcium carbonate to calcium oxide and carbon dioxide gas. From Fig. 9(a), the highest mass loss (~ 13%) was observed for untreated Blessington sand in the temperature range between 600 and 800 °C, and translates to a carbonate content of ~ 29.4% in the soil sample, which is consistent with the carbonate content of 29% (Table 1) determined from the acid digestion test for the untreated Blessington soil sample. Mass loss in bio-cemented Blessington soil samples was about 14–15%, which is ~ 1–2% higher compared to untreated soil sample and equates to about 3–8% of precipitated calcite content, depending on the treatment formulation of the soil columns. Furthermore, from Fig. 9(c), it may be observed that there were few smaller peaks of DTG around 300–500 °C which could be due to loss of bound water in hydrated minerals in the temperature range of 200–400 °C, and also due to decomposition of secondary mineral phases such as dolomite, present in the Blessington soil, which can occur at a temperature as low as 470 °C102,103,104. In contrast, from Fig. 9(b) and (d), it can be seen that most of the mass loss for concrete and silica sands occurred between 600 and 800 °C, and there were fewer DTG peaks below 600 °C. The untreated concrete sand showed a mass loss of 2.4% and the MICP treated concrete sand samples showed a mass loss of ~ 7%, while the untreated silica sand showed negligible loss in mass of 0.1% and MICP treated silica sand showed ~ 2.1%, which is equivalent to ~ 4.5% of precipitated calcite, and comparable to the precipitated calcite profile shown in Fig. 2.
Thermogravimetric measurements for pure calcium carbonate, untreated, and MICP treated Blessington, concrete, and Ottawa sands. (A) Normalized mass fraction curves for untreated and MICP treated Blessington soil samples, (B) Normalized mass fraction curves for untreated and MICP treated concrete and Ottawa F-110 sand samples, (C) DTG (first differential of mass loss) curves for untreated and MICP treated Blessington soil samples, and (D) DTG curves for untreated and MICP treated concrete and Ottawa F-110 sand samples.
The loss on ignition (LOI) test results are presented in Table 6. Compared to the TGA test, LOI is a more representative test for loss in mass because the size of soil samples used for testing is larger (in the range of 10–15 g for LOI compared to range of 30–60 mg for TGA). However, the mass loss as a function of temperature is not measured in the LOI test, only the final masses after combustion. From Table 6, it can be noted that the untreated Blessington showed a carbonate content of 30%, whereas untreated Ottawa F-110 sand lost 0.2% of its mass on heating to 950 °C, which agree with the TGA results obtained. Concrete sand showed a loss in mass of 3%, comparable to 2.5% from TGA result, which might indicate that it contains secondary minerals that are undergoing decomposition. The precipitated calcite contents of bio-cemented samples also agree well with the TGA results and the precipitated calcite profiles from acid digestion tests reported earlier.
Microscale tests
X-ray diffraction
X-ray diffraction (XRD) was performed on soil samples to obtain their mineralogical composition, and to confirm the presence of the precipitated calcite mineral phase in the MICP treated soil samples. The XRD scan of pure calcium carbonate, from 2 theta angle of 4° to 80° serves as reference pattern for calcite peaks (Fig. 10(a)). The XRD scan of untreated Ottawa F-110 sand consisted of essentially purely quartz mineral phase (Fig. S4). Comparing it with the XRD scan of MICP treated Ottawa F-110 sand (Fig. 10(b)), the presence of calcite peaks was noted, especially the significant peak at 29.5°, which corresponds to 100% relative intensity in the pure calcite XRD pattern. Rietveld refinement was performed to quantify the mineral phases, which indicated a calcite content of 3.8% in the MICP treated Ottawa F-110 sand sample. This agreed well with the previous calcite measurements made from acid digestion, TGA, and LOI tests. XRD patterns of untreated concrete sand (Fig. S5) showed the presence of quartz and albite minerals, with some vermiculite as minor phase, while MICP treated concrete sands (Fig. 10(c) and Fig. S5) showed the presence of calcite peaks and calcite content of 6.8–7.8%, which was slightly higher compared to the acid digestion results but similar to TGA and LOI results.
For the XRD pattern of untreated Blessington soil, most of the significant peaks in the XRD pattern were identified and designated, with the exception of a few small peaks (Fig. S6). The Blessington soil is a multiphase mineralogical material, with quartz and calcite being the dominant phases, and albite, muscovite, and dolomite being the secondary mineral phases. Muscovite peak at 8.89°, albite peaks at 22.1° and 27.98°, and dolomite peak at 31° were noted. XRD patterns of MICP treated Blessington soil samples with different yeast extract concentrations of 0.02, 0.2 (Fig. 10(d)), and 2 g/L are shown in Figures S6(b), 10(d) and S6(c), and S6(d) respectively. Rietveld analysis of BS_0.2_350_1:1 and BS_2_350_1:1 showed calcite content of 31.8% and 32.1%, which is about 7.8% and 8.1% precipitated calcite in the MICP treated soil samples, which is comparable with previously obtained results from acid digestion, TGA, and LOI tests. However, Rietveld analysis of BS_0.02_50_1:1 shows a calcite content of 19% which is low compared to previous results; this could have been an error resulting from overcompensation of quartz phase in the soil sample. Hence Rietveld analysis, which is a semi-quantitative approach, was used with caution to draw conclusions on quantitative analysis.
