Abstract
Oryza. sativa subsp. indica, a subspecies of Asian cultivated rice, plays a crucial role in global rice production, particularly in the utilization of hybrid vigor. However, the genetic basis of hybrid sterility among indica-indica intrasubspecific hybrids remains elusive, hindering the effective exploitation of heterosis in rice breeding programs. In this study, a near isogenic line (NIL) was developed using the indica variety Swarna as the donor parent and the indica variety IR64 as the recurrent parent. A novel locus, designated S68, responsible for intrasubspecific hybrid sterility was mapped to the region between RM14247 and RM3413 on the short arm of chromosome 3, spanning approximately 190 kb. S68 followed one-locus allelic interaction model. In IR64/NIL-S68 hybrids, the gametes from IR64 exhibit a transmission advantage, while those from Swarna are aborted in the heterozygote. This is the first hybrid sterile locus identified in indica-indica intrasubspecific hybrids, which enhances our understanding of the genetic mechanisms underlying intrasubspecific hybrid sterility and the genetic divergence within indica rice, thereby providing guidance for hybrid breeding programs within the indica subspecies.
Similar content being viewed by others
Introduction
Rice (Oryza sativa L.) is widely cultivated and serves as the staple food for more than half of the global population. With the growth of the global population and the reduction of arable land, it is crucial to enhance rice yield to ensure food security. However, rice yield have plateaued, showing no significant improvement1. The crucial factor lies in the preference of breeders for specific parental lines, leading to a decline in genetic diversity and serious homogenization among varieties2. Due to its widespread geographical distribution and the influence of artificial selection, O. sativa exhibits abundant genetic diversity and characteristics of population differentiation. Asian cultivated rice comprises five subspecies: temperate japonica, tropical japonica, indica, aus and basmati3,4,5,6,7,8,9. Further, based on genome sequencing, indica subspecies have been classified into either two or five groups, indicating significant genetic diversity and substantial divergence within rice subspecies 10,11. Indica subspecies play a pivotal role in achieving yield breakthrough in rice. One major breakthrough stemmed from the utilization of the semidwarf gene during the 1950s and 1960s, which enhanced lodging resistance and increased yield by approximately 20%12. Another significant breakthrough emerged from the utilization of heterosis since the 1970s, which led to the widespread adoption of hybrid varieties derived from indica-indica crosses, resulting in an approximate yield increase of about 20%13,14. Thus, thoroughly mining and utilizing favorable genes and strong heterosis between divergent germplasm within subspecies are crucial pathways to increasing yield. However, hybrid sterility limits the utilization of the abundant genetic diversity and hybrid vigor present within these divergent groups. Therefore, identifying intrasubspecific hybrid loci and discovering wide compatibility alleles or neutral alleles are essential strategies to overcome hybrid sterility.
Hybrid sterility is the major form of postzygotic reproductive isolation in rice15. Great progress has been made in understanding interspecific and intraspecific hybrid sterility. By now, over 60 hybrid sterility loci between interspecific and intraspecific crosses have been identified16. More than 30 hybrid sterile loci were responsible for hybrid sterility in the crosses between indica and japonica subspecies15. Twenty-two hybrid sterility loci have been identified in the crosses between indica and temperate japonica cultivars, such as Sa, Sc, Sd, S11, S33, S34, S35, f5/Sb/S24, INK, Pf5.2, Pf10, and S25/Se/qS12/pf12 for male gamete sterility and f1, f3, S5, S8, S9, S10, S30, S31, qSS-8a/f8, Sf9, and HSA1 for female gamete sterility17,18,19,20,21,22,23,24,25,26. Five loci were responsible for hybrid sterility in the hybrids between temperate japonica and aus, such as, DPL1/DPL227. S7 and S15 accounted for female sterility in hybrids between indica and aus28. While S9, S16, S17, S29, S31, S32, qSS-2, and qSS-8b are involved in gamete abortion in hybrids between tropical japonica and temperate japonica24,29,30,31. Furthermore, S7, S8, S9, and S35(t) regulated female sterility in the crosses between tropical japonica and indica21,25,26,32,33, and S7 and S9 controlled hybrid sterility between tropical japonica and aus25,28. A recent study indicated that S67 contributed to pollen sterility in the F1 hybrids between temperate japonica and basmati34. These findings demonstrate the prevalence of hybrid sterility between subspecies and suggest that hybrid sterility loci may have contributed to the evolutionary divergence of these subspecies in rice.
