Abstract
The positive sense RNA virus, hepatitis C virus (HCV), is affiliated with the Flaviviridae family. Approximately 1% of people around the globe experience the impact of the HCV, which can coax them into potentially fatal conditions, including cirrhosis and carcinoma. The second-highest hepatitis C infection burden lies in Pakistan. The case of a HCV-infected patient who additionally has obesity or concurrent medical conditions like diabetes, HBV infection, or HIV infection is susceptible to becoming direr. Direct-acting antiviral medications replaced interferons as a staple of the treatment plan since they have fewer, milder side effects and a higher SVR rate in patients. The present study sought to assess the modifications to glucose homeostasis in non-diabetic chronic HCV-infected patients getting DAA treatment and the factors that independently pertain to insulin resistance. The study enrolled 250 patients, with 190 individuals having HCV-positive PCR results. The analysis included CBC, LFTs, glycaemic and insulin measurements, and the insulin resistance index calculation. Key cardiometabolic risk factors crucial for defining MASLD were assessed, including BMI measurement, evaluation of type 2 diabetes, and lipid profile analysis. The same tests were repeated following DAA therapy, and HOMA-IR was computed to compare pre-and post-treatment results. Among the 250 recruited patients, 190 were detected as HCV positive by the PCR assay, 57% (110 patients) were women, 43% (80 patients) were men, and patients were 47 years old on average. The patients showed high BMI (average 26.28 kg/m2) and signs of severe insulin resistance (HOMA-IR > 2.5). Multivariable logistic regression analysis pointed out that elevated baseline levels of triglycerides, ALT, ALP, cholesterol, and total bilirubin were independently associated with high insulin resistance. A notable improvement in HOMA-IR from 13.63 ± 2.63 to 3.16 ± 1.52 (p < 0.005) was spotted after administering interferon-free antiviral therapy for 3 months. The presence of high BMI, hyperlipidemia, and elevated levels of ALP, ALT, and AST in non-diabetic HCV-infected patients were independently associated with IR. In patients who previously had a higher IR index, there was a decrease in the HOMA-IR index after infection clearance by direct-acting antivirals.
Similar content being viewed by others
Introduction
According to the studies, Pakistan is the residence of the second-highest proportion of chronic HCV cases, and noteworthy risk factors there revolve around blood transfusions, confinement in hospitals, orthodontic treatments, injections, and a history of surgery1. Cirrhosis, the 11th most recognized contributor to liver disease and liver cancer, the 16th most prevalent, atone for 2 million deaths concerning liver disease each year. The risk of liver disease is exacerbated by drinking liquor, adiposity, and diabetic complications. Drug-induced damage to the liver is progressively becoming a hallmark of infection caused by HCV infection. Although transplantation of the liver is the second most frequently performed organ transplant, only under 10% of the world’s transplant demands are addressed. To foster better public health, the issues mentioned earlier require being remedied2. Host lipid metabolism regulates the replication and life cycle of the hepatitis C virus3. It is well known that the hepatitis C virus depends profoundly on lipid metabolism to invade and augment new cells4. Worn-out HCV-specific CD8 + T lymphocytes advocate for memory-like and terminally knackered fractions in chronic HCV infection5.
With 3% of the globe’s population6 and 6% of the Pakistani populace infected, HCV infection is a significant threat to public health worldwide. It consists of 67 subtypes and 7 genotypes. It is vital to be aware of the HCV genotype to come up with a successful treatment plan7 and gauge the extent to which it is effective. The genotype 3a is the most widespread8, and Pakistan has the second-highest prevalence6. Host lipid metabolism impacts the replication and life cycle of the hepatitis-C virus6, miR-122 binding helps stabilize RNA9, and translation is contingent on IRES10. Insulin resistance, glucose homeostasis instability11, and a greater likelihood of type 2 diabetes all appear to be attributed to chronic HCV infection12.
Architectural (core, E1, and E2) and regulatory (NS2-NS5B) genes influence HCV-induced insulin resistance and diabetes mellitus, with core and non-structural NS5A proteins exerting a substantial effect on glucose metabolism13. Diabetes increases the prospect of developing carcinoma in CLD cases, with the most significant risk being caused by HCV infection and NAFLD, followed by ALD and HBV. On the other hand, HCV and NAFLD bolster the likelihood of diabetes, and concomitant Diabetes Mellitus (DM) could serve as a precursor to the severity of CLD. In light of this, at least some manifestations of DM are caused by reverse causality14. Diabetes and chronic liver disease (CHC) are two fatal ailments that claim a substantial number of lives around the world15. Several cohort studies provide evidence that chronic HCV infection drives up the risk of developing micro and macrovascular events in diabetic patients and the risk of developing diabetes in non-diabetic subjects (by contributing to insulin resistance as well as fostering cell dysfunction). In addition to a reduced incidence of liver-related consequences, sustained virological response, which may be acquired by most patients through the administration of direct antiviral drugs, significantly improves glycaemic control and lowers the risk of repercussions in patients with diabetes16.