Scanning electron microscopy
Scanning electron microscopy (SEM) images were obtained to gain insight into the interaction of microbes with soil particles and precipitation of calcite crystals, calcite crystal shape and morphology, calcium carbonate polymorphs, and cementation bonding of soil particles. For this purpose, high resolution SEM images of MICP treated Blessington soil were obtained using a Field Emission electron microscope (FE-SEM) (Fig. 11). High magnification FE-SEM image of bacteria present on the surface of calcite crystals is shown in Fig. 11(a). The bacteria were well preserved and their interaction with the calcite crystals was clearly seen. Some of the bacteria were engulfed in the precipitated calcite while some were growing and about to divide. It is probable that these surviving bacteria would perform urea hydrolysis for the next cementation treatment and continue the MICP process, and confirms that bacteria act as nucleation sites and mediate the MICP process. Furthermore, it is interesting to see an ordered arrangement of calcite particles, closely packed together, which shows that calcite precipitation and crystal growth is continuous (morphology of precipitated calcite crystals on the surface of a soil particle can be observed in Fig. 11(b)). Calcite crystal precipitation was quite high and covered the entire surface of some soil particles.
Scanning electron microscopy views of MICP treated Blessington sand: (A) Presence of microbes (shown in orange color) on the surface and within the calcite crystals, (B) Morphology of precipitated calcite crystals on the surface of a soil particle, (C) Muscovite particle cemented between two other soil particles, (D) Larger, blocky calcite crystals in the cemented samples (E) Aragonite polymorph and cemented soil particles, and (F) Low magnification view showing a representative view of cemented soil unit: widespread precipitated calcite crystals, cementation bonds and matrix, and calcium carbonate polymorphs.
SEM imaging revealed a muscovite particle cemented between soil particles into a cementation matrix (Fig. 11(c)), and larger, blocky calcite crystals were seen in the MICP treated samples along with bacteria (Fig. 11(d)). In some cases, aragonite, a calcium carbonate polymorph, formed large crystals that bonded soil particles (Fig. 11(e) at low magnification). The crystal shape and morphology of aragonite is distinct from calcite crystals, and the precipitation of calcium carbonate metastable phases such as vaterite and aragonite is likely due to minor changes in aqueous geochemistry and supersaturation conditions, owing to microbial activity, that can allow for simultaneous precipitation and dissolution of these polymorphs. However, the impact of microbial activity on the precipitation of these polymorphs needs further understanding6,105. A representative SEM image showing the cementation bonding between soil particles and the calcite cementation matrix showed widespread calcite precipitation on the surface of the larger soil particles (Fig. 11(f)). Calcite crystal precipitation and growth resulted in the formation of a cementation matrix that engulfed many particles, bonded them together, and resulted in the formation of bigger cemented particle units which was responsible for the increases in measured shear-wave velocity.
Energy X-ray dispersive spectroscopy
SEM–EDS was performed to map elemental distribution and identify precipitated calcite and cementation bonds between soil particles, and provided complimentary evidence to the SEM results. The SEM–EDS layered image, individual elemental maps, and energy spectra obtained on cemented Blessington and Ottawa F-110 soil specimens are shown in Fig. 12 and Figures S(7–10). The major elemental maps (Figures S7(c-f) and S9(c-f)) were silicon, oxygen, calcium, and aluminum and the peaks for each of these elements were identified in the energy spectra (Figures S8 and S10). It can be observed from the layered image (Fig. 12(b)), the presence of two particles that appear to be mainly composed of calcium and oxygen appearing yellowish green in color, while other particles around them appear to be silica rich appearing reddish pink in color with some calcium present on top. This suggests that these two particles which are not rich in silica might be the natural carbonate particles present in the Blessington soil that have undergone change in morphology due to the MICP process. The difference in morphology between these two particles and the other silica rich particles can be observed in the patterns of precipitated calcite crystals. The carbonate rich particles show a highly ordered arrangement of calcite crystals which tend to resemble crystal growth rather than newly nucleated calcite crystals like those present on the silica particles seen in Fig. 12(e and f). This suggests that the carbonate particles could be serving as preferential sites with lower activation energy barrier for calcite precipitation, undergoing a complete morphological transformation, whereas in the case of silica-rich particles, the bacteria might serve as the nucleation sites and hence the calcite crystals have to first nucleate at those locations and then the crystal growth continues, which would explain the widespread calcite precipitation seen on the carbonate-rich particles compared to the silica-rich particles.
Energy X-ray dispersive spectroscopy (SEM–EDS) of MICP treated Blessington and Ottawa F-110 sand for comparison of precipitated calcite morphology. (A,B) SEM view and SEM–EDS layered image, respectively, of MICP treated Blessington sand showing the detected elements. (C,D) Silicon and Calcium element map scan, respectively, of MICP treated Blessington sand show in (A,B). (E–F) SEM view and SEM–EDS layered image, respectively, of MICP treated Ottawa F-110 sand showing the detected elements. The difference in precipitated calcite morphology on natural carbonate and silica particles can be observed from SEM–EDS and elemental scans.
