Abstract
Prosopis juliflora is a major invasive species in tropical and subtropical regions. This study explores the link between precipitation, soil moisture, and plant growth, focusing on the invasive P. juliflora in a simulated altered rainfall regime. The study was conducted simultaneously by using three rainout shelters, Normal Rainfall (NR, 1000 mm) (ii) High Rainfall (HR, 1400 mm) (iii) Low Rainfall (LR, 500 mm). Each rainout shelter was divided into three subplots measuring 2 × 2 m to establish P. juliflora by transplanting 20 seedlings per 4 m2. After 1 years of establishments, plant growth, ecophysiological attributes and soil properties were recorded as per standard protocols. Increased rainfall led to higher soil moisture and promoted P. juliflora growth, biomass, and photosynthetic activity. Conversely, decreased rainfall triggered drought stress, impacting gas exchange and reducing growth. Statistical analysis showed significant (p < 0.05), SM, soil inorganic-N, N-mineralization, growth measurements, photosynthesis rate (Aarea), transpiration rate (E), height (H), diameter (D), root: shoot ratio, and biomass (AGB + BGB) exhibited the highest values in the HR plots during the rainy season and the lowest values in the LR plots during the summer season, following the trend HR > NR > LR. When different rainfall conditions were regressed against P. juliflora biomass, significantly positive linear relationships were observed. The findings suggest that P. juliflora adapts to changing rainfall regimes by allocating resources strategically. While it thrives under high precipitation, it exhibits surprising drought tolerance, potentially colonizing drier regions. Declining monsoon rainfall and increased precipitation intensity may boost P. juliflora’s invasiveness.
Similar content being viewed by others
Introduction
The intricate interplay between global climate change and its multifaceted impacts on rainfall patterns has garnered substantial attention in recent years. The current understanding of the impact of ongoing climate change indicates shifts in rainfall patterns including profound disparities in its distribution1. Coupled Model Intercomparison Project Phase 5 (CMIP5) has forecasted augmented periods of aridity within tropical climes2,3. Elevating temperatures are expected to amplify extreme rainfall occurrences in Asia, notably the East and South Asian regions4,5. A temperature elevation of 1.5 °C in East and Central Asia will correspondingly spur a 28% and 15% surge in extreme rainfall, as compared to the 1971–2000 benchmark4. The ramifications of this irregular rainfall distribution have prompted widespread concern, as it may trigger cascading effects on ecosystems, biodiversity, and water availability.
Amidst the wealth of research into elevated temperature and atmospheric CO2, scant attention has been accorded to the intricate interplay between predicted precipitation regime alterations and terrestrial ecosystems6. The sensitivity of global ecosystems, particularly tropical ones, to annual precipitation and its temporal distribution, underscores their vulnerability to disruption7,8,9. This heightened susceptibility is attributed to the low adaptive capacity of tropical ecosystems, augmenting the potential impact of rainfall regime changes8,10,11.
The far-reaching effects of altered precipitation are epitomized in its direct influence on soil moisture dynamics, leaching, erosion, and subsequent impacts on nutrient uptake and ecosystem productivity12. These intricate changes reverberate through inorganic nutrient pools, effectively reconfiguring the N-cycling of ecosystems12,13. Moreover, the repercussions extend to plant physiology, species composition, and community structure, with both long-term shifts and acute variations brought about by rainfall variability14,15,16,17.
This intricate interplay between altered precipitation patterns and their ecological ramifications is further compounded by the intrusion of invasive plant species (IPS), which have emerged as potent agents of global change18,19,20,21,22. The incursion of IPS triggers an array of negative consequences, ranging from ecological imbalances and biodiversity loss to disruptions in ecosystem services and human health hazards22,23,24,25. As invasive species has the potential to alter soil nutrient dynamics, particularly in the context of nitrogen cycling, their impacts extend from changes in nitrogen mineralization rates to shifts in plant community structure and stability22,26,27,28,29,30. Despite the substantial progress in understanding these phenomena, a crucial gap exists in comprehending the intricate interactions between rainfall patterns, invasive species, and soil-climate dynamics. In recent years a few precipitation manipulations experiments have been done across global grasslands. However, there is a dearth of insights into how variations in rainfall patterns interact with invasive plants, soils, and climate to orchestrate ecosystem processes, particularly the nutrient cycle. The current research aimed to address this gap by investigating how rainfall variability modulates soil nutrient pools, mineralization processes, and the biomass of the invasive Prosopis juliflora plant. By providing insight from an invasion experiment under varying precipitation conditions—representing drought, current, and future rainfall scenarios—the present study is an attempt to understand the broader ecological consequences of changing rainfall regimes in tropical ecosystems, particularly within the Indian subcontinent.
Results
Soil physicochemical properties
The soil had a composition of 4% sand, 85% silt, and 11% clay. Soil bulk density (BD) ranged from 1.21 ± 0.03 to 1.29 ± 0.01 g cm⁻3, and water holding capacity (WHC) varied from 43.47 ± 0.24% to 45.21 ± 0.57%. Soil organic carbon (SOC) ranged from 0.78 ± 0.15 to 1.37 ± 0.28 mg g−1, total organic carbon (TOC) from 6.37 ± 0.03 to 7.07 ± 0.09 mg g−1, total nitrogen (TN) from 0.45 ± 0.02 to 0.65 ± 0.02 mg g−1, and the C: N ratio of soil ranged from 10.83 ± 0.43 to 14.24 ± 0.75 across all precipitation plots with P. juliflora (Table 1). After two years of experimentation, no significant changes in soil physicochemical properties were observed (Supplementary Table 1).
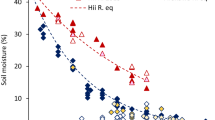
Soil moisture and temperature varied significantly among different simulated rainfall treatments with P. juliflora. Soil moisture showed significant variation with both the season and the type of simulated rainfall (LR, NR, HR) (Supplementary Tables 2 and 3a). Soil moisture exhibited the highest values in the HR plots during the rainy season and the lowest values in the LR plots during the summer season, following the trend HR > NR > LR (Fig. 1a). Compared to NR conditions, soil moisture decreased by 25% under LR conditions and increased by 28% under HR conditions.
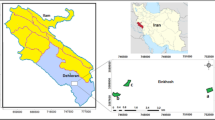
(a) Map of India, (b) Map of Uttar Pradesh, (c) Map of Banaras Hindu University (BHU) within Varanasi district, (d) Studied Plots in Botanical Garden, BHU (Pj = Prosopis juliflora) (This map was created using QGIS, an open-source Geographic Information System software and base map is sourced from OPENSTREETMAP (OSM), a publicly accessible and open-source platform31. The data and base map are openly licensed under the Open Data Commons Open Database License (ODbL) and Creative Commons Attribution-ShareAlike (CC-BY-SA). The attribution for the base map and data is as follows: Base map and data from OPENSTREETMAP (OSM) and OPENSTREETMAP (OSM) Foundation (CC-BY-SA). https://www.openstreetmap.org and contributors). The source is cited as: OPENSTREETMAP (OSM) contributors. OPENSTREETMAP database [PostgreSQL via API]. OPENSTREETMAP (OSM) Foundation: Cambridge, UK; 2021 [cited 22 Dec 2021]. Available under the Open Database Licence from: openstreetmap.org. Data mining by Overpass turbo.
