Abstract
Restless legs syndrome (RLS) and acute ischemic stroke are prevalent conditions in neurology. Several studies have indicated a relationship between RLS, acute ischemic stroke, and vitamin D levels. This study aimed to evaluate vitamin D levels in patients with RLS after acute ischemic stroke, compared to those with only acute ischemic stroke, to determine if there is a significant association. A case-control study included 270 patients with acute ischemic stroke and 53 patients who developed RLS following acute ischemic stroke. The International Restless Legs Syndrome Study Group (IRLSSG) scale was used to assess RLS severity, and vitamin D levels were measured (levels below 20 ng/ml were considered insufficient). Patients with RLS after acute ischemic stroke had significantly lower vitamin D levels (P < 0.001) and a higher incidence of vitamin D insufficiency (P < 0.001) compared to those with only acute ischemic stroke. The multivariate logistic regression model adjusted for the age, female, smoking status, alcohol consumption, history of hypertension, history of diabetes, low density lipoprotein-cholesterol (LDL-C), Homocysteine (HCY), vitamin D, vitamin D (OR = 0.673, 95% CI: 0.610–0.743, P < 0.001) and sex remained (OR = 4.217, 95% CI: 1.390-12.791, P = 0.011) significantly associated with RLS following acute ischemic stroke. Among RLS patients, those with (extremely) severe RLS had lower vitamin D levels than those with mild to moderate RLS (P < 0.001). Vitamin D levels were negatively correlated with IRLSSG scores after adjusting for confounding factors. The area under the curve (AUC) was 0.915 (95% CI 0.873–0.958), and the cut-off value for vitamin D was 18.15 ng/ml, with a sensitivity of 84.4% and specificity of 90.6%. Adults with acute post-ischemic stroke RLS generally have lower vitamin D levels than those with ischemic stroke alone, and lower vitamin D levels are linked to more severe RLS symptoms. Vitamin D levels and sex are important influencing factors for the occurrence of RLS following acute ischemic stroke. Vitamin D level is an independent predictor of RLS after acute ischemic stroke and has high predictive value.
Similar content being viewed by others
Introduction
Stroke encompasses various causes of cerebrovascular damage, leading to focal or widespread injury to brain tissue, with clinical symptoms lasting over 24 h or resulting in death. It is marked by high rates of incidence, disability, recurrence, and mortality1. Strokes are classified as either hemorrhagic or ischemic, with ischemic strokes being the most common. Globally, ischemic stroke is a leading cause of death and long-term disability2. Its causes include conditions such as atherosclerosis affecting cerebral vessels, small vessel occlusion, and cardiac embolism3.
RLS is a frequent neurological condition characterized by uncomfortable sensations and paresthesia in the lower limbs, particularly during rest or nighttime sleep. Patients often experience a strong urge to move their legs and may repeatedly move or hit them to alleviate discomfort, which tends to worsen at night and improve with movement4. While the exact cause of RLS is not fully understood, genetic factors, dysfunction of dopaminergic neurons, and brain iron deficiency are known contributors5.
Vitamin D deficiency has emerged as a significant systemic risk factor for stroke6,7. Numerous studies have shown that low vitamin D levels are associated with a greater risk of vascular diseases, including stroke8,9,10,11,12. Moreover, research has indicated a strong connection between reduced vitamin D levels and RLS13,14. The relationship between vitamin D and RLS after acute ischemic stroke has been less extensively studied. Therefore, further investigation into the role of vitamin D as an influencing in RLS patients after acute ischemic stroke is crucial. This study aimed to evaluate vitamin D levels in patients with RLS following acute ischemic stroke, as well as in those with only acute ischemic stroke, to determine if a significant association exists. We measured vitamin D levels in patients with acute post-ischemic stroke RLS, comparing them to patients with acute ischemic stroke treated concurrently in the hospital. Additionally, we assessed RLS severity using the IRLSSG scale and plotted a receiver operating characteristic (ROC) curve to evaluate the predictive value of vitamin D for RLS after acute ischemic stroke.