Shear-wave velocity measurements
The variation of shear-wave velocity with every cementation treatment, measured at mid-height of the columns using bender element sensors, for different treatment formulations of Blessington and concrete sands is shown in Fig. 13. The Vs profile of BS_2_350_1:1 started at a low Vs ~ 70 m/s and reached ~ 775 m/s for the tenth cementation treatment which was higher than all the other Blessington MICP soil columns tested. This can be attributed to higher precipitated calcite content observed for this column, approx. 9% compared to 5% (at mid-height) for the rest of Blessington soil columns (Fig. 4), resulting in more inter-particle bonds and producing a more complete cementation matrix as observed from SEM.
Comparing Vs profiles between carbonate-rich soil and silica-rich sands for the same treatment formulations: for yeast extract concentration of 0.2 g/L (BS_0.2_350_1:1 and OS_0.2_350_1:1), the increase in calcite precipitation (at mid-height) in Blessington soil was ~ 0.6% compared to F-110 sand, which was not sufficient to increase the stiffness of the Blessington column to equal/greater than Ottawa sand column. However, when the yeast extract concentration was increased to 2 g/L (BS_2_350_1:1 and OS_2_350_1:1), the increase in calcite precipitation in Blessington soil was ~ 4% (at mid-height) compared to F-110 sand, which resulted in a steady increase in Vs of the Blessington soil column, achieving Vs values similar to F-110 sand column towards the end of cementation treatment (8th cycle onwards) and equaling it at 10th cementation cycle, despite containing 30% fines, because a higher amount of calcite content would be needed to establish cementation bonds between Blessington soil particles as compared to F-110 sand which contains sand particles of approx. uniform size and almost no fines content. The measured Vs values for column BS_2_350_1:1 does indicate that increase in precipitated calcite content in Blessington was responsible for stiffening of soil particle contact points due to formation of cementation bonds, thus leading to a steady increase in Vs values.
Comparing Vs profiles of Blessington and concrete sands, it can be seen that the concrete sand Vs profile was higher on average by 128 m/s for initial packing and across all cementation treatments, with the exception of BS_2_350_1:1. The higher Vs of concrete sand could be because of fewer inter-particle contacts due to its larger particle size compared to Blessington sand. However, the Vs values were not as high as BS_2_350_1:1 column because of lower precipitated calcite content in concrete sand which was on average 5% compared to 9% in BS_2_350_1:1 column. Furthermore, some of the precipitated calcite would result in particle coating on the surface of bigger particles and not entirely in inter-particle bonds.
The initial Vs for BS_0.02 and BS_0.2 columns was the range of 70–135 m/s, and the final Vs values were in the range of 366–530 m/s. It can be observed that BS_0.02_50_1:1 column with lower yeast extract concentration and lower concentration of stimulation showed the lowest values of Vs across all cementation treatments. This agrees well with previous chemical measurements which indicated partial urea hydrolysis for this column, and with precipitated calcite profile which was found to be lower than other Blessington columns. However, the Vs profile of column BS_0.02_350_1.4:1 was similar with that of Blessington columns treated with 0.2 g/L yeast extract concentration which indicated that even though the yeast extract was 0.02 g/L, the higher urea concentrations during stimulation and cementation treatments enabled development of ureolytic microbial community to produce cementation levels that were similar to BS_0.2 columns. The columns BS_0.2_50_1:1 and BS_0.2_350_1.4:1 had almost identical Vs profiles, and almost parallel to the Vs profile of BS_0.2_350_1:1 column. These results indicate that similar levels of precipitated calcite, approx. 4–6%, across different Blessington columns produced a consistent increase in Vs with every cementation treatment, and the differences in Vs profiles observed could be due to changes in specimen preparation and initial soil packing. Comparing the Vs profiles of columns treated with commercial grade chemicals, significant changes were not observed either for Blessington or concrete sands which agrees well with the earlier results in the present study.
The plots of Vs with precipitated calcite content, and change in Vs per treatment with precipitated calcite content for Blessington soil columns are shown in Figures S11 and S12, respectively. The obtained correlations are as follows:
\({\text{V}}_{\text{s}}\left(\frac{\text{m}}{\text{s}}\right)=91.1\text{ x Precipitated calcite content},\text{ CC }(\text{\%})\), R2 = 0.99,
\(\Delta {\text{V}}_{\text{s}}\left(\frac{\text{m}}{\text{s}}\right)=8.25\text{ x Precipitated calcite content},\text{ CC }(\text{\%})\), R2 = 0.98.
The correlations suggest that Vs increases linearly with precipitated calcite content, provided it is the same type of soil and other experimental testing parameters are held constant. Higher levels of cementation for Blessington soil columns were achieved by treating with three different yeast extract concentrations 0.02, 0.2, and 2 g/L which resulted in increasing Vs values at the end of the tenth cementation treatment. Hence, only the calcite content was varied while keeping the other parameters constant, which quantifies the effect of variation of calcite content on shear-wave velocity for Blessington soil. For an average precipitated calcite content of 5% corresponding to BS_0.02 and BS_0.2 columns, a final Vs of ~ 450 m/s and a delta Vs per treatment of ~ 40 m/s was obtained. With an increase in precipitated calcite content to 9% corresponding to BS_2 column, a final Vs of ~ 775 m/s and a delta Vs per treatment of ~ 70 m/s was obtained. Furthermore, comparing the rate of increase in Vs of Blessington soil with a larger database (Figure S11), it is evident that the increase in Vs is lower for Blessington soil, because the database predominantly comprises of MICP tests on Ottawa 20/30, Ottawa 50–70, and Nevada sands which are larger in particle size and hence fewer interparticle contacts, which therefore show a higher rate of increase in Vs compared to Blessington soil.