Soil temperature also varied strongly, with the highest values observed in low rainfall plots during the summer season and the lowest values in high rainfall plots during the winter season (Fig. 1b). Both seasonality and simulated rainfall affected soil moisture similarly (Figs. 2 and 3), impacting soil dynamics and P. juliflora growth. Therefore, in this manuscript, we will focus on the effect of simulated rainfall on soil properties, physiology, and growth attributes.
(a) Soil moisture in different precipitation treatments (LR, NR, HR). (b) Soil temperature in different precipitation treatments (LR, NR, HR). Bars affixed with different letter are significantly different from each other (P < 0.05). Values are mean ± SE. (Rainfall i.e. LR, NR and HR pooled across two-year seasonal data sets of 2021–22).
Soil N pools and processes
Figures 4 and 5 shows that soil inorganic-N (NO2–N + NH4N) and N-mineralization (nitrification + ammonification) varied significantly under different simulated rainfall conditions (LR, NR, and HR) and also varied significantly with the season (Supplementary Tables 3a and 3b). Compared to NR plots, soil inorganic-N decreased by 13.55% in LR plots and increased by 11.34% in HR plots. Soil inorganic-N (available nitrogen) was highest in the HR plots across all three seasons, following the trend HR > NR > LR (Fig. 4). Soil inorganic-N ranged from 5.24 ± 0.08 to 10.42 ± 0.09 mg g−1 LR plots, 6.02 ± 0.10 to 11.88 ± 0.14 mg g−1 in NR plots, and 7.05 ± 0.05 to 13.04 ± 0.05 mg g−1 in HR plots (Table 2).
Annual mean value (2021–2022) of (a) nitrate–N (NO3–N) andammonium-N (NH4–N) (b) nitrification (NF) and ammonification (AF) across different simulated rainfall. Bars affixed with different letter are significantly different from each other for (a) total inorganic-N and (b) N-mineralization (P < 0.05). Values are mean ± SE. (Rainfall i.e. LR, NR and HR pooled across two-year seasonal data sets of 2021–22).
N-mineralization ranged from 3.27 ± 0.23 to 11.31 ± 0.09 mg g−1 in LR plots, 4.49 ± 0.30 to 12.52 ± 0.10 mg g−1 in NR plots, and 5.50 ± 0.15 to 13.78 ± 0.14 mg g−1 in HR plots (Table 2). When compared with NR plots, N-mineralization decreased by 12.12% in LR plots and increased by 19.82% in HR plots. Annual mean N-mineralization was highest in higher rainfall plots across all three seasons, following the trend HR > NR > LR (Fig. 4). Data on inorganic-N and N-mineralization under simulated rainfall conditions (LR, NR, and HR) showed a significant positive correlation with soil moisture (Fig. 5).
The correlation analysis revealed a significantly positive relationship between soil moisture and NO3–N, NH4–N, TIN, NF, AF, and N-MIN, with correlation coefficients ranging from r = 0.85 to r = 0.95 and P-values less than 0.01. Conversely, soil temperature exhibited a significantly negative correlation with NO3–N, NH4–N, and TIN, with correlation coefficients ranging from r = − 0.29 to r = − 0.36 and P-values less than 0.05. Soil moisture demonstrated a positive linear relationship with NO3–N, NH4–SN, TIN, NF, AF, and N-MIN during regression analysis, with R2 values ranging from 0.76 to 0.85 (Table 3; Fig. 6). These findings underscore the intricate interplay between soil moisture, nitrogen forms, temperature, and various mineralization processes.
In the P. juliflora invaded plots, microbial biomass carbon (MBC) and nitrogen (MBN) varied significantly with the rainfall pattern (Supplementary Table 3b). Figure 6 shows that MBC and MBN were significantly and negatively correlated with soil moisture when rainfall plot data for 2021–2022 were pooled (Fig. 6) MBC and MBN exhibited a reverse trend with soil moisture content, with maximum values in low rainfall (LR) plots and minimum values in high rainfall (HR) plots. During the study period, MBC ranged from 281.33 ± 1.28 to 553.07 ± 2.17 μg g−1 in HR and LR plots, respectively. MBN ranged from 31.15 ± 1.34 to 55.17 ± 1.31 μg g−1 in HR and LR plots, respectively (Table 2).
Compared to the normal rainfall (NR) plots, MBC increased by 5%, 4%, and 7% in the LR plots during the winter, summer, and rainy seasons, respectively. In HR plots, MBC decreased by 8%, 8%, and 6% in the winter, summer, and rainy seasons, respectively. Similarly, compared to the NR plots, MBN increased by 10%, 18%, and 5% in the LR plots during the winter, summer, and rainy seasons, respectively. In HR plots, MBN decreased by 7%, 10%, and 14% in the winter, summer, and rainy seasons, respectively. Since both MBC and MBN were highest in invasive plant-infested plots receiving the minimum rainfall across all three seasons, the general trend observed was LR > NR > HR (Table 2).
Soil moisture exhibited a statistically significant negative correlation with MBC (r = − 0.94, P < 0.01) and MBN (r = − 0.88, P < 0.01) (Supplementary Table 2). Regression analysis of soil moisture with MBC (R2 = 0.62) and MBN (R2 = 0.76) revealed a negative linear relationship (Table 3; Fig. 6). N-mineralization exhibited a negative linear relationship during regression analysis with MBC (R2 = 0.96) and MBN (R2 = 0.77) (Fig. 7).
Plant growth and functional traits
The experiment resulted in significant effects on physiological properties, growth measurements, and functional traits of P. juliflora. Among physiological properties and growth measurements, photosynthesis rate (Aarea), transpiration rate (E), height (H), diameter (D), root: shoot ratio, and biomass (AGB + BGB) varied significantly (Fig. 7), exhibiting a positive response to changes in precipitation (Table 4). Maximum values for photosynthesis rate, transpiration rate, height, diameter, root: shoot ratio, and biomass were observed in HR, demonstrating a general trend of HR > NR > LR (Table 4, Fig. 8).
However, water use efficiency (WUE) and root length (RL) showed a negative response to changes in precipitation, being highest in LR plots and lowest in HR plots, with the general trend LR > NR > HR (Table 4). Stomatal conductance was not found to vary significantly across the simulated rainfall plots.
Among leaf attributes, leaf area (LA), specific leaf area (SLA), leaf carbon content (LCC), and leaf C: N ratio varied positively significant with simulated rainfall, showing the general trend HR > NR > LR (Supplementary Tables 4 and 5a, b; Table 4). Conversely, leaf dry matter content (LDMC) and leaf nitrogen (LN) had significant negative responses, with the highest values found in plots receiving low rainfall, following the trend LR > NR > HR (Supplementary Tables 4 and 5a, b; Table 4).