Materials and methods
Study population
This case-control study adhered to STROBE guidelines and was in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). This study was approved by the Institutional Review Committee of Tangshan Workers’ Hospital (protocol code. 2023LSYLD176). The patient’s written informed consent was obtained. Patients with cerebral infarction who were treated and hospitalized in Tangshan Workers’ Hospital from July 2023 to July 2024 were collected. Inclusion criteria were as follows: (1) confirmed by computed tomography (CT) and diffusion-weighted imaging (DWI); (2) Glasgow coma score > 12; (3) onset of symptoms within 3 days; (4) age 18 years or older. Exclusion criteria included: (1) diagnosis of post-infarction cerebral hemorrhage, traumatic brain injury; (2) patients taking drugs that affected serum vitamin D levels; (3) patients with a medical history, as judged by researchers, unsuitable for enrollment due to conditions such as malignant tumors or autoimmune diseases; (4) patients on immunosuppressants treatments before admission; (5) Parkinson’s disease (PD), peripheral neuropathy, pregnancy, myelopathy and radiculopathy; (6) severe renal, liver, or cardiac dysfunction15.
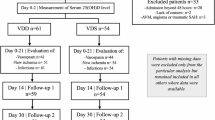
Diagnosis of post-stroke RLS was made according to the IRLSSG diagnostic criteria in 20144. A total of 323 patients with cerebral infarction were included, comprising 53 in the RLS after acute ischemic stroke group and 270 in the acute ischemic stroke group. This study was approved by the hospital’s ethics committee, and all subjects provided informed consent. The flowchart of this study is shown in Fig. 1.
Baseline clinical data collection
The clinical data of patients were collected, including: age, sex, body mass index (BMI), history of alcohol consumption, history of smoking, history of hypertension, and history of diabetes. Additionally, The laboratory tests included serum glucose, serum cholesterol, triglyceride, LDL-C, high density lipoprotein-cholesterol (HDL-C), Glycated hemoglobin (HbA1c), HCY and vitamin D.
Laboratory assessment
To measure 25-hydroxyvitamin D (25- (OH) D) levels, we used the Enzyme-Linked Immunosorbent Assay (ELISA). Additionally, in our hospital, insufficient vitamin D level has been defined as < 20ng/ml respectively.
Diagnosis of RLS
Each patient was then verified for a diagnosis of RLS, according to current IRLSSG criteria: (1) An urge to move the legs usually but not always accompanied by or felt to be caused by uncomfortable and unpleasant sensations in the legs; (2) The urge to move the legs and any accompanying unpleasant sensations begin or worsen during periods of rest or inactivity such as lying down or sitting; (3)The urge to move the legs and any accompanying unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues; (4) The urge to move the legs and any accompanying unpleasant sensations during rest or inactivity only occur or are worse in the evening or night than during the night; (5) The occurrences of the above features are not solely accounted for as symptoms primary to another medical or behavioral condition (e.g. myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping)4.
Two trained medical specialists, Xiuling Wu and Tianyang An, conducted face-to-face interviews with potential participants to confirm their eligibility and establish a diagnosis of RLS based on the updated IRLSSG diagnostic criteria.
IRLSSG rating scale
All patients with RLS completed the 10-item IRLSSG rating scale, which was validated and shown to be reliable for the assessment of RLS severity. Each item was graded on a scale of 0 to 4. Disease severity was classified using the following scale: mild, 0–10 points; moderate, 11–20 points; severe, 21–30 points; and very severe, 31–40 points16.
Statistical analysis
The SPSS Statics 22.0 software for analysis. Representation of non-normally distributed data in interquartile range (M, P25, P75) and normally distributed date in mean ± standard deviation (SD). The student’s-t test for normally distributed continuous data, while The Mann-Whitney U test for the non-normally distributed date. The Chi-square test was utilized to analyze the categorical variables. Spearman or Pearson correlation analysis was used for analysis between vitamin D level and IRLSSG scale score. To determine the predictive value of vitamin D levels for RLS following acute ischemic stroke, ROC curve analysis was used for evaluation. P < 0.05 was deemed statistically significant.