By now, the fifteen loci, including S5, Hsa1a/Hsa1b, Sa, S7, Sc, Se/pf12/RHS12 and DPL1/DPL2 for intraspecific hybrid sterility, S27/S28, ESA, DGS1/DGS2, S1, qHMS1, qHMS7, S22A and S22B for interspecific hybrid sterility, have been cloned and characterized17,18,19,32,33,35,36,37,38,39,40,41,42,43,44,45. Among these loci, S5, S7, HSA1, ESA, Sa, Sc, S1, qHMS7, pf12/RHS12/Se fitted to the one-locus spore gametophytic interaction model, whereas S27/S28, DGS1/DGS2 and DPL1/DPL2 followed by the duplicate gametophytic lethal model15,17,18,19,27,32,33,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49. So far, only four QTLs in indica-indica hybrids have been identified, Ef1 affecting embryo sac fertility, Pf3 regulating pollen fertility and Sf3 responsible for spikelet fertility, were discovered in the MH63/ZS97 combination50. And the S40(t) locus for pollen fertility was found in the hybrid between the indica variety MH63 and another indica upland rice variety LuYin4651. Thus, the genetic basis of hybrid sterility in indica-indica populations still remains unclear.
In this study, IR64, a representative indica variety developed by the International Rice Research Institute, served as the recurrent parent. Swarna, another prominent indica rice variety in India, is famous for its low glycemic index and semi dwarf characteristics, and was used as the donor parent52. A near iso-genic line was developed through successive crossing and backcrossing from F1 to BC6F1 generations. A novel locus S68 was located in the region between markers RM14247 and RM3413 on the short arm of chromosome 3. This result will help us elucidate the molecular mechanism of hybrid sterility in indica-indica hybrids, and provide new evidence for divergence within the indica subspecies.
Results
Characterization of the F1 hybrids derived from a cross between IR64 and NIL-S68
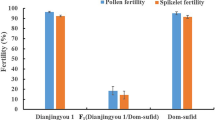
No significant differences were observed in plant height, tiller number, heading date, the number of panicles, and spikelet fertility among IR64, NIL and F1 hybrids (Fig. 1a–c, g). Furthermore, the average pollen fertility of IR64 and NIL-S68 was 98.67% and 98.02%, respectively. Whereas the F1 hybrids exhibited a significant proportion of smaller pollen grains, accounting for 53.43% of the total pollen grains (Fig. 1d–g). To investigate whether the smaller pollen grains have ability to germination, the pollen germination experiment was performed. The results revealed significant differences in pollen germination rates among two parental lines and their F1 hybrids. The recurrent parent IR64 exhibited the highest germination rate at 98.0%, followed by NIL-S68 at 75.2%. In contrast, the F1 hybrids had the lowest germination rate, at only 51.6%, and the smaller pollen grains were abortive and had no ability to germination (Fig. 1h). These results indicate that F1 hybrids between IR64 and NIL-S68 exhibite the pollen sterility due to the low germination rate.
The phenotype comparison among IR64, NIL-S68 and hybrid F1. (a–c) The plant architecture exhibits no significant differences among IR64 (a), F1 (b) and NIL-S68 (c). Scale bar, 10 cm. (d–f) Pollen fertility of IR64 (d), F1 (e) and NIL-S68 (f). The green arrows denote to normal pollen grains, the red arrows indicate the aborted pollen grains. Scale bar, 100 μm. (g) Quantitative analysis of pollen fertility and spikelet fertility. (h) Analysis of pollen germination rate of IR64, F1 and NIL-S68.
Subsequently, cytological analysis was performed to investigate pollen abortion in hybrids. Scanning electron microscopy experiments (SEM) revealed no significant difference in anthers among the two parents and their F1 hybrids (Fig. 2a–c), whereas the aborted pollen grains of the hybrid F1 were smaller, wrinkled and shrunken compared to the normal pollen grains of IR64 and NIL-S68 (Fig. 2d–i). Transmission electron microscopy experiment demonstrated that abundant starch granules were accumulated in the pollen grains of IR64 and NIL-S68, while few starch granules were observed in the aborted pollen of F1 hybrids (Fig. 2j–l). In addition, the aborted pollen wall in the F1 hybrids was thicker than those of the two parents, IR64 and NIL-S68 (Fig. 2m–o). These results suggested that starch granules accumulation and pollen wall development were defective in F1, leading to pollen abortion. Based on the above data, the F1 hybrids derived from the cross between IR64 and Swarna exhibited low germination rate due to starch accumulation and pollen cell development malformation.