Prosopis biomass showed a significant positive correlation with soil moisture (r = − 0.88, P < 0.01), soil inorganic nitrogen (r = − 0.88, P < 0.01), and nitrogen mineralization (r = − 0.88, P < 0.01) (Supplementary Table 2; Fig. 9), while exhibiting a significant negative correlation with microbial biomass carbon (MBC) (P < 0.01) and microbial biomass nitrogen (MBN) (P < 0.01) (Supplementary Tables 2b, 3 and 4). Regression analysis revealed strong positive linear relationships between Prosopis biomass and soil moisture (R2 = 0.83), soil inorganic nitrogen (R2 = 0.77), and nitrogen mineralization (R2 = 0.79) (Figs. 9a, b, c). In contrast, negative linear correlations were observed with MBC (R2 = 0.51) and MBN (R2 = 0.76).
Interaction among variables
This research demonstrated that the variability of rainfall, and consequently soil moisture, significantly influenced soil properties and processes, as well as nutrient cycling within the ecosystem. The variables soil ST, NH4–N, NO3–N, NF, AF, and N-MIN exhibited a significant positive correlation with soil moisture (Supplementary Tables 2, 3a, b). The analysis of the plant’s eco-physiological and growth traits indicated that Aarea exhibited a positive correlation with E, LA, and SLA, while demonstrating a negative correlation with WUE (Supplementary Tables 4 and 5a, b). A robust positive correlation exists between Prosopis biomass and its height, diameter, leaf area, and specific leaf area (Supplementary Tables 4 and 5a, b).
Discussion
Precipitation is a critical factor influencing land surface hydrology and terrestrial water and energy cycles32. This study demonstrates the strong link between precipitation and soil moisture (SM) (Fig. 2). Soil moisture exhibited a seasonal pattern, with highest values during the high-rainfall (HR) season and lowest during the low-rainfall (LR) summer season (HR > NR > LR). This aligns with previous findings by8 who reported significant impacts of rainfall variations on soil moisture and plant production.
Increased rainfall leads to higher soil moisture due to increased infiltration, benefiting plant growth and agriculture by providing adequate water to crops. However, excessive precipitation can saturate the soil, depriving plant roots of oxygen and nutrients, leading to water stress or death. Conversely, decreased rainfall results in lower soil moisture, triggering drought conditions that negatively impact plant development and agricultural output. Climate change, with its associated alterations in precipitation patterns and temperature, further disrupts these dynamics33.
Both seasonality and simulated rainfall significantly affect soil moisture and consequently, the growth of P. juliflora. While precipitation patterns are changing globally, impacting soil nitrogen availability, little is known about how soil nitrogen systems respond to drought stress under climate change34. Our findings suggest that in dry tropical grasslands, soil characteristics significantly influence total inorganic nitrogen (N) and N-mineralization. Precipitation indirectly affects soil N-mineralization through variations in soil moisture, temperature, nitrate leaching, and carbon (C) and nitrogen concentrations35,36.
The link between precipitation and net nitrification is complex and depends on factors like soil moisture, rainfall frequency and intensity, and seasonality37,38. In this study, soil moisture had a significant positive effect on the nitrification rate during the rainy season, but not during the dry season (HR > NR > LR). This might be due to higher microbial nitrate immobilization exceeding gross nitrate mineralization during dry periods when soil moisture is low39.
Our findings reveal a seasonal pattern of accessible nutrient pools and mechanisms related to N dynamics, likely driven by variations in soil moisture due to changing rainfall patterns40,41. Plant nitrogen uptake was lowest in the summer (highest available N) and highest during the rainy season due to increased plant growth. This seasonality aligns with observations in other tropical Indian soils40,42.
The study demonstrates a positive correlation between N-content and N-mineralization with soil moisture in P. juliflora-invaded plots. Despite being drought-tolerant, P. juliflora biomass decreased by 26.90% in LR plots compared to NR plots but increased by 78.45% in HR plots. This 51.55% increase in biomass with increased precipitation likely explains the high N-content observed in HR plots. Additionally, the higher photosynthetic rate in plots receiving more precipitation, a limiting factor for plant N-fixation, might contribute to the high inorganic-N content in HR plots despite the high growth requirement for this nutrient.
Microbial biomass, measured by microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN), represents a significant pool of nutrients, especially in less fertile ecosystems like disturbed forests and dry tropical forests43,44. In dry tropical forests, MBN content can range from 20–80 μg gˉ1 of dry soil, accounting for a substantial portion of the total soil nitrogen (1.7–9.6%)45,46,47. This study reveals changes in microbial biomass C and N content with variations in rainfall patterns. For instance, changes in soil moisture significantly affected the microbial biomass content in the study plots.
In the present study, higher microbial biomass was found in the plots receiving the least rainfall (LR) and during the dry season with lower soil moisture, resulting in significant negative relationships between MBC and soil moisture across all rainfall treatments. During the wet season with optimal plant growth, microbial biomass is mineralized at a faster pace to readily available forms to support plant growth. Similar patterns have been reported by several studies from different parts of India48. Under circumstances of low moisture, microbial biomass serves as a reservoir for nutrients that plants are incapable of absorbing. When the soil becomes dry, microorganisms enhance their stress pathways to safeguard their molecular structures29,33. In extreme instances, this may lead to a state of hibernation, resulting in an elevation of the carbon and nitrogen levels in microbial biomass33,49. Have33 suggested that the increased nutritional requirements of plants during the rainy season restrict the availability of nutrients for soil microorganisms, resulting in a decrease in microbial biomass.
The present study found a significant positive relationship between MBC and MBN, which aligns with previous research by50 and33. This positive correlation might be due to the fact that C and N ratios are conserved across most organisms and vary only when microbial composition changes due to the altered dominance of fungi and bacteria. This C: N ratio governs various processes related to decomposition and mineralization of organic matter, as well as the assimilation and immobilization of nutrients by plants and microbes, respectively.
This study investigated how P. juliflora captures resources through root uptake in response to changes in precipitation patterns. We simulated rainfall scenarios (low, normal, high) and observed significant effects on the plant’s gas exchange, water use efficiency (WUE), growth parameters, and root development. In our study increasing rainfall significantly increased P. juliflora biomass, height, and diameter, while decreasing rainfall significantly reduced these parameters. These findings align with long-term studies on terrestrial ecosystems51,52.
Increased precipitation may enhance photosynthetic rates through expanded leaf area, leading to higher biomass accumulation53,54. In this study also, high rainfall plots exhibited increased photosynthetic rates, stomatal conductance, and transpiration rates compared to lower rainfall plots. However, WUE showed an opposite trend, with the highest values observed in low rainfall plots and the lowest in high rainfall plots. This suggests that increased precipitation directly affects the pace of photosynthesis33 but may also lead to less efficient water use.
Low rainfall plots had reduced photosynthesis, stomatal conductance, transpiration, height, diameter, and biomass. Leaves with a larger surface area can enhance gas exchange and photosynthesis55. Our findings support this, with P. juliflora leaves showing increased surface area under higher rainfall, leading to higher photosynthetic rates. This suggests the species’ adaptability to varying precipitation levels.
Low moisture conditions significantly impact gas exchange and photosynthetic rates56. Drought-tolerant plants like P. juliflora may respond by increasing WUE, as seen in this study. This allows them to conserve water through stomatal closure, leaf area adjustments, and root-to-shoot ratio changes57. Our results align with this concept, with WUE highest in low rainfall plots.