Results
Baseline information
The summary of participant demographic and clinical characteristics for the overall sample (N = 323) was presented in Table 1. The subjects were divided into two groups: the RLS after acute ischemic stroke group (N = 53) and the pure acute ischemic stroke group (N = 270). Among the RLS after acute ischemic stroke group, 23 women (43.40%) were included. The sample of persons with RLS following acute ischemic stroke reported a mean age of (61.89 ± 12.26) years. A total of 270 cases were enrolled in the pure acute ischemic stroke group, including 76 women (28.15%), with a mean age of (61.94 ± 10.42) years. Significant difference was found in Alcohol consumption (P = 0.03) and sex between the two groups (P = 0.028). Patients with RLS following acute ischemic stroke had significantly lower vitamin D levels (P < 0.001) and a higher incidence of vitamin D insufficiency (P < 0.001) compared to those with only acute ischemic stroke.
Comparison of clinical data of patients with mild to moderate RLS and those with (extremely) severe RLS
Among 53 patients with RLS following acute ischemic stroke, 34 patients (64.15%) had mild to moderate RLS, including 12 women, with a mean age of (59.77 ± 11.51) years were presented in Table 2. There were 19 (35.85%) (Extremely) severe patients with RLS, including 11 women, with a mean age of (65.68 ± 12.95) years. A statistically significant difference in smoking status was found between the two groups (P = 0.023). No significant difference was found in sex and age between the two groups (P > 0.05). The vitamin D levels of (Extremely) severe were significantly lower in severe patients with RLS than in mild to moderate patients with RLS (P < 0.001).
Multivariate logistic regression analysis of possible factors for RLS following acute ischemic stroke
The multivariate logistic regression model adjusted for the age, female, smoking status, alcohol consumption, history of hypertension, history of diabetes, LDL-C, vitamin D and HCY, vitamin D (OR = 0.673, 95% CI: 0.610-0.743, P < 0.001), smoking status(OR = 3.942, 95% CI: 1.358-11.441, P = 0.012) and sex remained (OR = 4.217, 95% CI: 1.390-12.791, P = 0.011) significantly associated acute post-ischemic stroke RLS. The multivariate logistic regression analysis was shown in Table 3.
Pearson correlation analysis of IRLSSG score with vitamin D level in patients with RLS
Pearson correlation analysis showed that vitamin D was negatively correlated with IRLSSG score (P = 0.031). The partial correlation analysis was used to show that vitamin D was still negatively associated with IRLSSG score (P = 0.015), after adjustment for the factors of age, sex, history of hypertension, history of diabetes, smoking, drinking, HDL-C, LDL-C, HCY, Cholesterol, Triglyceride, Glucose, HbA1c. The correlation analysis was shown in Table 4.
ROC analysis
Patients were grouped according to the diagnostic criteria established by the IRLSSG. Those diagnosed with RLS following acute ischemic stroke were assigned to the case group, while those with acute ischemic stroke alone were assigned to the control group. ROC analysis was performed to determine the predictive value of serum vitamin D for patients with RLS following acute ischemic stroke, which was shown in Fig. 2; Table 5. The area under the curve (AUC) was 0.915 (95% CI 0.873–0.958). In addition, the cut-off value of vitamin D was 18.15 ng/ml with a sensitivity of 84.4% and specificity of 90.6%.
Discussion
To our knowledge, this is the first case-control study examining the association between Vitamin D levels and RLS following acute ischemic stroke. First, the mean vitamin D levels in patients with acute post-ischemic stroke RLS were significantly lower than those in the control group. Additionally, vitamin D levels were inversely correlated with IRLSSG scores. Based on this, we hypothesize that vitamin D deficiency may play a key role in the patients with RLS following acute ischemic stroke.
A meta-analysis of 9,590 participants revealed that individuals with RLS had significantly lower vitamin D levels13. Previous research has also shown that vitamin D deficiency is more common in ischemic stroke patients compared to the general population17. These studies suggest a potential link between vitamin D levels and the presence of RLS following acute ischemic stroke.
