Abstract
The systemic immune-inflammation index (SII) is a newly identified marker of inflammation., and the relationship between chronic bronchitis (CB) and inflammation is closely associated. However, the influence of SII on CB remains unclear at present.This cross-sectional study was conducted using data from individuals with complete SII and CB records from the 2001–2018 National Health and Nutrition Examination Survey (NHANES). Binary weighted logistic regression was employed to investigate the relationship between SII and CB risk. Additionally, restricted cubic spline regression models and segmented regression models were used to examine nonlinear relationships and threshold effects. Receiver operating characteristic (ROC) curves were adopted to evaluate the predictive value of SII for CB. Stratified analysis was adopted to assess the association between SII and CB in different populations. After adjusting for all covariables, there was a significant positive relevance observed between log-transformed SII (log (SII)) with CB (OR = 1.52, 95% CI: 1.27–1.82, P < 0.001). A nonlinear dose–response relationship with the threshold of 8.14 was observed between log (SII) and CB risk. When log (SII) exceeded 8.14, each unit increase in log (SII) was associated with a 1.31-fold increase in the risk of CB (OR = 1.31, 95% CI: 1.22–1.40, P < 0.001). Furthermore, ROC curves revealed strong predictive capability of SII for CB (AUC = 0.729). Elevated SII levels are associated with an increased prevalence of CB. Furthermore, a non-linear association exists between SII and the increased risk of CB.
Similar content being viewed by others
Introduction
Chronic bronchitis (CB) is a frequent but variable phenomenon of chronic obstructive pulmonary disease (COPD) and leads to more frequent exacerbations, accelerate lung function descend, decrease health-related quality of life, and potentially higher mortality rates1,2,3,4 The classic definition of CB describes it as having a history of a productive cough with sputum for at least three months over two consecutive years5 CB develops due to an excessive production and secretion of mucus by goblet cells. This leads to a progressive obstruction of airflow as small airways become blocked, accompanied by structural changes in the epithelium and shifts in airway surface tension, thereby heightening the vulnerability to airway collapse6 In COPD, inflammation in the airways is primarily marked by the presence of neutrophils, macrophages, and CD8 + cells7 Therefore, the treatment of chronic bronchitis focuses not only on reducing excessive mucus production, improving mucus consistency, alleviating cough, and restoring ciliary function, but also on reducing inflammation8
The systemic immune-inflammation index (SII) is a newly and comprehensive inflammatory biomarker derived from lymphocyte (LYM), neutrophil(NE), and platelet(PLT) counts9 The SII is often employed as a predictor of mortality in cancer patients, as elevated SII values correlate with a higher risk of death10,11 Additionally, a growing body of research suggests that SII can be utilized to forecast the severity of various diseases and track the effectiveness of treatments, such as, non-alcoholic fatty liver disease (NAFLD), hyperlipidemia and rheumatoid arthritis12,13,14 Although these studies were cross-sectional studies, they provided robust evidence supporting the association between SII and the aforementioned diseases. Liu et al. identified a non-linear correlation between the SII and rheumatoid arthritis, demonstrating that an SII value exceeding 578.25 significantly elevates the risk of rheumatoid arthritis. Furthermore, an inverted U-shaped relationship was observed between SII and the controlled attenuation parameter (CAP) in transient elastography, a commonly employed diagnostic tool for NAFLD, with a critical threshold at 687.059 (1000 cells/µl). Additionally, an inverted U-shaped association was also established between SII and hyperlipidemia, with an inflection point at 479.15. This finding indicated that SII levels below 479.15 constitute an independent risk factor for hyperlipidemia. Inflammation is a key factor in the progression of CB, with the recruitment of circulating inflammatory cells and the increase of inflammatory mediators playing an important role in the progression of airway fibrosis15 However, the association of SII and CB is still unclear.
Therefore, we examined the association between SII and CB in this study, employing a large sample of people from the National Health and Nutrition Examination Survey (NHANES).