Unconfined compressive strength
The results of unconfined compressive strength (UCS) testing are shown in Fig. 14 for the specimens BS_0.02_50_1:1, BS_0.02_350_1.4:1, and BS_0.2_350_1.4:1. From earlier test results, it was known that BS_0.02_50_1:1 column achieved only partial urea hydrolysis until the fifth cementation treatment, and hence lower cementation and lower strength was expected. Increasing the urea concentration, as in column BS_0.02_350_1.4:1 resulted in better urea hydrolysis during stimulation phase compared to BS_0.02_50_1:1, and a higher precipitated calcite profile was observed. Increasing the yeast extract concentration to 0.2 g/L resulted in complete urea hydrolysis throughout all treatment cycles. The UCS curves reflected these results at a macroscopic scale; lowest UCS of 0.4 MPa was obtained for BS_0.02_50_1:1, 0.58 MPa for BS_0.02_350_1.4:1, and highest UCS of 0.68 MPa for BS_0.2_350_1.4:1.
These values of UCS compared reasonably well with previous results from literature data: Al Qabany and Soga42 have performed UCS testing at different relative densities and chemical formulation for a British standard grade D silica sand with d50 = 165 µm and d90 = 250 μm. At 250 mM urea and CaCl2 treatment, the UCS was reported to be 0.3 MPa for a precipitated calcite content of 40 kg/m3, 0.6 MPa at 68 kg/m3 calcite content, and it increased to as high as 1.4 MPa for 100 kg/m3 calcite content. For the same 250 mM treatment formulation, UCS results of Blessington sand ranged from 0.4–0.68 MPa for a precipitated calcite content of 60–80 kg/m3, which are comparable to the results reported by Al Qabany and Soga (2013)42, although the Blessington sand may be a finer sand (d50 = 110 µm and d90 = 250 µm) than British grade D silica sand (d50 = 165 µm and d90 = 250 μm).
Conclusions
The present study was performed to elucidate the effect of natural carbonates on the MICP process, with the goal of extending the MICP process to carbonate-rich soils and understanding the key factors controlling the process. To this end, macroscale MICP column experiments were performed with the carbonate-rich Blessington soil and compared against benchmark silica-rich soils. Following conclusions can be drawn from the present study:
-
1.
Natural carbonates can have a significant impact on the MICP process efficiency, increasing it by as much as 23% and precipitated calcite contents by as high as 82% when compared to benchmark silica-rich F-110 sand, for similar treatment formulations.
-
2.
The results suggest that natural carbonate particles can act as preferential sites lowering the energy barrier required for calcite precipitation during the MICP process, and thus propagating the growth of calcite crystals on pre-existing calcite mineral surface, as observed through SEM and SEM–EDS. As a result, precipitated calcite crystals with different surface morphologies and cementation bonds between carbonate-rich Blessington soil particles were documented.
-
3.
The yeast extract concentrations applied during MICP treatments were found to be the key factor controlling the development of urea degradation capacity during MICP treatment as observed through chemical measurements. For the same flow rate conditions, increasing yeast extract concentration from 0.2 g/L to 2 g/L resulted in a high increase in MICP efficiency in carbonate-rich Blessington soil from 48 to 72% compared to 44% to 49% in silica-rich F-110 sand, and the increase in calcite precipitation was as high as 80% in Blessington sand compared to 27% in Ottawa F-110 sand, which shows the compounding effect of yeast extract concentration and natural carbonates on the MICP process.
-
4.
Shear-wave velocity measurements showed that the additional increase in precipitated calcite content observed in Blessington soil treated with yeast extract concentration of 2 g/L was responsible for stiffening of soil particle contact points due to formation of cementation bonds, thus leading to a steady increase in Vs values. Additionally, in the case of Blessington soil, for precipitated calcite contents ranging from 3.5 – 9%, shear-wave velocity was found to increase linearly with increases in precipitated calcite content.
Data availability
All data generated or analyzed during this study are provided within the manuscript and supplementary information files.
References
Huang, L., Krigsvoll, G., Johansen, F., Liu, Y. & Zhang, X. Carbon emission of global construction sector. Renew. Sustain. Energy Rev. 81, 1906–1916 (2018).
Carter, M. S. et al. Microbially induced calcium carbonate precipitation by Sporosarcina pasteurii: A case study in optimizing biological CaCO3 precipitation. Appl. Environ. Microbiol. 89, e0179422 (2023).
Castro-Alonso, M. J. et al. Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: Microbiological and molecular concepts. Front. Mater. https://doi.org/10.3389/fmats.2019.00126 (2019).
Dhami, N. K., Reddy, M. S. & Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. https://doi.org/10.3389/fmicb.2013.00314 (2013).
Mujah, D., Shahin, M. A. & Cheng, L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol. J. 34, 524–537 (2017).