Optimal allocation theory suggests that plants allocate resources strategically. In drought conditions, plants prioritize root growth to maximize water uptake58,59. This may explain the observed decrease in root length under higher simulated rainfall, as plants allocate more resources to aboveground growth when water is readily available60 also reported decreased water uptake by P. juliflora during winter, supporting this concept.
Changes in precipitation can affect plant growth rates61. While some species experience reduced growth under drought, others show resilience or even enhanced growth53,54. Our study confirms this, with simulated rainfall impacting P. juliflora growth, biomass, height, diameter, and invasiveness62. However, root length responded negatively to increased simulated rainfall. Changes in precipitation can significantly impact productivity, biomass, and soil processes62.
Plants adapt to changing conditions by adjusting biomass allocation and root morphology. Fine root diameters and long root lengths are associated with drought tolerance, allowing access to deeper soil water63. Conversely, in water-rich environments, plants may prioritize shallower roots for efficient water uptake64,65. Eco physiological traits like photosynthesis and stomatal conductance are linked to leaf-level processes5. This explains the higher photosynthetic rates and stomatal conductance observed in high precipitation plots compared to low precipitation plots. Drought-stressed leaves may have lower photosynthetic activity, while wetter conditions may encourage increased stomatal conductance and carbon uptake66.
ALinking the soil dynamics, growth and physiology of Prosopis under simulated rainfall
Tropical regions characterized by monsoonal climates exhibit pronounced seasonal variations, extreme water limitation during the summer or dry period, and substantial biological activity during the rainy or wet period67. Our study elucidated the impact of seasonality on components of soil dynamics (TIN, MBC, MBN, N-MIN), Prosopis growth, and its ecophysiology. Across all simulated rainfall conditions (LR. NR, HR), we observed a positive correlation between Prosopis biomass, photosynthetic rate, leaf area, specific leaf area, leaf carbon content, and C:N ratio and soil moisture, soil inorganic nitrogen, and nitrogen mineralization (Supplementary Tables 2, 3, 4, and 5). Conversely, we observed a significant negative correlation between MBC, MBN, LDMC, and LN. This finding aligns with previous studies on nearby tropical soils9,29,30,33,68. This underscores the dependence of the soil nutrient pool, microbial biomass, growth, and physiology on adequate soil moisture availability9,68.
Our study corroborates climate-induced alterations in precipitation patterns, which have facilitated the proliferation of invasive plant species (IPS) such as Leptochloa chinensis and Marsilea quadrifolia in rice paddy areas by enhancing their biomass, nutrient content, N-mineralization, leaf area, specific leaf area, and photosynthetic rate69. Consequently, the interaction between precipitation and IPS poses a significant threat to the survival of numerous species. Furthermore, the interplay between elevated temperatures, arid conditions, and productive species may disrupt host ecosystems, resulting in detrimental effects on biodiversity and, subsequently, ecosystem structure and function70.
In India’s tropical ecosystem, indigenous and invasive springtails exhibit divergent phenotypic plasticity. Specifically, climate change-induced warming enhances desiccation tolerance and survival in invasive species, while reducing survival in native species under arid conditions71. Prosopis juliflora, in particular, exhibits resistance to high temperature and humidity conditions. Consequently, the impact of rainfall variability on their distribution and adaptation strategies is expected to become more pronounced in the future72.
P. juliflora is a widespread invasive plant in India that particularly thrives in arid regions. While it flourishes under high rainfall due to increased soil nitrogen availability, it also exhibits a surprising tolerance for drought. Studies show a significant increase in its below-ground biomass during simulated droughts, suggesting an adaptation for resource conservation during water scarcity73,74. This adaptability allows P. juliflora to potentially colonize drier areas, including naturally arid regions or those projected to become drier due to climate change. Trends indicate a significant decline in southwest monsoon rainfall across India over the past three decades (1989–2018) and increased precipitation intensity in arid regions of the country75. These contrasting trends, coupled with the predicted rise in extreme weather events – intense droughts and heavy rainfall episodes – may enhance the invasiveness of P. juliflora.
Materials and methods
Study site
The study was conducted in experimental plots at the Banaras Hindu University Botanical Garden (25°16′3.3″N and 82°59′22.7″E), Varanasi, India (Fig. 1), Located in the middle Indo-Gangetic plains, the area sits at an elevation of approximately 86.7 m above mean sea level. The map in Fig. 1 was created using QGIS, an open-source Geographic Information System software and base map is sourced from OpenStreetMap (OSM), a publicly accessible and open-source platform.
Climatic conditions
The climate is monsoonal with an annual rainfall of approximately 950 mm. Around 80% of this rainfall occurs between June and September, while the remaining 20% is distributed over the other two seasons, spanning the remaining eight months. The year is divided into three distinct seasons: (i) winter (November to February) characterized by cooler temperatures, with a mean monthly minimum of 10.2 °C (ii) summer (April to June) marked by high temperatures, with a mean monthly maximum of 36.9 °C, and (iii) warm rainy season (July to September) dominated by heavy monsoonal rainfall. The months of March and October serve as transition periods, bridging the gap between winter and summer, and between the rainy and winter seasons, respectively.
Soil
The soil on the Banaras Hindu University campus is classified as Banaras Type III and falls under the inceptisol category76. This soil is characterized by a deep, pale brown color and a silty loam texture, with a neutral reaction. Generally, the soil is alluvial, well-drained, and moderately fertile. It has low nitrogen availability and medium phosphorus and potassium availability77. The soil pH ranges from neutral to alkaline. Table 1 provides the observed soil physicochemical properties at the beginning of the experiment in the experimental plots.
Experimental design
The experimental design for the study was based on a randomized block design, spanning two years (2021 and 2022) and involving three distinct rainfall conditions: normal rainfall (NR): 1000 mm, low rainfall (LR): 500 mm, and high rainfall (HR): 1400 mm (hereafter denoted as LR, NR, and HR). A rainout shelter facility was established within the Botanical Garden of the Banaras Hindu University, India, to simulate varying precipitation levels as indicated above. Prior to establishing the experimental plots, the site soil was thoroughly mixed and homogenized. Each rainout shelter block (9 m × 8 m) was subdivided into six smaller subplots (2 m × 2 m), separated by a 1 m wide buffer zone. Three randomly selected subplots under each simulated rainfall regime were transplanted with Prosopis juliflora, ensuring a density of 20 seedlings per subplot and a 0.5-m spacing between each plant. Figure 1 provides a graphical representation of the experimental layout.
The study aimed to examine the effects of various rainfall conditions on the soil properties related to P. juliflora plants. We carefully managed the plant density in each subplot to encourage a shrubby development pattern rather than a woody one78. We systematically eliminated surplus plants not included in the trial from each plot. The rainout shelter, covered by a transparent polythene sheet to permit natural light, maintains similar air temperature and relative humidity to the external environment due to adequate ventilation.