Research has shown significant sex differences in the prevalence of vitamin D deficiency among RLS patients. Studies indicate that women with RLS tend to have lower vitamin D levels18,19, whereas no significant difference in vitamin D levels were found between men with RLS and those without it18. This suggests that the link between vitamin D deficiency and RLS may be sex-specific, with women being more vulnerable to this deficiency, which could contribute to the onset of the syndrome. Our study found that, compared to men, women have an increased risk of developing RLS following acute ischemic stroke.
Vitamin D levels have also been linked to disease severity. One study found that RLS patients had significantly lower serum vitamin D levels compared to controls, with an inverse relationship between vitamin D levels and RLS severity20. In addition, Wali et al. studied the impact of vitamin D on disease severity in 12 RLS patients, observing that as vitamin D levels increased, the severity of RLS decreased21. Similarly, our study also demonstrated an association between serum vitamin D levels and RLS following acute ischemic stroke.
The underlying mechanisms of RLS occurrence following acute ischemic stroke remain unclear. Vitamin D has anti-inflammatory and immune-regulating properties. Cytokines, which are small regulatory proteins involved in inflammatory processes, play a role in regulating neuron activity in both the peripheral and central nervous systems22. A previous review noted that IL-6 acts as a pro-inflammatory agent following ischemic stroke23. Increased serum IL-6 levels have been observed in RLS patients24, suggesting that inflammatory processes may contribute to the development of RLS25,26,27. As an immunoregulatory molecule, vitamin D may inhibit cellular inflammatory factors such as IL-6 and TNF-α28,29. Thus, we speculate that vitamin D levels may be related to the development of RLS following acute ischemic stroke.
However, this study has several limitations. The data were extracted from patients’ medical records during hospitalization, resulting in the absence of long-term follow-up data. The occurrence of RLS after discharge could affect data accuracy. Furthermore, the study’s small sample size limits the generalizability and strength of the findings. Further research should involve multicenter studies to address these limitations.
Conclusions
Vitamin D levels are typically lower in adults with RLS following acute ischemic stroke compared to those with ischemic stroke alone, and lower vitamin D levels are associated with more severe RLS symptoms. Vitamin D levels and sex are important influencing factors for the occurrence of RLS following acute ischemic stroke. Vitamin D level is an independent predictor of RLS after acute ischemic stroke and has high predictive value. Further research is needed to explore the relationship between serum vitamin D levels and factors such as sleep quality, depression, and anxiety.
Data availability
All data generated or analyzed during this study could be acquired from the corresponding author through e-mail.
References
Feigin, V. L. et al. Global and regional burden of stroke during 1990–2010: find global burden of disease study 2010. Lancet 383 (9913), 245–254 (2014).
Pu, L. et al. Projected global trends in ischemic stroke incidence, deaths and Disability-Adjusted life years from 2020 to 2030. Stroke 54 (5), 1330–1339 (2024).
Miceli, G. et al. Artificial intelligence in acute ischemic stroke subtypes according to toast classification: a comprehensive narrative review. Biomedicines 11 (4), 1138 (2023).
Allen, R. P. et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated international restless legs syndrome study group (IRLSSG) consensus criteria-history, rationale, description, and significance. Sleep. Med. 15, 860–873 (2014).
An, T. et al. Associations of anxiety and depression with restless leg syndrome: a systematic review and meta-analysis. Front. Neurol. 15, 1366839 (2024).
Kim, H. A. et al. Vitamin D deficiency and the risk of cerebrovascular disease. Antioxidants 9, 327 (2020).
Yarlagadda, K. et al. Vitamin D and stroke: effects on incidence, severity, and outcome and the potential benefits of supplementation. Front. Neurol. 11, 384 (2020).
Zhou, R. et al. Lower vitamin D status is associated with an increased risk of ischemic stroke: A systematic review and Meta-Analysis. Nutrients 10 (3), 277 (2018).