Improvements in insulin resistance are brought about by sustained virological responses in infected individuals17; direct-acting antiviral therapy effectively eliminates infection in 95% of patients, even in those with severe liver disease17. Short courses of antivirals (DAAs) have redefined chronic HCV infection treatment, but limitations with locating patients, treatment accessibility, and cost containment persist17. In this study, glucose homeostasis was evaluated in non-diabetic chronic HCV infection patients getting DAA treatment as well as factors that independently contributed to insulin resistance.
Methods
Study design
A cross-sectional investigation was conducted in this study. HCV-infected cases were recruited for the research. Laboratory testing comprising glycaemic parameters, lipid profiles, and liver function tests was carried out before the first administration of the direct-acting antiviral medication and after 12 weeks of treatment. HOMA-IR indices were calculated and compared before and after treatment with direct-acting antivirals. Participant consent was obtained as per World Health Organization proforma.
Enrolment of patients
Between March and May 2023, at the Minhaj Diagnostic Centre in Lahore, 190 consecutive HCV positive cases were recruited for this study. The enrolled patients satisfied the inclusion criteria of the study, i.e.; (1) were > 20 years old; (2) had detectable viral load quantified by PCR; (3) agreed to adhere to the medication and lab tests; (4) submitted informed consent; and (5) were not already diabetic. PCR was performed, and the results confirmed the infection in all enrolled patients. Patients were not accepted to take part in this study if they: (1) skipped lab tests; (2) have or had diabetes; (3) had co-infection with HBV or HIV; (4) had any other concurrent infection; (5) had a history of alcohol consumption or (6) were equal to or younger than 20 years old. Patients were administered DAA regimens of Sofosbuvir (400 mg) and Daclatasvir (60 mg) for 12 weeks.
Laboratory examination
In a PCR assay, serum HCV RNA levels were measured with sensitivity as low as 15 IU/ml using Qiagen extraction kit and AmpliSens amplification kits. The data was analysed using BIO-RAD CFX manager. Before and at 12 weeks of treatment, the recruited patients’ CBC profile was assessed using Sysmax KX-21, indices of glycemia, bilirubin, albumin, ALP, AST, ALT, cholesterol, and triglyceride, levels were measured using Selectra Pro-S. Insulin was measured by Beckman Coulter analyser and HOMA-IR was enumerated using the following formula:
In patients with HOMA-IR greater than the cutoff value of 2.5, we perused the aspects cognate with insulin resistance. All laboratory tests were repeated once after the patients completed their DAA therapy for 3 months.
Statistical analysis
Categorical data were delineated as numbers (percentages), and quantitative variables depicted as mean ± standard deviation, and the latter were compared using the student’s t-test. We implemented multivariable logistic regression modeling to pinpoint variables independently related to a high baseline IR. We employed the Wilcoxon signed rank test for the pre-post-therapy comparison of numeric variables. With a significance threshold set at p ≤ 0.05, all statistical analyses were classified as two-sided hypotheses. Statistical analyses were conducted with SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
In the intervening period, IFN-free direct-acting antivirals were administered to 190 enrolled HCV patients. The baseline characteristics, along with the results, are presented in Table 1. Results presented a gender split of 110 females (57.9%) and 80 males (42.1%). The mean viral load in patients before starting treatment was 45 × 105 IU/mL and after 3 months of DAAs therapy, the infection was cleared in all patients and a viral load was not detected.
Parameters consorted with insulin resistance
Before the onset of the DAA treatment, all the enrolled patients had notably high IR indices (HOMA-IR > 2.5). Table 2 shows the univariate analysis depicting the variables related to high HOMA-IR. High baseline levels of cholesterol, ALP, AST, ALT, and triglycerides were all profoundly attributable to high IR. The p-value < 0.005 refers to the statistical significance of the relationship between high HOMA-IR values and the related variables.
A multivariable logistic regression analysis (Table 3) in which the variables were selected for their clinical relevance and strong correlations with insulin resistance found in univariate analysis, and the outcomes suggested that high levels of ALT, ALP, AST, cholesterol, and triglycerides at baseline were all independently linked with alarming insulin resistance (all p < 0.005).