Cytological observation of pollen grains in IR64, F1 and NIL-S68. (a–c) Anther morphology of IR64, F1 and NIL-S68, respectively. Scale bar, 200 μm. (d–f) The morphology of mature pollen grains from IR64, F1 and NIL as analyzed by SEM, respectively. Scale bar, 20 μm. Green arrows indicated the normal pollen grains and red arrows noted the aborted pollen grains. (g–i) The magnified mature pollen grain observed by SEM from IR64 (g), F1 (h) and NIL-S68 (i), respectively. Scale bar, 5 μm. (j–l) The transverse section of mature pollen grains from IR64 (j), F1 (k) and NIL-S68 (l), as observed by TEM. Scale bar, 5 μm. (m–o) Thinner pollen wall in IR64 (m) and NIL-S68 (o), and thicker pollen wall in F1 (n). Scale bar, 500 nm. Ex, exine; In, intine; Te, tectum; Ne, nexine; Ba, bacula.
Genetic mode of the S68 locus
In order to determine whether S68 acted as a single locus, pollen fertility was investigated in BC6F2 population derived from the cross between IR64 and Swarna. The result indicated that the population segregating for semi-sterile and fertile pollen exhibited a bimodal distribution and conformed to a 1:1 ratio in the BC6F2 generation (χ2(1:1) = 5.83, P = 0.015), suggesting that pollen semi-sterility in the F1 hybrids was controlled by a single locus, which was designated as S68 (Fig. 3a).
Genetic analysis and chromosome introgression. (a) Phenotypic segregation in BC6F2 population. (b) Detection of genetic background and target fragment introgression by Rice 1 K Liquid Chips. Green (A) represents homozygous IR64 genotype; Blue (B) depicts homozygous Swarna genotype; Red (H) notes heterozygous genotype; Black (N) represents undetected genotype. Orange box indicates the target introgression fragments. PS, Pollen semi-sterility; PF, Pollen fertility.
To detect the genetic background and target introgression fragments, pollen fertile plants and sterile plants were genotyped using Rice 1 K Liquid Chip. The results showed that approximately 92.5% of the genetic background in BC6F2 individuals was identical to that of the recurrent parent IR64 (Fig. 3b). Furthermore, heterozygous introgression fragments on the short arm of chromosome 3 were detected in all semi-sterile plants, whereas all pollen fertile individuals exhibited the homozygous IR64 genetic background at the same position. Correlation analysis showed that the SNP markers at this location was significantly negatively correlated with pollen fertility (r = − 0.813**). Linkage analysis indicated that S68 for intrasubspecific hybrid sterility was tightly linked with RM14241 on the short arm of chromosome 3. Homozygous alleles from both IR64 and NIL-S68 exhibited normal pollen fertility, whereas individuals possessing heterozygous alleles displayed significantly reduced pollen fertility, characterized by semi-sterility (Table 1). It suggested that S68 located near RM14241 marker. In addition, the number of plants harboring the homozygous IR64 alleles was significant higher that of the homozygous Swarna alleles, suggesting that gametes from IR64 gained the transmission advantage in the hybrids, and interaction between S68-IR64 and S68-Swarna resulted in the abortion of the male gametes carrying the S68-Swarna allele in the heterozygotes, thereby leading to segregation distortion in the progeny (Table 1). Genetic pattern of S68 was consistent with one-locus allelic interaction mode53.
Molecular mapping and gamete transmission of the S68 locus
To map S68, a total of 41 SSR markers within introgression interval on chromosome 3 were selected to screen polymorphisms between IR64 and NIL-S68. Then, 15 polymorphic markers were used to genotype individuals in the mapping population (Table S1, Fig. S1). Based on the genotype and phenotype data, S68 was restricted to a 0.4 cM region flanked by RM14247 and RM3413, with physical distance between the two markers approximate 190 kb (Fig. 4). No hybrid sterility locus has been reported within this region, thus, S68 is a novel locus for intrasubspecific hybrid sterility within the indica subspecies.
To investigate whether segregation distortion caused by male gametes abortion, reciprocal test was performed to further analyze gamete transmission. When F1 hybrids were used as the male parent to cross with the recurrent parent IR64, the number of plants carrying IR64 alleles was significant higher that of heterozygous plants (χ2(1:1) = 113.32, P = 2.46E−25). Conversely, when F1 hybrids served as the female parents, the ratio of the number of homozygous IR64 alleles to that of heterozygous alleles fitted a 1:1 (χ2(1:1) = 0.87, P = 0.65) (Fig. 5). It suggested that the male gametes from IR64 had a transmission advantage in the hybrids, whereas female gametes from F1 hybrids had an equal ability to transmit their alleles to the next generation.