DeJong, J. T. et al. State of the Art: MICP soil improvement and its application to liquefaction hazard mitigation. Proc. 20th ICSMGE 405–508 (2022).
De Jong, J. T. et al. Biogeochemical processes and geotechnical applications: progress, opportunities and challenges. In Bio- and Chemo-Mechanical Processes in Geotechnical Engineering (ed. Laloui, L.) 143–157 (ICE Publishing, 2014).
DeJong, J. T., Fritzges, M. B. & Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 132, 1381–1392 (2006).
Seifan, M. & Berenjian, A. Microbially induced calcium carbonate precipitation: A widespread phenomenon in the biological world. Appl. Microbiol. Biotechnol. 103, 4693–4708 (2019).
Ivanov, V. & Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Biotechnol. 7, 139–153 (2008).
Chu, J., Stabnikov, V. & Ivanov, V. Microbially induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol. J. 29, 544–549 (2012).
Hoffmann, T. D., Reeksting, B. J. & Gebhard, S. Bacteria-induced mineral precipitation: A mechanistic review. Microbiology (N. Y.) https://doi.org/10.1099/mic.0.001049 (2021).
Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 13, 59–67 (2018).
Fu, T., Saracho, A. C. & Haigh, S. K. Microbially induced carbonate precipitation (MICP) for soil strengthening: A comprehensive review. Biogeotechnics 1, 100002 (2023).
DeJong, J. T., Mortensen, B. M., Martinez, B. C. & Nelson, D. C. Bio-mediated soil improvement. Ecol. Eng. 36, 197–210 (2010).
Stocks-Fischer, S., Galinat, J. K. & Bang, S. S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 31, 1563–1571 (1999).
Whiffin, V. S., van Paassen, L. A. & Harkes, M. P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 24, 417–423 (2007).
Harkes, M. P., van Paassen, L. A., Booster, J. L., Whiffin, V. S. & van Loosdrecht, M. C. M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 36, 112–117 (2010).
Montoya, B. M. & DeJong, J. T. Stress-strain behavior of sands cemented by microbially induced calcite precipitation. J. Geotech. Geoenviron. Eng. 141, 1–10 (2015).
Graddy, C. M. R., Gomez, M. G., DeJong, J. T. & Nelson, D. C. Native bacterial community convergence in augmented and stimulated ureolytic MICP biocementation. Environ. Sci. Technol. 55, 10784–10793 (2021).
Mortensen, B. M., Haber, M. J., DeJong, J. T., Caslake, L. F. & Nelson, D. C. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 111, 338–349 (2011).
Montoya, B. M., De Jong, J. T. & Boulanger, R. W. Dynamic response of liquefiable sand improved by microbial-induced calcite precipitation. In Bio- and Chemo-Mechanical Processes in Geotechnical Engineering (ed. Montoya, B. M.) 125–135 (ICE Publishing, 2014). https://doi.org/10.1680/bcmpge.60531.012.
Zhang, W., Ju, Y., Zong, Y., Qi, H. & Zhao, K. In situ real-time study on dynamics of microbially induced calcium carbonate precipitation at a single-cell level. Environ. Sci. Technol. 52, 9266–9276 (2018).
Ghosh, T., Bhaduri, S., Montemagno, C. & Kumar, A. Sporosarcina pasteurii can form nanoscale calcium carbonate crystals on cell surface. PLoS ONE 14, 1–15 (2019).
Mitchell, A. C. & Ferris, F. G. The influence of Bacillus pasteurii on the nucleation and growth of calcium carbonate. Geomicrobiol. J. 23, 213–226 (2006).
Zambare, N. M., Naser, N. Y., Gerlach, R. & Chang, C. B. Mineralogy of microbially induced calcium carbonate precipitates formed using single cell drop-based microfluidics. Sci. Rep. 10, 17535 (2020).
Wang, Y., Soga, K., DeJong, J. T. & Kabla, A. J. Microscale Visualization of Microbial-Induced Calcium Carbonate Precipitation Processes. J. Geotech. Geoenviron. Eng. 145, (2019).
Dhami, N. K., Alsubhi, W. R., Watkin, E. & Mukherjee, A. Bacterial community dynamics and biocement formation during stimulation and augmentation: Implications for soil consolidation. Front. Microbiol. 8, (2017).
Gomez, M. G., Graddy, C. M. R., DeJong, J. T. & Nelson, D. C. Biogeochemical changes during bio-cementation mediated by stimulated and augmented ureolytic microorganisms. Sci. Rep. 9, 11517 (2019).
Gomez, M. G. et al. Large-Scale Comparison of Bioaugmentation and Biostimulation Approaches for Biocementation of Sands. J. Geotech. Geoenviron. Eng. 143, (2017).
Dubey, A. A. et al. Biocementation mediated by native microbes from Brahmaputra riverbank for mitigation of soil erodibility. Sci. Rep. 11, 15250 (2021).
Gomez, M. G., Graddy, C. M. R., DeJong, J. T., Nelson, D. C. & Tsesarsky, M. Stimulation of native microorganisms for biocementation in samples recovered from field-scale treatment depths. J. Geotech. Geoenviron. Eng. 144, 1–13 (2018).
Lauchnor, E. G., Topp, D. M., Parker, A. E. & Gerlach, R. Whole cell kinetics of ureolysis by Sporosarcina pasteurii. J. Appl. Microbiol. 118, 1321–1332 (2015).