Method
NHANES is a nationwide, continuous surveillance program with a multistage design, supported by the National Center for Health Statistics (NCHS) under the Centers for Disease Control and Prevention. NHANES data were gathered via structured personal interviews conducted at participants’ residences, health assessments at mobile examination centers, and laboratory testing of samples.
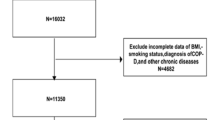
Our investigation into the association between the SII and CB employed NHANES data spanning from 2001 to 2018. We enrolled adult participants aged 18 and older with CB who underwent mobile examinations, which offered comprehensive data on blood and physical measurements. Furthermore, we excluded individuals without covariable data and a total of 40,135 individuals were ultimately enrolled for further research. (Fig. 1).
Data collection
Data on demographics, physical examinations, laboratory blood tests, and medical histories were collected from the participants. (1) Demographic data included sex, age, race, education, marital status, income-to-poverty ratio (PIR), smoking status, drinking status. Race was divided into four sections of White, Black, Mexican and other. Educational levels were classified as less than high school, high school diploma, and more than high school. Marital status is considered as married/living with partner, widowed/divorced/separated and never married. PIR are divided into < 1, 1–3, or > 3. Smoking status was classified as non-smoker and smoker. Drinking status was recorded as mild, moderate and heavy drinker. (2) Physical examination included BMI and physical activity was divided into no or less, moderate and heavy. (3) Clinical indicators such as White blood cell (WBC), Red blood cell (RBC), HGB Hemoglobin (HGB), PLT, NE, LYM, Eosinophil (EOS), Monocyte (MON) was collected. (4) Medical history included diabetes, hypertension and cardiovascular disease (CVD). The definition of diabetes, hypertension and CVD was self-reported diagnosis.
Assessment of SII and definitions of CB
Based on former researches, the calculation formula of SII is PLT count × NE count/LYM count9 Due to the skewed distribution of SII values, a logarithm (log) transformation was used to approximate a normal distribution. The transformed data were then divided into quartiles (Q1, Q2, Q3, and Q4).
The CB status was determined using variable MCQ160k from the 2001–2018 NHANES questionnaire data. Those who answered “yes” to the question "Ever told you had chronic bronchitis?" were classified as having CB.
Statistical analysis
Given that the NHANES database was conducted using complex, multi-step sampling, our study utilized MEC exam weights for analysis. Continuous variables in baseline characteristics were presented as median (interquartile range) and categorical variables as number (percentage). The groups were compared using analysis of Wilcoxon rank sum test the χ2 tests.
Three models were developed with increasing levels of adjustment for potential outcome confounders: crude was unadjusted, model 1 was adjusted for sex, age, race, education, marital status, PIR, BMI, physical activity, model 2 was adjusted for sex, age, race, education, marital status, PIR, BMI, physical activity, smoke, alcohol, hypertension, diabetes and CVD. Logistic regression models were used to assess odds ratios (OR) for the association between SII index and CB. Additionally, linear trends across SII quartiles were evaluated by treating the median value of each quartile as a continuous variable. To assess potential nonlinear dose–response relationships between the SII and the risk of CB by adjusting for multiple variables, restricted cubic splines (RCS) were employed. Four knots were positioned at the 5th, 35th, 65th, and 95th percentiles, and observations with the top and bottom 1% of SII index values were excluded. The likelihood ratio test was employed to assess nonlinearity. Stratified analysis was carried out for significant covariates, considering potential effect modifiers such as sex, age, race, education, marital status, PIR, BMI, physical activity, smoke, alcohol, hypertension, diabetes and CVD.
All analyses of our data were conducted using R software (version 4.3.2), and statistical significance was assessed with a two-tailed P value of 0.05.