Leung, R. Y. et al. Serum 25-hydroxyvitamin D and the risk of stroke in Hong Kong Chinese. Thromb. Haemost. 117 (1), 158–163 (2017).
Xu, J. et al. Neurosteroids: A novel promise for the treatment of stroke and post-stroke complications. J. Neurochem. 160 (1), 113–127 (2022).
Wu, C. et al. Association between serum levels of vitamin D and the risk of Post-Stroke anxiety. Med. (Baltim). 95(18), e3566 (2016).
Han, B. et al. Low serum levels of vitamin D are associated with post-stroke depression. Eur. J. Neurol. 22 (9), 1269–1274 (2015).
Mansourian, M. et al. Are serum vitamin D, calcium and phosphorous associated with restless leg syndrome? A systematic review and meta-analysis. Sleep. Med. 75, 326–334 (2020).
Wali, S. et al. The association between vitamin d level and restless legs syndrome: A population-based case-control study. Clin. Sleep. Med. 14 (4), 557–564 (2018).
Liu, H. et al. Analysis of serum vitamin D level and related factors in patients with restless legs syndrome. Front. Neurol. 12, 782565 (2021).
Abetz, L. et al. The reliability, validity and responsiveness of the international restless legs syndrome study group rating scale and subscales in a clinical-trial setting. Sleep. Med. 7 (4), 340–349 (2006).
Marek, K. et al. The role of vitamin D in stroke prevention and the effects of its supplementation for post-stroke rehabilitation: A narrative review. Nutrients 14 (13), 2761 (2022).
Balaban, H. et al. Serum 25-hydroxyvitamin D levels in restless legs syndrome patients. Sleep. Med. 13 (7), 953–957 (2012).
Celik, K. et al. Serum endocan levels in women with restless legs syndrome. Neuropsychiatr. Dis. Treat. 11, 2919–2925 (2015).
Wali, S. et al. The association between vitamin d level and restless legs syndrome: A population-based case-control study. Sleep. Med. 14 (4), 557–564 (2018).
Wali, S. et al. The effect of vitamin D supplements on the severity of restless legs syndrome. Sleep. Breath. 19 (2), 579–583 (2015).
Zhang, J. M. et al. Cytokines, inflammation, and pain. Int. Anesthesiol Clin. 45 (2), 27–37 (2007).
Zhu, H. et al. Interleukins and ischemic stroke. Front. Immunol. 13, 828447 (2022).
Higuchi, T. et al. Association of restless legs syndrome with oxidative stress and inflammation in patients undergoing Hemodialysis. Sleep. Med. 16 (8), 941–948 (2015).
Hassan, N. et al. Systemic lupus and risk of restless legs syndrome. JRheumatol 38 (5), 874–876 (2011).
Weinstock, L. B. et al. Crohn’s disease is associated with restless legs syndrome. Inflamm. Bowel Dis. 16 (2), 275–279 (2010).
Weinstock, L. B. et al. Celiac disease is associated with restless legs syndrome. Dig. Dis. Sci. 55 (6), 1667–1673 (2010).
Zhang, Y. et al. Vitamin D inhibits monocyte/macrophage Proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 188 (5), 2127–2135 (2012).
Jablonski, K. L. et al. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57 (1), 63–69 (2011).
Funding
This study was supported by Hebei province administration of traditional Chinese medicine scientific research project(2023204).
Author information
Authors and Affiliations
Contributions
T.y.A, the first author, was responsible for registering, drafting the manuscript and conducting the literature search. L. Y, X.l Wu,Y.m. M,H.y.S contributed to analyze the data and evaluate the methodological quality of the included studies. B.q.L is the corresponding author and was responsible for manuscript revision and manuscript submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
An, T., Yuan, L., Wu, X. et al. The association between vitamin D and restless legs syndrome following acute ischemic stroke. Sci Rep 15, 14867 (2025). https://doi.org/10.1038/s41598-025-98055-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98055-3
Keywords
This article is cited by
-
Restless legs syndrome and periodic limb movements of sleep — the relationship with stroke and other cerebrovascular disease
Nature Reviews Neurology (2026)