Mobley, H. L. T. & Hausinger, R. P. Microbial ureases: Significance, regulation, and molecular characterization. Microbiol. Rev. 53, 85–108 (1989).
Mobley, H. L., Island, M. D. & Hausinger, R. P. Molecular biology of microbial ureases. Microbiol. Rev. 59, 451–480 (1995).
Krajewska, B. & Ureases, I. Functional, catalytic and kinetic properties: A review. J. Mol. Catal. B Enzym. 59, 9–21 (2009).
Gat, D., Ronen, Z. & Tsesarsky, M. Soil bacteria population dynamics following stimulation for ureolytic microbial-induced CaCO 3 precipitation. Environ. Sci. Technol. 50, 616–624 (2016).
Proto, C. J., DeJong, J. T. & Nelson, D. C. Biomediated permeability reduction of saturated sands. J. Geotech. Geoenviron. Eng. 142, 1–11 (2016).
Feng, K. & Montoya, B. M. Drained shear strength of MICP sand at varying cementation levels. In IFCEE 2015 (ed. Feng, K.) 2242–2251 (American Society of Civil Engineers, 2015). https://doi.org/10.1061/9780784479087.208.
Feng, K. & Montoya, B. M. Quantifying level of microbial-induced cementation for cyclically loaded sand. J. Geotech. Geoenviron. Eng. 143, 1–4 (2017).
Al Qabany, A., Soga, K. & Santamarina, C. Factors affecting efficiency of microbially induced calcite precipitation. J. Geotech. Geoenviron. Eng. 138, 992–1001 (2012).
Al Qabany, A. & Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. In Bio- and Chemo-Mechanical Processes in Geotechnical Engineering (ed. Laloui, L.) 107–115 (ICE Publishing, 2014). https://doi.org/10.1680/bcmpge.60531.010.
Terzis, D. & Laloui, L. 3-D micro-architecture and mechanical response of soil cemented via microbial-induced calcite precipitation. Sci. Rep. 8, 1–11 (2018).
Martinez, B. C. et al. Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J. Geotech. Geoenviron. Eng. 139, 587–598 (2013).
Lin, H., Suleiman, M. T., Brown, D. G. & Kavazanjian, E. Mechanical behavior of sands treated by microbially induced carbonate precipitation. J. Geotech. Geoenviron. Eng. 142, 1–13 (2016).
Feng, K. & Montoya, B. M. Influence of confinement and cementation level on the behavior of microbial-induced calcite precipitated sands under monotonic drained loading. J. Geotech. Geoenviron. Eng. 142, 1–9 (2016).
Chu, J., Ivanov, V., Naeimi, M., Stabnikov, V. & Liu, H. L. Optimization of calcium-based bioclogging and biocementation of sand. Acta Geotech. 9, 277–285 (2014).
Barkouki, T. H. et al. Forward and inverse bio-geochemical modeling of microbially induced calcite precipitation in half-meter column experiments. Transp. Porous Media 90, 23–39 (2011).
Wilson, J. L. Carbonate Depositional Environments and Diagenesis. in (eds. Palaz, I. & Marfurt, K. J.) (Society of Exploration Geophysicists, Tulsa, OK, 1997). https://doi.org/10.1190/1.9781560802099.
Flügel, E. Microfacies of Carbonate Rocks Vol. 976 (Springer, 2010).
Tong, C.-X. et al. On compression behavior and particle breakage of carbonate silty sands. Eng. Geol. 297, 106492 (2022).
Xiao, P., Liu, H., Xiao, Y., Stuedlein, A. W. & Evans, T. M. Liquefaction resistance of bio-cemented calcareous sand. Soil Dyn. Earthq. Eng. 107, 9–19 (2018).
Xiao, P., Liu, H., Stuedlein, A. W., Evans, T. M. & Xiao, Y. Effect of relative density and biocementation on cyclic response of calcareous sand. Can. Geotech. J. 56, 1849–1862 (2019).
Zhang, X., Chen, Y., Liu, H., Zhang, Z. & Ding, X. Performance evaluation of a MICP-treated calcareous sandy foundation using shake table tests. Soil Dyn. Earthq. Eng. 129, 105959 (2020).
Gomez, M. G., DeJong, J. T., Anderson, C. M., Nelson, D. C. & Graddy, C. M. Large-scale bio-cementation improvement of sands. In Geotechnical and Structural Engineering Congress 2016 (ed. Gomez, M. G.) 941–949 (American Society of Civil Engineers, 2016). https://doi.org/10.1061/9780784479742.079.
Gomez, M. G. & DeJong, J. T. Engineering properties of bio-cementation improved sandy soils. In Grouting 2017 (ed. Gomez, M. G.) 23–33 (American Society of Civil Engineers, 2017). https://doi.org/10.1061/9780784480793.003.
San Pablo, A. C. M. et al. Meter-Scale Biocementation Experiments to Advance Process Control and Reduce Impacts: Examining Spatial Control, Ammonium By-Product Removal, and Chemical Reductions. J. Geotech. Geoenviron. Eng. 146, (2020).
Lee, M. et al. Investigating ammonium by-product removal for ureolytic bio-cementation using meter-scale experiments. Sci. Rep. 9, 18313 (2019).