Results
Clinical and biochemical characteristics in non-CB and CB groups
Data were generated from 40,135 patients with CB (19,338 males and 20,797 females), including 2326 participants with CB and 37,809 participants without CB. Demographic and clinical characteristics of the two groups were compared in Table 1. Compared with non-CB individuals, CB patients tended to be female, older, Mexican, married individuals, middle-PIR, smoker, mild-drinker. Meanwhile, individuals with CB exhibited higher SII, NLR (neutrophil-to-lymphocyte ratio), PLR (platelet-to-lymphocyte ratio) and LMR (lymphocyte-to-monocyte ratio), and a higher prevalence of diabetes.
Moreover, to characterize the baseline traits of CB patients across different SII levels, we categorized them into four quartiles(Q) according to the SII index (Supplementary Table 1). Patients with higher SII level are likely to be female, Mexican, middle-PIR, mild-drinker, and to be higher lower BMI. Interestingly, CB patients with higher SII level exhibited lower rates of hypertension, diabetes and CVD.
Association between SII and CB
In both univariate and multivariate logistic regression, SII had a greater risk association with CB compared to PLR, NLR and LMR (Table 2). Sex, age, race, marital status, PIR, smoke, CVD remained significantly associated with the odds of CB in weighted multivariable analysis (Supplementary Table 2).
Table 3 showed the detailed results of the weighted multivariate logistic regression analysis of SII in CB. When the SII was considered a continuous variable, regression analysis revealed a significant association between the log (SII) and CB in crude [1.35 (1.25 ~ 1.46)] and adjust models [For model 1:1.27 (1.17 ~ 1.37); For model 2: 1.23 (1.14 ~ 1.33)].
In the crude and adjust models, there were upward trends between the SII and CB (all P for trend < 0.001). For sensitivity analysis, we also treated the SII as a categorical variable (quartiles). A significant positive association between log (SII) and the prevalence of CB when compared to the lowest quartile was observed. [OR: crude model: 1.77 (1.5 ~ 2.09), model 1: (1.59 (1.34 ~ 1.9) and model 2: (1.52 (1.27 ~ 1.82)].
Furthermore, we investigated the predictive value of SII for CB using ROC curves and SII exhibited strong predictive capability (AUC = 0.729) (Fig. 2).
Using restricted cubic splines to analyze the association between SII and CB
In adjusted model 2, RCS analysis revealed a non-linear relationship between SII and the risk of CB. (p < 0.001, p-nonlinear = 0.004) (Fig. 3). Moreover, in the segmented regression and threshold analysis (Table 4), an inflection point value of 8.14 for log (SII) was revealed. When the log (SII) value surpassed 8.14, every additional unit increase in log (SII) corresponded to a 1.31-fold rise in the risk of CB. (OR = 1.31, 95% CI. 1.22–1.40, P < 0.001) (log-likelihood test < 0.001).
The subgroup analysis and interaction test
The subgroup analysis demonstrated that the relationship of SII with CB was consistent in most subgroups. (Fig. 4) SII was shown significant correlation with CB in subgroups stratified by sex, age, race, education, marital status, PIR, smoke, hypertension, diabetes and CVD. However, in patients with a BMI < 18.5, those who engage in extensive physical activity, and heavy drinkers, SII is not associated with CB. The interaction test showed that the interaction between SII and education is significant in CB patients.
Discussion
After normalizing the SII by log-transformation, our analysis demonstrated a significant positive correlation between SII and the risk of CB, even after adjusting covariates among US population. When compared to other inflammatory markers such as NLR, PLR and MLR, SII proved stronger association with CB. Additionally, a non-linear relationship between SII and CB was identified, with the lowest risk of CB occurring at a log (SII) value of 8.14. Above this threshold, a positive correlation was noted. Moreover, subgroup analysis verified that the association of SII with CB was no difference in most subgroups.