II and IS represents homozygous IR64 genotype and heterozygous genotype.
Discussion
Hybrid sterility is the major obstacle to utilizing heterosis and transferring favorable genes in both interspecific and intraspecific crosses in rice. Heterosis primarily relies on the abundant diversity within the indica subspecies, however, the genetic basis of hybrid sterility between indica varieties remains unclear. In this study, we identified a novel hybrid sterility locus as S68, which affected pollen fertility in the cross between two indica varieties IR64 and Swarna. Cytological analysis indicated that starch granules accumulation and pollen wall development were defective in F1 hybrids (Fig. 2). It is inferred that the accumulation of pollen starch granules in the F1 hybrids could be insufficient to provide the necessary energy for its development, and the excessively thick pollen wall may hinder pollen germination and fertilization, ultimately leading to partial pollen abortion in F1 hybrids. Genetic analysis demonstrated that the ratio of fertile individuals to sterile individuals fitted into 1:1, and male gametes from Swarna was selectively aborted in the hybrids (Table 1), consistent with a one-locus sporophyte-gametophyte allelic interaction model53. Subsequently, S68 was mapped to a 190 kb region between RM14247 and RM3413 on the short arm of chromosome 3 (Fig. 4). To date, only four hybrid sterility QTLs (Ef1, Pf3, Sf3, S40(t)) have been identified in indica-indica crosses. Specifically, Ef1 is located on the short arm of chromosome 1 (3.518–4.579 Mb), and Sf3 is mapped to a region on the long arm of chromosome 3 (32.849–35.142 Mb), and both of loci affect female gamete fertility in the hybrids. Pf3 responsible for hybrid pollen sterility is restricted in a region spanning 0.396–3.477 Mb on the short arm of chromosome 350. The S40(t) locus for hybrid pollen sterility is confined to a region is restricted in a region spanning about 28.805 Mb on the long arm of chromosome 351. Thus, the S68 locus is a novel hybrid sterility locus controlling pollen fertility in indica-indica hybrids.
Asian cultivated rice classified into five subspecies was well-documented at molecular and genome levels, including temperate japonica, tropic japonica, indica, aus and basmati3,4,5,6,7,8,11. Hybrid sterility is the major form of reproductive isolation and serves as the indicator of species formation54. Many loci for hybrid sterility have been identified in crosses between subspecies in rice. These hybrid sterility loci provide evidence for subspecies differentiation and divergence. There has been ongoing controversy regarding the existence of differentiation within the indica subspecies. One previous report identified ind I and ind II within the indica subspecies according to whole genome sequencing data10. While another study indicates that there were five clusters within indica subspecies based on 3010 genomes, including XI-1A, XI-1B, XI-2, XI-3 and XI-adm11. These results suggested that the indica subspecies exhibits substantial differentiation due to geographic adaptation and successive divergent selection. Based on these classifications, Zhenshan97 and Minghui63 belong to ind I/XI-IA and ind II/XI-adm, respectively11. Ef1, Sf3 and Pf3 are responsible for the hybrid sterility in the cross between Zhenshan97 and Minghui6350, it provided the evidence that those three QTLs contributed to the divergence between ind I/XI-IA and ind II/XI-adm within indica. In this study, the recurrent parent IR64, bred by the International Rice Research Institute, belongs to XI-1B subgroup, while the donor parent Swarna, a popular cultivated indica rice variety with a low glycemic index (GI) and semi-dwarf super rice variety in India, is categories into the XI-adm subgroup11. Thus, the discovery of S68 will greatly aid our understanding of reproductive isolation between the XI-1B and XI-adm subgroups, and provide evidence of genetic differentiation and divergence within the indica subspecies. It is worth noting that Swarna variety is developed by two parent lines, Mahsuri and Vasista, while Mahsuri is a hybrid line derived from the cross between an indica and a japonica varieties, and one of the parents is famous japonica variety Taichung6555. Whether the introgression of Taichung65 contributes to hybrid sterility in the cross between Swarna and IR64 remains unclear. Systematic exploration of hybrid sterility loci will contribute to a comprehensive elucidation of differentiation and diversity within the indica subspecies.