Lee, M. et al. Improving the spatial control of soil biocementation using indigenous microorganisms: Column experiments and reactive transport modeling. Eng. Geol. 318, 107104 (2023).
Ferris, F. G., Phoenix, V., Fujita, Y. & Smith, R. W. Kinetics of calcite precipitation induced by ureolytic bacteria at 10 to 20°C in artificial groundwater. Geochim. Cosmochim. Acta 68, 1701–1710 (2004).
Lee, M. & Gomez, M. G. Removal of ammonium by-products produced during biocementation soil improvement using rinse injection strategies. Soil Use Manag. https://doi.org/10.1111/sum.12984 (2024).
Lee, M., Kolbus, C. M., Yepez, A. D. & Gomez, M. G. Investigating ammonium by-product removal following stimulated ureolytic microbially-induced calcite precipitation. In Geo-Congress 2019 (ed. Lee, M.) 260–272 (American Society of Civil Engineers, 2019). https://doi.org/10.1061/9780784482117.026.
Knorst, M. T., Neubert, R. & Wohlrab, W. Analytical methods for measuring urea in pharmaceutical formulations. J. Pharm. Biomed. Anal. 15, 1627–1632 (1997).
Krom, M. D. Spectrophotometric determination of ammonia: A study of a modified Berthelot reaction using salicylate and dichloroisocyanurate. Analyst 105, 305 (1980).
Keeney, D. R. & Nelson, D. W. Nitrogen—Inorganic Forms. in Amer. Soc. Agronomy, Madison, Wis.(2nd ed., 1982) 643–698. https://doi.org/10.2134/agronmonogr9.2.2ed.c33 (1982).
ASTM D4373. Standard Test Method for Rapid Determination of Carbonate Content of Soils. Preprint at https://doi.org/10.1520/D4373-21 (2021).
ASTM D7348. Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues. Preprint at https://doi.org/10.1520/D7348-21 (2021).
Brignoli, E., Gotti, M. & Stokoe, K. Measurement of shear waves in laboratory specimens by means of piezoelectric transducers. Geotech. Test. J. 19, 384–397 (1996).
Lee, J.-S. & Santamarina, J. C. Bender elements: Performance and signal interpretation. J. Geotech. Geoenviron. Eng. 131, 1063–1070 (2005).
Montoya, B. M. et al. Fabrication, operation, and health monitoring of bender elements for aggressive environments. Geotech. Test. J. 35, 103300 (2012).
Cheng, L., Cord-Ruwisch, R. & Shahin, M. A. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 50, 81–90 (2013).
Zehner, J., Røyne, A. & Sikorski, P. Calcite seed-assisted microbial induced carbonate precipitation (MICP). PLoS ONE 16, e0240763 (2021).
Fujita, Y., Grant Ferris, F., Daniel Lawson, R., Colwell, F. S. & Smith, R. W. Calcium carbonate precipitation by ureolytic subsurface bacteria. Geomicrobiol. J. 17, 305–318 (2000).
Bang, S. S., Galinat, J. K. & Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb. Technol. 28, 404–409 (2001).
Verdoes, D., Kashchiev, D. & van Rosmalen, G. M. Determination of nucleation and growth rates from induction times in seeded and unseeded precipitation of calcium carbonate. J. Cryst. Growth 118, 401–413 (1992).
Lioliou, M. G., Paraskeva, C. A., Koutsoukos, P. G. & Payatakes, A. C. Heterogeneous nucleation and growth of calcium carbonate on calcite and quartz. J. Colloid Interface Sci. 308, 421–428 (2007).
Jaho, S. et al. Experimental investigation of calcium carbonate precipitation and crystal growth in one- and two-dimensional porous media. Cryst. Growth Des. 16, 359–370 (2016).
Nooraiepour, M., Masoudi, M., Shokri, N. & Hellevang, H. Probabilistic nucleation and crystal growth in porous medium: New insights from calcium carbonate precipitation on primary and secondary substrates. ACS Omega 6, 28072–28083 (2021).
Li, Q., Fernandez-Martinez, A., Lee, B., Waychunas, G. A. & Jun, Y.-S. Interfacial energies for heterogeneous nucleation of calcium carbonate on Mica and Quartz. Environ. Sci. Technol. 48, 5745–5753 (2014).
Fernandez-Martinez, A., Hu, Y., Lee, B., Jun, Y.-S. & Waychunas, G. A. In situ determination of interfacial energies between heterogeneously nucleated CaCO3 and quartz substrates: Thermodynamics of CO2 mineral trapping. Environ. Sci. Technol. 47, 102–109 (2013).
Freeman, C. L. & Harding, J. H. The transformation of amorphous calcium carbonate to calcite and classical nucleation theory. J. Cryst. Growth 603, 126978 (2023).
De Yoreo, J. J. & Vekilov, P. G. Principles of crystal nucleation and growth. Rev. Mineral Geochem. 54, 57–93 (2003).
Meldrum, F. C. Calcium carbonate in biomineralisation and biomimetic chemistry. Int. Mater. Rev. 48, 187–224 (2003).
Weiner, S., Levi-Kalisman, Y., Raz, S. & Addadi, L. Biologically formed amorphous calcium carbonate. Connect. Tissue Res. 44, 214–218 (2003).