A large number of studies have connected inflammation and immune system to the risk and progression of CB and COPD16,17 The interactions of most risk factors such as cigarette smoke, airway macrophages with epithelial cells can stimulate the release of inflammatory factors, such as IL-8, TNF-α and LTB4, which triggers the recruitment, infiltration, and activation of airway NE. These activated NE subsequently generate proteases, oxidants, MPO, IL-8 and LTB4 to perpetuate inflammation response, excessive mucus production, and tissue damage in CB18 The resolution of inflammation is essential for keeping the balance between the protective effects of the immune response and the potential for tissue damage. If acute inflammation is not properly resolved, it can lead to chronic inflammation or even autoimmunity19 Platelets may contribute to the pathogenesis of CB and COPD through alveolar integrity destruction, pulmonary vascular remodeling, and hypoxia disorder20 Moreover, epithelial cell and macrophage release chemokines to activate T lymphocytes, resulting in cytotoxic reaction that contribute to apoptosis of alveolar cells21 Therefore, these inflammatory cells and their associated ratios could potentially function as biomarkers for CB in clinical practice.
As a novel systemic immune-inflammatory marker, the SII index has been used to predict the prognosis of various diseases. For instance, it is utilized for patients undergoing radical resection of hepatocellular carcinoma and other cancer patients, which offers strong predictive stability and could reliably represent the balance between the inflammatory and immune responses9,22,23 Compared to the earlier NLR, which only considers NE and LYM, the SII includes PLT in the equation, providing a better reflection of the disease state24 Platelets are closely involved in immune regulation, inflammatory response, thrombosis, and tissue repair and remodeling, and therefore are closely related to the body state. A cross-sectional study in US revealed that SII may be a better systemic inflammation warning marker for hypertension25 Xu et.al demonstrated that, compared to NLR and PLR, SII was superior to predict the risk of COPD among patients aged 40 and above in the United States26 Our study also proved a stronger association between SII and CB than NLR, PLR and MLR.
Moreover, we observed a non-linear relationship between log (SII) and CB. And when log (SII) is greater than 8.14, the risk of CB rises faster. Many studies have also confirmed that SII has a nonlinear relationship with various diseases. The relationship between SII and heart failure exhibited non-linearity, with an inflection point identified at 1104.7827 The relationship between log2-SII and all-cause mortality in patients with nonalcoholic fatty liver disease was nonlinear, with a threshold value of 8.828 When assessing the relationship between SII and thyroid function, the nonlinear relationship between FT3 and SII was similarly demonstrated29 Moreover, SII also has a nonlinear relationship with various pulmonary diseases30 Similar to our findings, these studies reveal a nonlinear relationship between SII and various diseases. This indicates that keeping SII within an optimal range might help reduce the incidence of diseases and improve their prognosis. However, in patients with a BMI < 18.5 in our study, SII was not associated with CB. In patients with BMI < 18.5, malnutrition or cachexia may alter immune-inflammatory responses. For instance, low BMI is associated with reduced platelet production31 and lymphopenia32, potentially diminishing SII’s sensitivity. Additionally, Chronic inflammation in underweight individuals may be driven by cytokines rather than being cell-mediated33.
The strong association between SII and CB is likely linked to inflammatory activation, immune dysregulation. In CB, macrophages are thought to play a key role in coordinating the inflammatory response34 NEs play an important role in acute inflammatory responses and are highly activated during acute exacerbations of COPD and CB, aiding in clearing pathogen clearance and releasing inflammatory factors35,36 Moreover, in patients with CB, the immune system is in a dysregulated state, with possible abnormalities in lymphocyte count and function, leading to elevated SII values17 Regulatory T cells (Tregs) are essential to maintain immunity homeostasis and immune tolerance. In a chronic inflammatory environment, Tregs may lose their ability to suppress the immune response37,38 The presence of dysfunctional Tregs may lead to an imbalance in inflammatory responses in patients with CB, resulting in abnormal SII values. Furthermore, CB may be accompanied by other complications, contributing partially to the abnormal SII values39.