Manipulated rain dose selections
The rainout shelters were constructed with a galvanized tubular steel frame supporting a clear plastic roof. The water amounts used for the precipitation treatments were determined based on rainfall patterns from the previous 20 years (2001–2020), utilizing daily meteorological data recorded by the Indian Meteorological Department (IMD) unit at the Department of Agronomy, Institute of Agriculture Sciences, BHU. The selected rainfall doses reflect the range observed over these two decades. The three precipitation doses applied were: (i) Normal Rainfall (NR): 1000 mm, (ii) High Rainfall (HR): 1400 mm (40% above the average), (iii) Low Rainfall (LR): 500 mm (50% below the average). We also calculated the average number of rainy days per month to create a watering calendar for 2021 and 2022. Manual watering was done using a sprinkler, adjusting the water volume for each plot’s surface area according to the patterns detailed in Table 5.
Sampling and analysis
Soil Analysis
To provide a baseline understanding of the soil properties before the commencement of the experimental treatments, initial soil characterisation was done for pH, soil texture, bulk density, and water holding capacity. Soil samples were collected from each replicate plot under different precipitation conditions. The samples were then sieved through a 2 mm mesh screen and stored at 4 °C for further analysis.
Soil texture was analyzed by the hydrometer method using unsieved soil from the replicate plots. A soil corer was used to measure the bulk density (BD), which is the weight of dry soil per unit volume, down to a depth of 10 cm79. The water holding capacity (WHC) was determined by use perforated circular brass boxes79,80 approach was used to quantify soil organic carbon (SOC), whereas the Kjeldahl method81was used to estimate total nitrogen (TN). The results of these analyses are detailed in Table 1.
Soil temperature (ST) was measured using a temperature probe. The soil pH was determined by a digital pH meter using a soil-solution ratio of 1:2.582. Field moist soil samples were divided into two parts: One part was used to estimate the total soil inorganic nitrogen (NO3–N + NH4–N) and the other part was used to evaluate the N-mineralization rate. NO3–N was quantified using the phenol disulphonic acid methodology80, and NH4–N was using the phenate method83. The buried bag technique was used to assess the N-mineralization in the soil84. NH4-N and NO3-N were measured twice: once during the initial zero-day sampling and once after the 30-day field incubation sampling. Net N-mineralization is the total rise in NH4-N and NO3-N concentrations during the duration of the field incubation46,85. The fumigation extraction method was used to estimate MBC as well as MBN86. Soil fumigation was carried out using pure liquid chloroform45. K2SO4 was used to extract the fumigated and non-fumigated soil both. MBC and MBN were determined from extracted soil by dichromate digestion87.
The estimation of microbial biomass carbon (MBC) was conducted using the technique described by79,80. The same methodology was used to determine the microbial biomass nitrogen (MBN) as the microbial biomass carbon (MBC) by using the same soil samples and the same extractant, K2SO4. According to86,87 it was ascertained using the CHCl3 fumigation extraction method.
Plant analysis
To study the physiological, leaf characteristics, and growth parameters during the rainy seasons of 2021 and 2022, three P. juliflora individuals were chosen from each replicate plot. Plant height (H) was measured from the ground to the inflorescence, and root length (RL) was measured from ground level to the taproot tip. In the laboratory, plants selected for physiological parameters were separated into stems, leaves, and roots, and their dry weights were recorded. Biomass assessment of P. juliflora included total biomass (AGB + BGB), i.e., above-ground biomass (AGB) and below-ground biomass (BGB), at the end of the growth season (September) in 2020 and 2021.
The LI-6400 gas exchange system (LI-COR, Lincoln, Nebraska, USA) was used to measure the photosynthetic rate (Aarea), stomatal conductance (gs), and transpiration rate (E) of the top, fully expanded, and healthy-looking leaves on sunny days with natural light. Measurements were taken in the mornings under clear skies (between 9:00 and 11:00 a.m. local time). Rates were measured at or near light saturation (mean PAR: 1305.2 ± 64 µmol m−2 s−1). The flow rate was maintained at 500 μmol s−1 at a CO2 concentration of 380 ± 10 µmol CO2 mol−1. Leaves were maintained in the chamber until their photosynthetic activity became consistent. Water use efficiency (WUE) was calculated as the ratio of carbon generated via photosynthesis (A) to water lost through transpiration (A/E).
Leaves collected after gas exchange measurement were placed in a plastic bag for future analysis. Leaf area (LA) was measured with a leaf area meter, and dry mass was obtained by oven-drying leaves at 70 °C for 48 h. Specific leaf area (SLA) was defined as area per unit mass (cm2 g−1), while leaf mass per unit area was calculated as 1/SLA33,88. Leaf dry matter content (LDMC) was calculated using the difference between a leaf’s oven-dry mass (mg) and its fresh, water-saturated mass (g)68,89,90,91. SLA, LAR (leaf area ratio), LDMC, and biomass were measured at each harvest of the same plant.
Dried P. juliflora leaf material from each plot was combined to determine organic-N concentration, and carbon content (C) was estimated using a CHN auto-analyser.
Statistical analysis
The impact of precipitation changes on P. juliflora plot soil properties and ecophysiology was investigated using multivariate analysis of variance (MANOVA). Tukey’s post hoc comparison test was performed to determine significant differences between means. Pearson correlation analysis and regression analysis were used to analyze how leaf nutrients (i.e., leaf N concentrations), photosynthetic qualities, and leaf structural features (i.e., SLA) were related in each simulated rainfall plot. All statistical analyses were performed using SPSS 16.
Conclusions
The precipitation plays a critical role in mediating soil moisture, which in turn significantly impacts soil nutrient pools and their dynamics. This study highlights the complex interplay between rainfall patterns, soil moisture, and microbial activity. Microbial biomass acts as a dynamic reservoir of nutrients, adapting to changing environmental conditions. The study further demonstrates that P. juliflora exhibits significant plasticity in its resource capture strategies in response to changing precipitation patterns. The plant can adjust gas exchange, water use efficiency, and root morphology to adapt to varying water availability. Understanding these adaptations is crucial for predicting the potential impact of changing precipitation patterns on P. juliflora invasion and its ecological consequences.
The present study explored the role of soil moisture as a mediator between rainfall and plant growth, particularly invasive P. juliflora in dry tropical grasslands. Rainfall patterns significantly impact soil moisture, which in turn plays a crucial role in nitrogen dynamics. It influences factors like nitrification and nitrogen availability for plants. The study found a positive correlation between soil moisture and N-mineralization, impacting P. juliflora growth and nitrogen content. Microbial biomass, a significant nutrient reservoir, also responds to changing rainfall patterns. A higher microbial biomass was found in drier conditions, possibly due to microbes acting as a nutrient sink when plants are unable to take them up.
The study also investigated P. juliflora's ecophysiological response to varying precipitation. Increased rainfall led to higher biomass, height, and diameter, while decreased rainfall had the opposite effect. This aligns with the observed changes in gas exchange and water use efficiency. Plants in drier conditions exhibited lower photosynthetic rates and prioritized water conservation strategies. Therefore, a predicted rise in extreme weather events – intense droughts and heavy rainfall episodes – may enhance the invasiveness of P. juliflora.
Data availability
The datasets are available from the corresponding author upon reasonable request.
References
IPCC: Climate change synthesis report. Contribution of Working Group I. II, & III to the Fifth Assessment Report of the International Panel on Climate Change. https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (2022).