To evaluate if there existed a significant difference in the measurements of cholesterol, triglycerides, ALT, AST, ALP, total bilirubin, albumin, and HOMA-IR in chronic HCV positive patients before and after taking direct-acting antivirals for three months, the Wilcoxon signed rank test was employed (Table 4). Conclusions from the analysis suggested that the test results from the pre-and post-treatment periods differed significantly (Fig. 1). Below are the results of the Wilcoxon Signed-Rank test for the comparison of pre- and post-treatment values of cholesterol, AST, ALT, ALP, triglycerides, total bilirubin, albumin, and HOMA-IR.
Wilcoxon signed-rank test results: SPSS analysis. HOMA-IR. The X-axis represents the measured parameters pre- and post-treatment. The Y-axis represents the frequency (number of observations) of pre- and post-treatment values for each variable, allowing direct visualization of data points’ distribution.
The outcome of viral elimination on HOMA-IR
The HOMA-IR index substantially dropped after treatment with direct-acting antivirals. With the infection clearance by DAA therapy, we noticed that the mean HOMA-IR significantly decreased to 3.16 ± 1.52 in individuals with an initial reading of HOMA-IR as high as 13.63 ± 2.63, p < 0.005. After 12 weeks of therapy, the subgroup analysis showed a statistically significant decrease in mean insulin resistance in patients.
Discussion
We presented a cross-sectional analysis of glycemic homeostasis in chronic HCV-infected patients of the Lahore population and the effect of DAA therapy. We identified that some factors sponsor insulin resistance in non-diabetic chronic patients with HCV infection. Elevated levels of ALT, AST, ALP, cholesterol, and triglycerides were independently correlated with insulin resistance. The connection between viral loads and HOMA-IR was not found on its own.
By repairing the virus-altered glucose homeostasis processes and removing HCV’s direct involvement in the onset of T2DM, infection clearance by DAA treatment results in a markedly decreased probability of the new T2DM cases, as reported by Adinolfi LE in the study18. The relationship between HCV genotype 1 and insulin resistance has also been examined in earlier research, however, the findings were inconclusive19,20,21,22. By interacting with host genetic and environmental variables that cause cytokine imbalance and hepatic steatosis, HCV contributes to IR23, and we suggest that a combination of factors, such as high triglycerides, obesity, high levels of ALT and cholesterol, and low levels of albumin, can lead to IR.
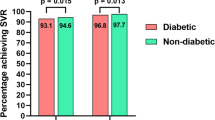
The course of HCV infection-related liver disease and extrahepatic symptoms are both adversely impacted by IR and T2DM24. In a significant real-world investigation, the non-diabetic patients who achieved SVR had a 21% lower risk of T2DM than those who did not reach SVR throughout an average of 3.7 years of follow-up25. We have observed the successful eradication of infection with direct-acting antivirals, and the patient’s insulin resistance was subsequently reduced.
Another study reported that glycaemic control improvement was pronounced in individuals with mild liver disease and was discordant with age, sex, or BMI26. However, additional research is required to assess how IR reversal affects the natural history of the hepatic and non-hepatic consequences of chronic HCV infection, especially in patients with advanced liver disease27. In recent years, direct-acting antivirals (DAAs) have considerably changed HCV treatment in various ways, including reducing treatment times, optimizing patient safety, increasing tolerance rates, and achieving remarkable SVR rates6,8,28. In cases of sustained virological response, a decline in the prevalence of T2DM and its implications as well as an improvement in glycemic control have been documented3,4,5,6.
The INF-free treatment causing infection clearance was identified in multivariate analysis as an independent factor affecting survival (p = 0.05), indicating a link between viral clearance and longer overall survival in HCC patients10. Other analyses highlight the significance of HCV viral clearance and the outstanding contribution of DAA to the decline in liver-related and non-liver-related mortality. Therefore, to more accurately analyze the long-term effects of DAA and create customized interventions based on the particular disease implicated, a clinical setting-specific mortality evaluation should be required over time. Such a strategy would significantly lower the global burden and mortality rate caused by HCV29,30.
This study focused on how HCV infection clearance directly influences IR in chronic HCV infected patients taking direct-acting antiviral therapy. By reducing the stress on β-cell function, HCV clearance through DAA therapy helps prevent insulin resistance-related pathological conditions. We demonstrated that HCV annihilation could partially reverse HCV-related IR, notably in patients with high baseline insulin resistance, HCV pathogenesis, and no prior treatment experience.