Nancollas, G. H. & Reddy, M. M. The crystallization of calcium carbonate. II. Calcite growth mechanism. J. Colloid Interface Sci. 37, 824–830 (1971).
Gong, Y. U. T. et al. Phase transitions in biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. 109, 6088–6093 (2012).
Rodriguez-Navarro, C., Burgos Cara, A., Elert, K., Putnis, C. V. & Ruiz-Agudo, E. Direct nanoscale imaging reveals the growth of calcite crystals via amorphous nanoparticles. Cryst. Growth Des. 16, 1850–1860 (2016).
Meldrum, F. C. & Cölfen, H. Controlling mineral morphologies and structures in biological and synthetic systems. Chem. Rev. 108, 4332–4432 (2008).
Gebauer, D., Völkel, A. & Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 1979(322), 1819–1822 (2008).
Bots, P., Benning, L. G., Rodriguez-Blanco, J.-D., Roncal-Herrero, T. & Shaw, S. Mechanistic insights into the crystallization of amorphous calcium carbonate (ACC). Cryst. Growth Des. 12, 3806–3814 (2012).
Addadi, L., Raz, S. & Weiner, S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv. Mater. 15, 959–970 (2003).
Rodriguez-Blanco, J. D., Shaw, S. & Benning, L. G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, viavaterite. Nanoscale 3, 265–271 (2011).
Söhnel, O. Electrolyte crystal-aqueous solution interfacial tensions from crystallization data. J. Cryst. Growth 57, 101–108 (1982).
Söhnel, O. & Mullin, J. W. A method for the determination of precipitation induction periods. J. Cryst. Growth 44, 377–382 (1978).
Koutsoukos, P. G. & Kontoyannis, C. G. Precipitation of calcium carbonate in aqueous solutions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 80, 1181 (1984).
De Yoreo, J. J., Waychunas, G. A., Jun, Y.-S. & Fernandez-Martinez, A. In situ investigations of carbonate nucleation on mineral and organic surfaces. Rev. Mineral Geochem. 77, 229–257 (2013).
Spanos, N. & Koutsoukos, P. G. Kinetics of precipitation of calcium carbonate in alkaline pH at constant supersaturation. Spontaneous and seeded growth. J. Phys. Chem. B 102, 6679–6684 (1998).
Kralj, D., Brečević, L. & Nielsen, A. E. Vaterite growth and dissolution in aqueous solution I. Kinetics of crystal growth. J. Cryst. Growth 104, 793–800 (1990).
Gómez-Morales, J., Torrent-Burgués, J. & Rodríguez-Clemente, R. Nucleation of calcium carbonate at different initial pH conditions. J. Cryst. Growth 169, 331–338 (1996).
Zhu, Y. et al. Sulfate-controlled heterogeneous CaCO 3 nucleation and its non-linear interfacial energy evolution. Environ. Sci. Technol. 55, 11455–11464 (2021).
U.S. Environmental Protection Agency. Aquatic Life Ambient Water Quality Criteria for Ammonia – Freshwater. EPA Publication No. 822-R-13–001 1–255 (2013).
Beck, C. W. Differential thermal analysis curves of carbonate minerals. Am. Miner. 35, 985–1013 (1950).
Wirth, X., Glatstein, D. A. & Burns, S. E. Mineral phases and carbon content in weathered fly ashes. Fuel 236, 1567–1576 (2019).
Villain, G., Thiery, M. & Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 37, 1182–1192 (2007).
Burdalski, R. J., Ribeiro, B. G. O., Gomez, M. G. & Gorman-Lewis, D. Mineralogy, morphology, and reaction kinetics of ureolytic bio-cementation in the presence of seawater ions and varying soil materials. Sci. Rep. 12, 17100 (2022).
Acknowledgements
This work was done as part of the Center-2-Center sub-project (PR75: C2C – ASU: Multi-scale Investigation of Bio-Based Mineral Precipitation in Carbonate Bearing Granular Soils and Construction Related Waste) of the Center for Bio-Inspired and Bio-Mediated Geotechnics (CBBG). The authors are grateful to Dr. Edward Kavazanjian Jr., Dr. Leon van Paassen, Dr. Frank McDermott, Dr. Mike Long, Dr. Shane Donohue, Dr. Rory Doherty, Dr. Shamsudeen Dandare, and Maria Judge for their insightful comments and discussion. This work was performed in part at the Georgia Tech Institute for Electronics and Nanotechnology, a member of the National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the National Science Foundation (Grant ECCS-2025462).
Author information
Authors and Affiliations
Contributions
SHS, VRY, JTD, and SEB were involved in the design, performance, and analysis of all data achieved in the presented study. CMRG performed additional chemical measurements and analyses presented in this paper. SHS wrote the first draft of the main manuscript text, and SHS and VRY prepared figures. The manuscript and figures were revised by MGG, JTD, and SEB. All authors were involved in the discussion and analysis of the achieved results as well as editing of the final manuscript text and figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shivaprakash, S.H., Yanez, V.R., Graddy, C.M.R. et al. Effect of natural carbonates on microbially induced calcite precipitation process. Sci Rep 15, 13290 (2025). https://doi.org/10.1038/s41598-025-97737-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97737-2