Duffy, P. B., Brando, P., Asner, G. P. & Field, C. B. Projections of future meteorological drought & wet periods in the Amazon. Proc. Natl. Acad. Sci. 112, 13172–13177. https://doi.org/10.1073/pnas.1421010112 (2015).
Chadwick, R., Good, P., Martin, G. & Rowell, D. P. Large rainfall changes consistently projected over substantial areas of tropical land. Nat. Clim. Change 6, 177. https://doi.org/10.1038/nclimate2805 (2016).
Zhang, W. et al. Anthropogenic influence on 2018 summer persistent heavy rainfall in central western China. Bull. Am. Meteor. Soc. 101, 65–70 (2020).
Zhang, W., Zhou, T., Zou, L., Zhang, L. & Chen, X. Reduced exposure to extreme precipitation from 0.5 C less warming in global land monsoon regions. Nat. Commun. 9, 3153 (2018).
Weltzin, J. F., Belote, R. T. & Sanders, N. J. Biological invaders in a greenhouse world: will elevated CO2 fuel plant invasions?. Front. Ecol. Environ. 11, 46–153. https://doi.org/10.2307/3867987 (2003).
Feng, X., Porporato, A. & Rodriguez-Iturbe, I. Changes in rainfall seasonality in the tropics. Nat. Clim. Change 3, 811–815. https://doi.org/10.1038/nclimate1907 (2013).
Afreen, T. & Singh, H. Does change in precipitation magnitude affect the soil respiration response? A study on constructed invaded & uninvaded tropical grassland ecosystem. Ecol. Ind. 102, 84–94. https://doi.org/10.1016/j.ecolind.2019.02.022 (2019).
Afreen, T., Rajak, P. & Singh, H. Invasion by Hyptis suaveolens modifies the effects of altered rainfall variability on nutrient cycling across seasons in a dry tropical grassland experiment. Ecoscience 30, 170–186. https://doi.org/10.1080/11956860.2024.2311824 (2023).
Deutsch, C. A. et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. 105, 6668–6672. https://doi.org/10.1073/pnas.0709472105 (2008).
Bouskill, N. J. et al. Belowground response to drought in a tropical forest soil. I. Changes in microbial functional potential & metabolism. Front. Microbiol. 7, 525. https://doi.org/10.3389/fmicb.2016.00525 (2016).
Cregger, M. A., McDowell, N. G., Pangle, R. E., Pockman, W. T. & Classen, A. T. The impact of precipitation changes on nitrogen cycling in a semi-arid ecosystem. Funct. Ecol. 28, 1534–1544. https://doi.org/10.1111/1365-2435.12282 (2014).
Shi, Y. et al. Interannual climate variability & altered precipitation influence the soil microbial community structure in a Tibetan Plateau grassland. Sci. Total Environ. 714, 136794. https://doi.org/10.1016/j.scitotenv.2020.136794 (2020).
Paruelo, J. M., Beltrán, A., Jobbágy, E., Sala, O. E. & Golluscio, R. A. The climate of Patagonia: General patterns & controls on biotic processes. Ecol. Aust. 8, 085–101 (1998).
Zhang, L. L., Zhang, L. M. & Tang, W. H. Rainfall-induced slope failure considering variability of soil properties. Geotechnique 55, 183–188. https://doi.org/10.1680/geot.55.2.183.59525 (2005).
Jin, Y. & Goulden, M. L. Ecological consequences of variation in precipitation: separating short-versus long-term effects using satellite data. Glob. Ecol. Biogeogr. 23, 358–370. https://doi.org/10.1111/geb.12135 (2014).
Greenwood, W. J. & Eimers, M. C. Hydroclimatic variability across the international Lake of the Woods watershed: Implications for nutrient export & climate sensitivity. J. Great Lakes Res. 49, 8–20 (2023).
Pimentel, D. et al. Economic & environmental threats of alien plant, animal, & microbe invasions. Agric. Ecosyst. Environ. 84, 1–20. https://doi.org/10.1016/S0167-8809(00)00178-X (2001).
Levine, J. M. et al. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 270, 775–781. https://doi.org/10.1098/rspb.2003.2327 (2003).
Sharma, G. P., Raghubanshi, A. S. & Singh, J. S. Lantana invasion: an overview. Weed Biol. Manag. 5, 157–165. https://doi.org/10.1111/j.1445-6664.2005.00178.x (2005).
Mwangi, E. & Swallow, B. Prosopis juliflora invasion & rural livelihoods in the Lake Baringo area of Kenya. Conserv. Soc. 6, 130–140 (2008).
Rajak, P., Afreen, T. & Singh, H. Assessing the impact of Invasive Alien Plant Species on environment ecosystem services & human health. Plant Arch. 21, 2155–2163. https://doi.org/10.51470/PLANTARCHIVES.2021.v21.S1.355 (2021).
Schei, P. J. Conclusions & recommendations from the UN/Norway conference on alien species. Sci. Int. 63, 32–36 (1996).
Hellmann, J. J., Byers, J. E., Bierwagen, B. G. & Dukes, J. S. Five potential consequences of climate change for invasive species. Conserv. Biol. 22, 534–543. https://doi.org/10.1111/j.1523-1739.2008.00951.x (2008).
Bellard, C. et al. Will climate change promote future invasions?. Glob. Change Biol. 19, 3740–3748. https://doi.org/10.1111/gcb.12344 (2013).
Ricciardi, A., Hoopes, M. F. & Marchetti, M. Progress toward understating the ecological impacts of non-native species. Ecol. Monogr. 83, 263–282. https://doi.org/10.1890/13-0183.1 (2013).
der Putten, V. et al. Plant–soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276. https://doi.org/10.1111/1365-2745.12054 (2013).
Maron, J. L., Klironomos, J., Waller, L. & Callaway, R. M. Invasive plants escape from suppressive soil biota at regional scales. J. Ecol. 102, 19–27. https://doi.org/10.1111/1365-2745.12172 (2014).
Afreen, T., Srivastava, P., Singh, H. & Singh, J. S. Effect of invasion by Hyptis suaveolens on plant diversity & selected soil properties of a constructed tropical grassland. J. Plant Ecol. 11, 751–760. https://doi.org/10.1093/jpe/rtx045 (2018).
Afreen, T. & Singh, H. Differential response of graminoid & forb species to precipitation variability in a constructed dry tropical grassland. Trop. Ecol. 65, 124–132. https://doi.org/10.1007/s42965-023-00313-w (2024).
OPENSTREETMAP (OSM) contributors. OPENSTREETMAP database [PostgreSQL via API]. OPENSTREETMAP (OSM) Foundation: Cambridge, UK; 2021 [cited 22 Dec 2021. Available under the Open Database Licence from: openstreetmap.org. Data mining by Overpass turbo. Available at http://overpass-turbo.eu/.
Sehler, R., Li, J., Reager, J. T. & Ye, H. Investigating relationship between soil moisture & precipitation globally using remote sensing observations. J. Contemp. Water Res. Educ. 168, 106–118. https://doi.org/10.1111/j.1936-704x.2019.03324.x (2019).