Indica rice represents an exceptional genetic resource, rich in genetic diversity due to its long history of differentiation, selection and adaptation56. Favorable genes from indica, such as Gn1a, DEP1, LAX1, SCM2 for panicle traits, GW5, GS3, GW6 for grain traits, Pi9/Pi2 for blast resistance, SD1 for lodging resistance, and NRT1.1B and OsNR2 for nitrogen use efficiency contributes greatly to breeding programs in northern China57,58,59,60,61. The widespread application of three-line hybrid rice and two-line hybrid rice varieties has revolutionized rice production by harnessing heterosis within indica rice varieties62. Indica-indica hybrid mainly utilize a wide range of genetic resources, pyramiding the superior genes from both parents and eliminating the effects of detrimental genes to achieve heterosis63. Thus, the systematical exploitation of favorable genes within different indica groups is a key strategy for overcoming the yield bottleneck of modern rice varieties. In this study, SSR marker RM3413 can be used for identification of hybrid fertility between two indica varieties at the S68 locus. In marker-assisted screening of indica rice varieties, co-migrating electrophoretic bands between two tested lines predict compatible crosses without hybrid sterility, while polymorphic banding patterns signal potential hybrid sterility due to allelic divergence at the S68 loci. Additionally, introgression lines or near iso-genic line can be used to break hybrid sterility between the XI-1B and XI-adm subgroups at the S68 locus by marker assisted selection breeding.
Understanding the genetic basis of hybrid sterility among different indica groups is essential for capitalizing on the hybrid vigor of indica-indica crosses. Previous reports indicated that the bridge parents developed by introgression S1-g allele from O. glaberrima into O. sativa could significant improve fertility in the hybrids between bridge parents and O. glaberrima64. In addition, marker-assisted pyramiding of hybrid sterility alleles (Sb, Sc, Sd, and S5ⁿ) in japonica backgrounds has successfully generated indica-compatible lines. These engineered lines demonstrate restored hybrid fertility, producing F₁ hybrids with fertile pollen and spikelets when crossed with indica varieties65. Another study revealed that pyramiding the natural neutral alleles of S5-n and f5-n, japonica alleles of Sc-j and pf12-j in the indica background could recover the pollen and embryo-sac fertility in the hybrids between pyramiding lines and japonica varieties17. Therefore, bridge parents developed by pyramiding different combinations of S68, Ef1, Sf3, S40(t) and Pf3 in indica subgroups would potentially overcome the intraspecific hybrid sterility among different subgroups within indica. Further, it is necessary to clone S68 and shed light on the genetic mechanism of S68 to create artificial wide-compatible lines, thereby utilizing the heterosis within different indica groups.
Materials and methods
Plant materials
IR64, an indica variety bred by the International Rice Research Institute as the female and recurrent parent, was crossed with Swarna, another indica variety from Indian, as the donor parent. Then, F1 hybrid as the female parent was successively backcrossed with IR64. Progenies with pollen semi-sterility were selected to backcrossing until the BC6F1 generation. Pollen fertile and semi-sterile individuals from the BC6F2 population were randomly selected for genotyping using Rice 1 K Liquid Chips (Huazhi Co, China). The individual with a homozygous genome fragment on the chromosome 3 was selected as a near isogenic line (NIL-S68), which was crossed with IR64 to produce the mapping population. A total of 990 individuals were used to map target loci for intraspecific hybrid sterility derived from the cross between IR64 and Swarna.
All plant materials were grown in the paddy field at Breeding Station, Yunnan Academy of Agricultural Sciences, Jinhong, Yunnan Province, P.R. China.
Phenotype evaluation
Pollen fertility was measured using anthers from spikelet at one day before flowering, and stored in 70% ethanol. Five anthers were taken from the spikelet and stained with I2-KI solution, and the sterile types were divided into typical, spherical, stained and small abortion types. About 300 pollen grains were observed under a light microscope.
Scanning electron microscopy
Stamens and mature pollen were fixed in 2.5% glutaraldehyde (pH7.4) for 24 h, then washed three times with 0.1 M phosphate buffer (pH7.2) and fixed in 1% osmic acid at 4 °C for 2 h. Samples were then gradient dehydrated in a series of ethanol concentrations and critical point-dried. The stamens and pollen grains were sputter-coated with gold for 30 s, and Images were observed using a scanning electron microscope (Gemini SEM 300, ZEISS, Germany).