There are several advantages in our study. Firstly, we demonstrated that abnormal SII index was associated with high risk of CB. Secondly, compared to other inflammatory markers such as NLR, PLR and MLR, SII revealed superior association with CB. Thirdly, we revealed the dose–effect relationship between CB and SII. However, there are also limitations in our study. Firstly, even after controlling for potential confounding factors, we cannot exclude the influence of certain unknown or unmeasured variables. Secondly, the diagnosis of CB relies on patient self-report, which may introduce bias into our study. Thirdly, due to the lack of objective measurement data for CB (e.g., spirometry) in the NHANES database, this study did not compare SII with other diagnostic methods. Fourthly, our study was a cross-sectional study, therefore, it cannot definitively confirm the temporality between SII and CB. Further validation of the predictive value of SII in CB is essential through future longitudinal and randomized controlled trials.
Conclusion
As a newly inflammatory biomarker, SII could be adopted to predict the risk of CB among adults in the United States. Nevertheless, further large-scale, extended-duration studies are required to validate these findings.
Data availability
The datasets generated and/or analysed during the current study are available in the NHANES repository [https://www.cdc.gov/nchs/nhanes/participant.htm].
References
Vestbo, J., Prescott, E. & Lange, P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity Copenhagen City Heart Study Group. Am. J. Respir. Crit. Care Med. 153(5), 1530–1535 (1996).
Burgel, P.-R. et al. Initiatives bronchopneumopathie chronique obstructive (BPCO) scientific committee cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest 135(4), 975–982 (2009).
Guerra, S. et al. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 64(10), 894–900 (2009).
Kim, V. et al. COPDGene Investigators the chronic bronchitic phenotype of COPD: an analysis of the COPDgene study. Chest 140(3), 626–633 (2011).
Ferris, B. G. Epidemiology standardization project (American thoracic society). Am. Rev. Respir. Dis. 118(6 Pt 2), 1–120 (1978).
Kim, V. & Criner, G. J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 187(3), 228–237 (2013).
Saetta, M. et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 160(2), 711–717 (1999).
Hartman, J. E., Garner, J. L., Shah, P. L. & Slebos, D.-J. New bronchoscopic treatment modalities for patients with chronic bronchitis. European Respir. Rev. An Off. J. Eur. Respir. Soc. 30(159), 200281 (2021).
Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res.:Off. J. Am. Assoc. Cancer Res. 20(23), 6212–6222 (2014).
Tian, B.-W. et al. Systemic immune-inflammation index predicts prognosis of cancer immunotherapy: systemic review and meta-analysis. Immunotherapy 14(18), 1481–1496 (2022).
He, L., Xie, X., Xue, J., Xie, H. & Zhang, Y. Association of the systemic immune-inflammation index with all-cause mortality in patients with arteriosclerotic cardiovascular disease. Front. Cardiovasc. Med.. 9, 952953 (2022).
Xie, R. et al. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front. Immunol. 13, 925690 (2022).
Mahemuti, N. et al. Association between Systemic Immunity-Inflammation Index and Hyperlipidemia: A Population-Based Study from the NHANES (2015–2020). Nutrients 15(5), 1177 (2023).
Liu, B., Wang, J., Li, Y.-Y., Li, K.-P. & Zhang, Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999–2018. Arthritis Res. Ther. 25(1), 34 (2023).
Sethi, S. et al. Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest 118(6), 1557–1565 (2000).
Barnes, P. J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 118(11), 3546–3556 (2008).
Cosio, M. G., Saetta, M. & Agusti, A. Immunologic aspects of chronic obstructive pulmonary disease. N. Engl. J. Med. 360(23), 2445–2454 (2009).
Quint, J. K. & Wedzicha, J. A. The neutrophil in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 119(5), 1065–1071 (2007).
Serhan, C. N. & Savill, J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6(12), 1191–1197 (2005).
Mallah, H., Ball, S., Sekhon, J., Parmar, K. & Nugent, K. Platelets in chronic obstructive pulmonary disease: An update on pathophysiology and implications for antiplatelet therapy. Respir. Med. 171, 106098 (2020).
Barnes, P. J. Inflammatory endotypes in COPD. Allergy 74(7), 1249–1256 (2019).