Afreen, T., Singh, V., Yadav, V. K., Singh, R. P. & Singh, H. Impact of rainfall variability on the ecophysiology of Hyptis suaveolens: a study in the constructed tropical grassland. Environ. Monit. Asses. 192, 1–11. https://doi.org/10.1007/s10661-020-08340-z (2020).
Chen, L. et al. Differential responses of net N mineralization & nitrification to throughfall reduction in a Castanopsis hystrix plantation in southern China. Forest Ecosyst. 6, 1–11. https://doi.org/10.1186/s40663-019-0174-2 (2019).
Paul, K. I. et al. Defining the relation between soil water content & net nitrogen mineralization. Eur. J. Soil Sci. 54, 39–47. https://doi.org/10.1046/j.1365-2389.2003.00502.x (2003).
Chen, J. et al. Soil nitrogen transformation responses to seasonal precipitation changes are regulated by changes in functional microbial abundance in a subtropical forest. Biogeosciences 14, 2513–2525. https://doi.org/10.5194/bg-14-2513-2017 (2017).
Yahdjian, L. & Sala, O. E. Size of precipitation pulses controls nitrogen transformation & losses in an arid Patagonian ecosystem. Ecosystems 13, 575–585. https://doi.org/10.1007/s10021-010-9341-6 (2010).
Jongen, M., Lecomte, X., Unger, S., Fangueiro, D. & Pereira, J. S. Precipitation variability does not affect soil respiration & nitrogen dynamics in the understory of a Mediterranean oak woodland. Plant Soil 372, 235–325. https://doi.org/10.1007/s11104-013-1728-7 (2013).
Yokobe, T., Hyodo, F. & Tokuchi, N. Seasonal effects on microbial community structure & nitrogen dynamics in temperate forest soil. Forests 9, 153. https://doi.org/10.3390/f9030153 (2018).
Singh, J. S., Raghubanshi, A. S., Singh, R. S. & Srivastava, S. C. Microbial biomass acts as a source of plant nutrients in dry tropical forest & savanna. Nature 338, 499–500 (1989).
Raizada, P., Raghubanshi, A. S. & Singh, J. S. Impact of invasive alien plant species on soil processes: A review. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 78, 288–298 (2008).
Roy, S. & Singh, J. S. Seasonal and spatial dynamics of plant-available N & P pools and N-mineralization in relation to fine roots in a dry tropical forest habitat. Soil Biol. Biochem. 27, 33–40. https://doi.org/10.1016/0038-0717(94)00138-Q (1995).
Vitousek, P. M. & Matson, P. A. Nitrogen transformations in a range of tropical forest soils. Soil Biol. Biochem. 20, 361–367. https://doi.org/10.1016/0038-0717(88)90017-X (1988).
Cheng, W. et al. Soil nitrogen, microbial biomass, & respiration along an arctic top sequence. Soil Sci. Soc. Am. J. 62, 654–662. https://doi.org/10.2136/sssaj1998.03615995006200030016x (1998).
Srivastava, S. C. & Singh, J. S. Carbon & phosphorus in the soil biomass of some tropical soils of India. Soil Biol. Biochem. 20, 743–774. https://doi.org/10.2307/2404213 (1988).
Raghubanshi, A. S. Dynamics of soil biomass C, N, & P in a dry tropical forest in India. Biol. Fertil. Soils 12, 55–59. https://doi.org/10.1007/BF00369388 (1991).
Tripathi, N. & Singh, R. S. Cultivation impacts nitrogen transformation in Indian forest ecosystems. Nutr. Cycl. Agroecosyst. 77, 233–243. https://doi.org/10.1007/s10705-006-9061-7 (2007).
Arunachalam, A. & Arunachalam, K. Influence of gap size & soil properties on microbial biomass in a subtropical humid forest of north-east India. Plant Soil 223, 187–195. https://doi.org/10.1023/A:1004828221756 (2000).
Jones, S. E. & Lennon, J. T. Dormancy contributes to the maintenance of microbial diversity. Proc. Natl. Acad. Sci. 107, 5881–5886. https://doi.org/10.1073/pnas.0912765107 (2010).
Singh, H. & Singh, K. P. Effect of residue placement & chemical fertilizer on soil microbial biomass under tropical dryland cultivation. Biol. Fertil. Soils 16, 275–281. https://doi.org/10.1007/BF00369304 (1993).
Xiong, X. et al. Reduced turnover rate of topsoil organic carbon in old-growth forests: a case study in subtropical China. For. Ecosyst. 8, 1–11. https://doi.org/10.1007/s10533-004-2222-3 (2021).
Zhang, X. et al. Observed changes in extreme precipitation over the Tienshan Mountains & associated large-scale climate teleconnections. J. Hydrol. 606, 127457. https://doi.org/10.1016/j.jhydrol.2022.127457 (2022).
Cheng, H., Gong, Y. & Zuo, X. Precipitation variability affects aboveground biomass directly & indirectly via plant functional traits in the desert steppe of inner Mongolia, Northern China. Front.Plant Sci. 12, 674527. https://doi.org/10.3389/fpls.2021.674527 (2021).
Wilcox, K. R. et al. Assessing carbon storage capacity & saturation across six central US grasslands using data–model integration. Biogeosciences 20, 2707–2725. https://doi.org/10.5194/bg-20-2707-2023 (2023).
Matzek, V. Superior performance & nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment. Biol. Invasions 13, 3005–3014. https://doi.org/10.1007/s10530-011-9985-y (2011).
Zouaoui, R. et al. Physiological & biochemical responses of Rhus tripartita (Ucria) grande under water stress. Pak. J. Bot. 51, 215–122 (2019).
Ikram, S., Bhattarai, S. & Walsh, K. B. Characterization of selected Mungbean genotypes for tolerance to waterlogging stress at pod filling stage. Agronomy 12, 1663. https://doi.org/10.3390/agronomy12071663 (2022).
Li, C. Genomic insight into changes of root architecture under drought stress in maize. Plant, Cell Environ. 46, 1860–1872. https://doi.org/10.1111/pce.14567 (2023).
Gupta, A. K. Drought stress & its tolerance mechanism in wheat. Int. J. Environ. Clim. Change 14, 529–544. https://doi.org/10.9734/ijecc/2024/v14i13866 (2024).
Shiferaw, W., Demissew, S., Bekele, T., Aynekulu, E. & Pitroff, W. Invasion of Prosopis juliflora & its effects on soil physicochemical properties in Afar region, Northeast Ethiopia. Int. Soil Water Conserv. Res. 9, 631–638. https://doi.org/10.1016/j.iswcr.2021.04.003 (2021).
Wright, I. J. & Westoby, M. Leaves at low versus high rainfall: coordination of structure, lifespan & physiology. New Phytol. 155, 403–416. https://doi.org/10.1046/j.1469-8137.2002.00479.x (2002).
Howari, F. M. Changes in the invasion rate of Prosopis juliflora & its impact on depletion of groundwater in the northern part of the United Arab Emirates. Plants 11, 682. https://doi.org/10.3390/plants11050682 (2022).
Maseda, P. H. & Fernández, R. J. Stay wet or else: Three ways in which plants can adjust hydraulically to their environment. J. Exp. Bot. 57, 3963–3977. https://doi.org/10.1093/jxb/erl127 (2006).