Transmission electron microscope
Mature pollen grains were fixed in 2.5% glutaraldehyde (pH7.4) for 24 h, washed three times with 0.1 M phosphate buffer (pH7.2), and fixed in 1% osmic acid at 4 °C for 2 h. Samples were gradient dehydrated in a graded series of ethanol and embedded in Epon-Araldite resin for infiltration and polymerization in a mold. Ultrathin sections were made and counterstained with 3% uranyl acetate and 2.7% lead citrate before being observed with a JEM1400 transmission electron microscope (JEOL, Japan).
DNA extraction, PCR protocol and SSR analysis
Genomic DNA from fresh leaf tissue was isolated using the CTAB method as described in previous report (2002)66. This DNA was subsequently employed for genotyping on Rice 1 k Liquid Chip (Huazhi Co, China). For simple sequence repeat (SSR) marker analysis, a rapid DNA extraction method was employed according to the protocol (1991)67. All SSR molecular markers used for mapping are derived from International Rice Genome Sequencing Project 68. PCR amplification was performed to detect the SSR markers, following the protocol established by McCouch et al69. The reaction mixture consisted of a 10 μl system: 0.3 μM of each Primer F and Primer R, 5 μl of 2 × TaqPCR StarMixase, and 25–50 ng of DNA template, adding nuclease-free water up to 10 μl. The PCR amplification protocol was as follows: initial denaturation at 95 °C for 5 min; followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, with a final extension at 72 °C for 5 min; the products were then held at 4 °C.
Electrophoretic analysis and linkage map construction
The PCR amplicons were resolved by electrophoresis on 8% polyacrylamide gels, and visualized post-electrophoresis using silver staining. Mapchart 2.2 software was used to construct a linkage map of polymorphic markers near the target locus.
Gamete transmission assay
F1-S68 as the female parent and male parent were crossed with IR64. The genotype of F1 populations was examined using SSR marker RM14241, and the transmission rate was analyzed according to the number of homozygous IR64 genotypes and the number of heterozygous genotypes.
Data availability
The datasets used and/or analysed during the current study available from the corresponding authors (taody12@aliyun.com); (zhangyu_rice@163.com) on reasonable request.
References
Van Nguyen, N. et al. Meeting the challenges of global rice production. Paddy Water Environ. 4, 1–9 (2006).
Zou, J. et al. Unlocking crop diversity: Enhancing variations through genome editing. Sci. Bull. 69, 281–284 (2024).
Garris, A. J. et al. Genetic structure and diversity in Oryza sativa L. Genetics 169, 1631–1638 (2005).
Huang, X. et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 490, 497–501 (2012).
Civan, P. et al. Three geographically separate domestications of Asian rice. Nat. Plants 1, 15164 (2015).
Glaszmann, J. C. Isozymes and classification of Asian rice varieties. Theor. Appl. Genet. 74, 21–30 (1987).
Kishor, D. S. et al. Evaluation of whole-genome sequence, genetic diversity, and agronomic traits of basmati rice (Oryza sativa L.). Front. Genet. 11, 86 (2020).
Kenneth, L. et al. Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. PNAS 106, 12273–12278 (2009).
Kovach, M. J. et al. New insights into the history of rice domestication. Trends Genet. 23, 578–587 (2007).
Xie, W. et al. Breeding signatures of rice improvement revealed by a genomic variation map from a large germplasm collection. Proc. Natl. Acad. Sci. 112, E5411–E5419 (2015).
Wang, W. et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557, 43–49 (2018).
Wu, B. et al. The history and prospect of rice genetic breeding in China. Yi Chuan 40, 841–857 (2018).
Yuan, L.-P. Development of hybrid rice to ensure food security. Rice Sci. 21, 1–2 (2014).
Qian, Q. et al. Breeding high-yield superior quality hybrid super rice by rational design. Natl. Sci. Rev. 3, 283–294 (2016).
Zhang, Y. et al. Understanding the nature of hybrid sterility and divergence of Asian cultivated rice. Front. Plant Sci. 13, 908342 (2022).
Ouyang, Y. et al. Hybrid sterility in plant: Stories from rice. Curr. Opin. Plant Biol. 13, 186–192 (2010).
Zhou, P. et al. A minimal genome design to maximally guarantee fertile inter-subspecific hybrid rice. Mol. Plant 16, 726–738 (2023).
Chen, J. et al. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica–japonica hybrids in rice. PNAS 105, 11436–11441 (2008).
Long, Y. et al. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. PNAS 105, 18871–18876 (2008).
Wang, J., Liu, K. D., Xu, C. G., Li, X. H. & Zhang, Q. The high level of wide-compatibility of variety ‘Dular’ has a complex genetic basis. Theor. Appl. Genet. 97, 407–412 (1998).