Meng, L., Yang, Y., Hu, X., Zhang, R. & Li, X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J. Transl. Med. 21(1), 79 (2023).
Ding, L. et al. Preoperative systemic immune-inflammation index as a significant prognostic factor after TURBT in patients with non-muscle-invasive bladder cancer: A retrospective study based on propensity score matching analysis. Cancer Med. 12(6), 7019–7028 (2023).
Ware, J., Corken, A. & Khetpal, R. Platelet function beyond hemostasis and thrombosis. Curr. Opin. Hematol. 20(5), 451–456 (2013).
Xu, J.-P. et al. Systemic inflammation markers and the prevalence of hypertension: A NHANES cross-sectional study. Hypertens. Res. Official J. Japanese Soc. Hypertension. 46(4), 1009–1019 (2023).
Xu, Y., Yan, Z., Li, K. & Liu, L. The association between systemic immune-inflammation index and chronic obstructive pulmonary disease in adults aged 40 years and above in the United States: a cross-sectional study based on the NHANES 2013–2020. Front. Med. 10, 1270368 (2023).
Zheng, H. et al. Associations between systemic immunity-inflammation index and heart failure: evidence from the NHANES 1999–2018. Int. J. Cardiol. 395, 131400 (2024).
Zhao, E., Cheng, Y., Yu, C., Li, H. & Fan, X. The systemic immune-inflammation index was non-linear associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann. Med. 55(1), 2197652 (2023).
Hu, X.-Y. et al. Association between the systemic immune-inflammation index and thyroid function in U.S Adults. Med Inflamm. 2023, 5831858 (2023).
Song, Y., Bai, X.-M. & Ma, J. The association of systemic immune-inflammation index with lung function, risk of COPD and COPD severity: A population-based study. PLoS ONE 19(6), e0303286 (2024).
Zabel, K. M. & Blankenship, J. C. High BMI: another barrier to rapid platelet inhibition after STEMI PCI. Cardiovasc. Drugs Ther. 38(1), 9–11 (2024).
Warnke, C. et al. Initial lymphocyte count and low BMI may affect fingolimod-induced lymphopenia. Neurology 83(23), 2153–2157 (2014).
McDade, T. W., Meyer, J. M., Koning, S. M. & Harris, K. M. Body mass and the epidemic of chronic inflammation in early mid-adulthood. Soc. Sci. Med. 2021(281), 114059 (1982).
Barnes, P. J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 138(1), 16–27 (2016).
Ge, L., Wang, N., Chen, Z., Xu, S. & Zhou, L. Expression of Siglec-9 in peripheral blood neutrophils was increased and associated with disease severity in patients with AECOPD. Cytokine 177, 156558 (2024).
Vaitkus, M. et al. Reactive oxygen species in peripheral blood and sputum neutrophils during bacterial and nonbacterial acute exacerbation of chronic obstructive pulmonary disease. Inflammation 36(6), 1485–1493 (2013).
Zhang, Y.-S. & Chen, Y.-Q. Dysfunctional regulatory T cell: May be an obstacle to immunotherapy in cardiovascular diseases. Biomed. Pharmacotherapy 173, 116359 (2024).
Ohkura, N. & Sakaguchi, S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res. 30(6), 465–474 (2020).
Negewo, N. A., Gibson, P. G. & McDonald, V. M. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology (Carlton, Vic). 20(8), 1160–1171 (2015).
Acknowledgements
This research was supported by the National Key Clinical Specialties Construction Program.
Author information
Authors and Affiliations
Contributions
LH wrote the main manuscript text, JZ designed and administration the text, SX and MF extracted data, LW and YS analysed and interpretated of the data,WG and ZL wrote and edited. All the authors participated in the discussions of the results and contributed to the manuscript.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, L., Gan, W., Xiao, S. et al. Association between systemic immune-inflammation index and chronic bronchitis: NHANES 2001–2018. Sci Rep 15, 14113 (2025). https://doi.org/10.1038/s41598-025-97895-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97895-3