Comas, L. H. et al. Root traits contributing to plant productivity under drought. Front. Plant Sci. 4, 442. https://doi.org/10.3389/fpls.2013.00442 (2013).
Xiang, W. et al. Stable isotopes of deep soil water retain long-term evaporation loss on China’s Loess Plateau. Sci. Total Environ. 784, 147153. https://doi.org/10.1016/j.scitotenv.2021.147153 (2021).
Singh, S. K. & Reddy, K. R. Regulation of photosynthesis, fluorescence, stomatal conductance & water-use efficiency of cowpea (Vigna unguiculata [L.] Walp.) under drought. J. Photochem. Photobiol. B: Biol. 105, 40–50. https://doi.org/10.1016/j.jphotobiol.2011.07.001 (2011).
Rohr, T., Manzoni, S., Feng, X., Menezes, R. S. & Porporato, A. Effect of rainfall seasonality on carbon storage in tropical dry ecosystems. JGR Biogeosci. 118, 1156–1167. https://doi.org/10.1002/jgrg.20091 (2013).
Mishra, A., Rajak, P., Raghubanshi, A. S. & Singh, H. Understanding competition between two invasive woody plants of India under an altered rainfall regime. Plant Ecol. https://doi.org/10.1007/s11258-024-01470-1 (2024).
Li, X. Q., Tang, S. C., Pan, Y. M., Wei, C. Q. & Lü, S. H. Increased precipitation magnifies the effects of N addition on performance of invasive plants in subtropical native communities. J. Plant Ecol. 15, 473–484. https://doi.org/10.1093/jpe/rtab103 (2022).
Turbelin, A. & Catford, J. A. Invasive plants and climate change. In Climate change 515–539 (Elsevier, 2021). https://doi.org/10.1016/B978-0-12-821575-3.00025-6.
Hussain, M. I., Shackleton, R., El-Keblawy, A., González, L. & Trigo, M. M. Impact of the invasive Prosopis juliflora on terrestrial ecosystems. Sustain. Agric. Rev. 52, 223–278. https://doi.org/10.1007/978-3-030-73245-5_7 (2021).
Salinger, M. J., Stigter, C. J. & Das, H. P. Agrometeorological adaptation strategies to increasing climate variability and climate change. Agric. For. Meteorol. 103, 167–184. https://doi.org/10.1016/S0168-1923(00)00110-6 (2000).
Erkin, F., Yue, D., Abdureyim, A., Huang, W. & Tayir, M. Link between the aboveground and belowground biomass allocation with growing of Tamarix sp. seedlings in the hinterland of Taklimakan Desert. China. Plos one 18, 289670. https://doi.org/10.1371/journal.pone.0289670 (2023).
Guasconi, D., Manzoni, S. & Hugelius, G. Climate-dependent responses of root and shoot biomass to drought duration & intensity in grasslands–a meta-analysis. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2023.166209 (2023).
The Ministry of Earth Sciences (MoES), Government of India has recently published a Climate Change report entitled "Assessment of Climate Change over the Indian Region" which covers all the aspects of regional climate change including the climatic extremes across India. https://pib.gov.in/Pressreleaseshare.aspx?PRID=1696515 (2021).
Agrawal, R. R. & Mehrotra C. L. Soil Work in Uttar Pradesh Vol II. (Department of Agriculture, UP, India, 1952).
Sagar, R. & Verma, P. Effects of soil physical characteristics & biotic interferences on the herbaceous community composition & species diversity on the campus of Banaras Hindu University, India. Environmentalist 30, 289–298. https://doi.org/10.1007/s10669-010-9276-7 (2010).
Elfeel, A. A. & Elmagboul, A. H. Effect of planting density on leucaena leucocephala forage and Woody stems production under arid dry climate. Int. J. Environ. Agric. Res. 2, 7–11 (2016).
Sagar, R., Singh, A. & Singh, J. S. Differential effect of woody plant canopies on species composition and diversity of ground vegetation, a case study. Trop. Ecol. 49, 189–197 (2008).
Piper, C. S. Soil & Plant Analysis (Interscience, New York, 1944).
Nelson, D. W. & Sommers, L. E. A simple digestion procedure for estimation of total nitrogen in soils & sediments. J. Environ. Qual 1, 423–425. https://doi.org/10.2134/jeq1972.00472425000100040020x (1972).
Jackson, M. L. Soil chemical analysis Prentice Hall. Inc. Englewood Cliffs 498, 183–204. https://doi.org/10.1002/jpln.19590850311 (1958).
Basak, N. et al. Assessing soil-quality indices for subtropical rice-based cropping systems in India. Soil Res. 54, 20–29. https://doi.org/10.1071/SR14245 (2015).
APHA (American Public Health Association). Standard methods for the examination of water & wastewater. Washington: American Public Health Association (1985).
Eno, C. F. Nitrate production in the field by incubating the soil in poly ethylene bags. Soil Sci. Soc. Am. Proc. 24, 277–279. https://doi.org/10.2136/sssaj1960.03615995002400040019x (1960).
Pastor, J., Aber, J. D., McClaugherty, C. A. & Melillo, J. M. Aboveground production & N & P cycling along a nitrogen mineralization gradient on Blackhawk Island Wisconsin. Ecology 65, 256–268. https://doi.org/10.2307/1939478 (1984).
Brookes, P. C., Landman, A., Pruden, G. & Jenkinson, D. S. Chloroform fumigation & the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 17, 837–842. https://doi.org/10.1016/0038-0717(85)90144-0 (1985).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707. https://doi.org/10.1016/0038-0717(87)90052-6 (1987).
Singh, V. & Singh, H. Leaf construction cost and related ecophysiological parameters of rice crop & its important weeds. Rice Sci. 19, 233–240. https://doi.org/10.1016/S1672-6308(12)60045-6 (2012).
Islam, T., Hamid, M., Nawchoo, I. A. & Khuroo, A. A. Leaf functional traits vary among growth forms & vegetation zones in the Himalaya. Sci. Total Environ. 906, 167274. https://doi.org/10.1016/j.scitotenv.2023.167274 (2024).
Rajak, P., Afreen, T., Raghubanshi, A.S. & Singh, H. Rainfall fluctuation causes the invasive plant Prosopis juliflora to adapt ecophysiologically and change phenotypically. Env. Mon. Asse. 197, 1–17. https://doi.org/10.1007/s10661-024-13393-5 (2025).
Acknowledgements
The authors would like to acknowledge Department of Science and Technology- Science and Engineering Research Board (DST-SERB), and Institute of Eminence (IoE)- Banaras Hindu University for providing research grants.
Author information
Authors and Affiliations
Contributions
All the authors contributed to study conceptualization. P.R. conducted the experiments, analysed the data and drafted the manuscript. A.S.R., H.S. and T.A. provided critical review and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rajak, P., Afreen, T., Raghubanshi, A.S. et al. The impact of rainfall variability on selected soil properties and ecophysiological traits in Prosopis juliflora invaded plots. Sci Rep 15, 31473 (2025). https://doi.org/10.1038/s41598-025-97750-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97750-5