Takahiko Kubo, Y. et al. A novel epistatic interaction at two loci causing hybrid male sterility in an inter-subspecific cross of rice (Oryza sativa L.). Genes Genet. Syst. 83, 443–453 (2008).
Takahiko Kubo, A. et al. Genetic characterization and fine mapping of S25, a hybrid male sterility gene, on rice chromosome 12. Genes Genet. Syst. 92, 205–212 (2017).
Zhu, S. et al. The origin of weedy rice Ludao in China deduced by genome wide analysis of its hybrid sterility genes. Breed. Sci. 55, 409–414 (2005).
Wan, J. & Ikehashi, H. Identification of a new locus S-16 causing hybrid sterility in native rice varieties (Oryza sativa L.) from Tai-hu Lake Region and Yunnan Province, China. Breed. Sci. 45, 461–470 (1995).
Wan, J., Yamaguchi, Y., Kato, H. & Ikehashi, H. Two new loci for hybrid sterility in cultivated rice (Oryza sativa L.). Theor. Appl. Genet. 92, 183–190 (1996).
Wan, J., Yanagihara, S., Kato, H. & Ikehashi, H. Multiple alleles at a new locus causing hybrid sterility between a Korean indica variety and a javanica variety in rice (Oryza sativa L.). Jpn. J. Breed. 43, 507–516 (1993).
Mizuta, Y. et al. Rice pollen hybrid incompatibility caused by reciprocal gene loss of duplicated genes. Proc. Natl. Acad. Sci. 107, 20417–20422 (2010).
Yanagihara, S., Kato, H. & Ikehashi, H. A new locus for multiple alleles causing hybrid sterility between an aus variety and javanica varieties in rice (Oryza sativa L.). Jpn. J. Breed. 42, 792–801 (1992).
Zhu, S. et al. A new gene located on chromosome 2 causing hybrid sterility in a remote cross of rice. Plant Breed. 124, 440–445 (2005).
Li, D. et al. Fine mapping of S32(t), a new gene causing hybrid embryo sac sterility in a Chinese landrace rice (Oryza sativa L.). Theor. Appl. Genet. 114, 515–524 (2006).
Wang, C. et al. Mapping segregation distortion loci and quantitative trait loci for spikelet sterility in rice (Oryza sativa L.). Genet. Res. 86, 97–106 (2005).
Chen, M. et al. A new gene controlling hybrid sterility in rice (Oryza sativa L.). Euphytica 184, 15–22 (2011).
Yu, Y. et al. Hybrid sterility in rice (Oryza sativa L.) involves the tetratricopeptide repeat domain containing protein. Genetics 203, 1439–1451 (2016).
Lv, Y. et al. Identification of a novel hybrid sterility locus S67 between temperate japonica subgroup and basmati subgroup in Oryza sativa L. Sci. Rep. 14, 28619 (2024).
Kubo, T. et al. Two tightly linked genes at the hsa1 locus cause both F1 and F2 hybrid sterility in rice. Mol. Plant 9, 221–232 (2016).
Shen, R. et al. Genomic structural variation-mediated allelic suppression causes hybrid male sterility in rice. Nat. Commun. 8, 1310 (2017).
Wang, D. et al. Two complementary genes in a presence-absence variation contribute to indica-japonica reproductive isolation in rice. Nat. Commun. 14, 4531 (2023).
Yu, X. et al. A selfish genetic element confers non-Mendelian inheritance in rice. Science 360, 1130–1132 (2018).
Sakata, M. et al. Domain unknown function DUF1668-containing genes in multiple lineages are responsible for F1 pollen sterility in rice. Front. Plant Sci. 11, 632420 (2021).
Yamagata, Y. et al. Mitochondrial gene in the nuclear genome induces reproductive barrier in rice. Proc. Natl. Acad. Sci. 107, 1494–1499 (2010).
Hou, J. et al. ESA1 is involved in embryo sac abortion in interspecific hybrid progeny of rice. Plant Physiol. 180, 356–366 (2019).
Nguyen, G. N. et al. Duplication and loss of function of genes encoding RNA polymerase III subunit C4 causes hybrid incompatibility in rice. G3 Genes Genomes Genet. 7, 2565–2575 (2017).
Xie, Y. et al. An asymmetric allelic interaction drives allele transmission bias in interspecific rice hybrids. Nat. Commun. 10, 2501 (2019).
Wang, C. et al. A natural gene drive system confers reproductive isolation in rice. Cell 186, 3577–3592 (2023).