Limitations
Despite the strengths of this study, certain limitations must be acknowledged. Due to the limited sample size and lack of diversity in terms of ethnic representation, geographic location, socioeconomic status, lifestyle, genetic variability, or presence of any coexisting medical condition like diabetes, the patients who took part in the study may not be a true representative of the wide-ranging populace. This study had a short duration; long-term follow-up studies might bring more information regarding the outcomes of therapy on glucose homeostasis in HCV infected patients. Some confounding factors, such as the use of glucose-lowering medicines like biguanides and thiazolidinediones or lifestyle factors that could potentially influence glucose levels, may also vary the relationship between direct-acting antiviral therapy and the outcome of this research interest. This study did not account for other potential influencing factors such as HCV genotype and liver disease severity, which could have enhanced the analysis if included. Evaluation of visceral adiposity could also provide a more comprehensive understanding of the factors affecting metabolic outcomes of the disease.
Conclusions
HCV-infected patients develop insulin resistance due to an array of conditions, including increased triglyceride levels, elevated ALT and cholesterol levels, and decreased albumin. Direct-acting antivirals significantly exterminated the virus, and patients’ insulin resistance was found to have substantially decreased. Patients’ post-treatment CBC and lipid profiles were normal or near-normal, and their mean HOMA-IR dropped.
Data availability
All the data is provied within the manuscript.
References
National Hepatitis Elimination Profile for Pakistan. Key Takeaways. https://www.globalhep.org/news/national-hepatitis-elimination-profile-pakistan-key-takeaways#:~:text=Pakistan%20has%20the%20second%20highest,history%20of%20surgery%20(9%25.
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. The burden of liver diseases in the world. J. Hepatol. 70 (1), 151–171. https://doi.org/10.1016/j.jhep.2018.09.014 (2019).
Gitto, S. et al. Worsening of serum lipid profile after direct acting antiviral treatment. Ann. Hepatol. 17 (1), 64–75. https://doi.org/10.5604/01.3001.0010.7536 (2018).
Lacerda, G. S. et al. Exploring lipid and apolipoprotein levels in chronic hepatitis C patients according to their response to antiviral treatment. Clin. Biochem. 60, 17–23. https://doi.org/10.1016/j.clinbiochem.2018.07.007 (2018).
Hensel, N. et al. Memory-like HCV-specific CD8+ T cells retain a molecular Scar after cure of chronic HCV infection. Nat. Immunol. 22 (2), 229–239. https://doi.org/10.1038/s41590-020-00817-w (2021).
Sahibzada, K. I. et al. Hepatitis C virus transmission cluster among injection drug users in Pakistan. PloS One. 17 (7), e0270910. https://doi.org/10.1371/journal.pone.0270910 (2022).
Zafar, A. et al. Prevalence and treatment of untypable HCV variants in different districts of Punjab, Pakistan. Viral Immunol. 31 (6), 426–432. https://doi.org/10.1089/vim.2017.0167 (2018).
Haqqi, A. et al. Prevalence of hepatitis C virus genotypes in Pakistan: current scenario and review of literature. Viral Immunol. 32 (9), 402–413. https://doi.org/10.1089/vim.2019.0058 (2019).
Gitto, S. et al. Worsening of serum lipid profile after direct acting antiviral treatment. Ann. Hepatol. 17 (1), 64–75 (2018).
Gebert, L. F. R., Law, M. & MacRae, I. J. A structured RNA motif locks Argonaute2:miR-122 onto the 5’ end of the HCV genome. Nat. Commun. 12 (1), 6836. https://doi.org/10.1038/s41467-021-27177-9 (2021).
Otto, G. Methylation regulates HCV genome translation. Nat. Rev. Microbiol. 19 (5), 284. https://doi.org/10.1038/s41579-021-00548-1 (2021).
Cheng, C. H. et al. Virus elimination by direct-acting antiviral agents impacts glucose homeostasis in chronic hepatitis C patients. Front. Endocrinol. 12, 799382. https://doi.org/10.3389/fendo.2021.799382 (2022).
Andrade, V. G. et al. Insulin resistance reduction after sustained virological response with direct acting antiviral: not every population improves. Arq. Gastroenterol. 55 (3), 274–278. https://doi.org/10.1590/S0004-2803.201800000-69 (2018).
Desbois, A. C. & Cacoub, P. Diabetes mellitus, insulin resistance, and hepatitis C virus infection: A contemporary review. World J. Gastroenterol. 23 (9), 1697–1711. https://doi.org/10.3748/wjg.v23.i9.1697 (2017).
Shin, H. S., Jun, B. G. & Yi, S. W. Impact of diabetes, obesity, and dyslipidemia on the risk of hepatocellular carcinoma in patients with chronic liver diseases. Clin. Mol. Hepatol. 28 (4), 773–789. https://doi.org/10.3350/cmh.2021.0383 (2022).