You, S. et al. A toxin-antidote system contributes to interspecific reproductive isolation in rice. Nat. Commun. 14, 7528 (2023).
Yang, J. et al. A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science 337, 1336–1340 (2012).
Li, J. et al. New insights into the nature of interspecific hybrid sterility in rice. Front. Plant Sci. 11, 555572 (2020).
Xie, Y. et al. Interspecific hybrid sterility in rice is mediated by OgTPR1 at the S1 locus encoding a peptidase-like protein. Mol. Plant 10, 1137–1140 (2017).
Koide, Y. et al. Lineage-specific gene acquisition or loss is involved in interspecific hybrid sterility in rice. Proc. Natl. Acad. Sci. 115, E1955–E1962 (2018).
Li, G. et al. Three representative inter and intra-subspecific crosses reveal the genetic architecture of reproductive isolation in rice. Plant J. 92, 349–362 (2017).
Xu, Y. Genetic analysis on hybrid sterility of Oryza sativa intraspecies, and between O. sativa and O. rufipogon (Master’s thesis of Yunnan Agricultural University, 2007).
Merugumala, G. R. et al. Molecular breeding of “Swarna”, a mega rice variety for lodging resistance. Mol. Breed. 39, 1–14 (2019).
Kitamura, E. Genetic studies on sterility observed n hybrids between distantly related varieties of rice, Oryza sativa L. Rep Chugoku Exp Sta 8, 141–205 (1962).
Zhao, Z. et al. Hybrid sterility genes with driving force for speciation in rice. Sci. Bull. 68, 1845–1848 (2023).
Patra, B. C. et al. Rice breeding in India: A journey from phenotype based pure-line selection to genomics assisted breeding. Agric. Res. J. 57, 816–825 (2020).
Lv, Q. et al. Resequencing of 1143 indica rice accessions reveals important genetic variations and different heterosis patterns. Nat. Commun. 11, 4778 (2020).
Zhao, D. S. et al. GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat. Commun. 9, 1240 (2018).
Liu, G. et al. Two broad-spectrum blast resistance genes, Pi9( t) and Pi2( t), are physically linked on rice chromosome 6. Mol. Genet. Genomics. 267, 472–480 (2002).
Gao, Z. et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 10, 5207 (2019).
Cui, D. et al. Genomic insights on the contribution of introgressions from Xian/Indica to the genetic improvement of Geng/Japonica rice cultivars. Plant Commun. 3, 100325 (2022).
Chen, Z. et al. Genomic decoding of breeding history to guide breeding-by-design in rice. Natl. Sci. Rev. 10(5), nwad029 (2023).
Gu, Z. et al. Structure and function of rice hybrid genomes reveal genetic basis and optimal performance of heterosis. Nat. Genet. 55, 1745–1756 (2023).
Zhang, G.-Q. Prospects of utilization of inter-subspecific heterosis between indica and japonica rice. J. Integr. Agric. 19, 1–10 (2020).
Deng, X. et al. The role of Sl-g alíele from Oryza glaberrima in improving interspecific hybrid sterility between O. sativa and O. glaberrim. Breed. Sci. 60, 342–346 (2010).
Guo, J. et al. Overcoming inter-subspecific hybrid sterility in rice by developing indica-compatible japonica lines. Sci. Rep. 6, 26878 (2016).
Susan, R. et al. Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res. 9, 199–207 (2002).
Edwards, K., Johnstone, C. & Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis (Oxford University Press, 1991).
International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature 436, 793–800 (2005).
McCouch, S. R. Gene nomenclature system for rice. Rice 1, 72–84 (2008).
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (Grant Nos. 31991221, 32460503), the Yunnan Provincial Science and Technology Department, China (202301BD070001-051, 202401AT070090), the Yunnan Provincial Government (YNWR-QNBJ-2018-359, 530000210000000013809).
Author information
Authors and Affiliations
Contributions
Y.Z and D.T conceived and designed the experiments. X.S, Y.Z developed the near isogenic lines, performed phenotype and genotype analysis, drafted the manuscript. Y.Y participated in near isogenic lines construction, phenotyping and genotyping. Q.P, J.Z, Y.L, X.J, J.L and X.D participated in raising the near iso-genic lines. Y.Z and D.T revised manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, X., Yang, Y., Pu, Q. et al. Molecular mapping of a novel locus S68 for intrasubspecific hybrid sterility in indica-indica hybrid. Sci Rep 15, 13536 (2025). https://doi.org/10.1038/s41598-025-98008-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98008-w