Ciardullo, S. et al. Hepatitis C virus infection and diabetes: A complex bidirectional relationship. Diabetes Res. Clin. Pract. 187, 109870. https://doi.org/10.1016/j.diabres.2022.109870 (2022).
Younas, S., Sumrin, A., Hussain, N. & Bilal, M. Identification of NS5B resistance against SOFOSBUVIR in hepatitis C virus genotype 3a, Naive and treated patients. J. Appl. Microbiol. 133 (5), 2826–2834. https://doi.org/10.1111/jam.15754 (2022).
Bartenschlager, R. et al. Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: considerations for scientists and funding agencies. Virus Res. 248, 53–62. https://doi.org/10.1016/j.virusres.2018.02.016 (2018).
Adinolfi, L. E. et al. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes. Metab. 22 (12), 2408–2416. https://doi.org/10.1111/dom.14168 (2020).
Mehta, S. H. et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatol. (Baltimore Md). 38 (1), 50–56. https://doi.org/10.1053/jhep.2003.50291 (2003).
Tsochatzis, E. et al. Serum HCV RNA levels and HCV genotype do not affect insulin resistance in nondiabetic patients with chronic hepatitis C: a multicentre study. Aliment. Pharmacol. Ther. 30 (9), 947–954. https://doi.org/10.1111/j.1365-2036.2009.04094.x (2009).
Knobler, H., Schihmanter, R., Zifroni, A., Fenakel, G. & Schattner, A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infection. Mayo Clin. Proc. 75 (4), 355–359. https://doi.org/10.4065/75.4.355 (2000).
Moucari, R. et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 134 (2), 416–423. https://doi.org/10.1053/j.gastro.2007.11.010 (2008).
Adinolfi, L. E., Restivo, L., Zampino, R., Lonardo, A. & Loria, P. Metabolic alterations and chronic hepatitis C: treatment strategies. Expert Opin. Pharmacother. 12 (14), 2215–2234. https://doi.org/10.1517/14656566.2011.597742 (2011).
Lonardo, A., Adinolfi, L. E., Petta, S., Craxì, A. & Loria, P. Hepatitis C and diabetes: the inevitable coincidence? Expert Rev. anti-infective Therapy. 7 (3), 293–308. https://doi.org/10.1586/eri.09.3 (2009).
Li, J. et al. Impact of sustained virological response on incidence of type 2 diabetes in hepatitis C patients. (2017).
Boraie, M. B., Elnaggar, Y. A., Ahmed, M. O. & Mahmoud, A. M. Effect of direct acting antiviral therapy of chronic hepatitis C virus on insulin resistance and Type2 DM in Egyptian patients (prospective study). Diabetes Metabolic Syndrome. 13 (4), 2641–2646. https://doi.org/10.1016/j.dsx.2019.07.032 (2019).
Russo, F. P. et al. Hepatitis C virus eradication with direct-acting antiviral improves insulin resistance. J. Viral Hepatitis. 27 (2), 188–194. https://doi.org/10.1111/jvh.13215 (2020).
Kamp, W. M., Sellers, C. M., Stein, S., Lim, J. K. & Kim, H. S. Impact of direct acting antivirals on survival in patients with chronic hepatitis C and hepatocellular carcinoma. Sci. Rep. 9 (1), 17081. https://doi.org/10.1038/s41598-019-53051-2 (2019).
Roelens, M. et al. All-cause mortality and causes of death in the Swiss hepatitis C cohort study (SCCS). Open. Forum Infect. Dis. 7 (8), ofaa308. https://doi.org/10.1093/ofid/ofaa308 (2020).
Acknowledgements
The authors extend their appreciation to the Center of Excellence in Biotechnology Research (CEBR), King Saud University, Riyadh, Saudi Arabia for supporting this research paper.
Author information
Authors and Affiliations
Contributions
Conception, data collection, analysis and drafting: S-J, S-U; Resources and supervision: S-U, M-I-U, A-B-W; Funding, critical review and editing: M-M-A, G-N, K-A-A, I-K. All the authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
for the study was obtained from the Ethical Research Committee of the University of Lahore. All the research protocols were evaluated, approved, and carried out according to the Ethical Research Committee guideline. Informed consents were obtained from all the participants before sampling.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jabeen, S., Khan, R., Alrashed, M.M. et al. Modulation of glucose metabolism and insulin resistance following hepatitis C virus clearance via direct-acting antivirals. Sci Rep 15, 14663 (2025). https://doi.org/10.1038/s41598-025-97827-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97827-1