Abstract
The role of Bone marrow mesenchymal stem cells (BMSCs) and their exosomes in regulating the host response to viral infections has garnered significant attention, yet research on their specific mechanisms in response to respiratory syncytial virus (RSV) infection remains limited. This study analyzes changes in cytokine levels and exosomal miRNA expression profiles in BMSCs supernatants following RSV infection. The findings reveal that RSV infection leads to a significant decrease in IL-4 levels in BMSCs supernatants, alongside notable increases in IL-6, IL-12, and IFN-γ levels. Additionally, expressions of RSV F protein, G protein, and N gene were detected in the exosomes. Further in vivo experiments demonstrated that exosomes from RSV-treated BMSCs significantly enhanced the inflammatory response in RSV-infected mice, indicated by elevated serum inflammatory cytokines, lung dysfunction, airway inflammation, and increased mucus secretion. In contrast, exosomes from untreated BMSCs showed minimal effects on airway inflammation and damage in infected mice. miRNA sequencing analysis of the exosomes identified differential miRNAs enriched in multiple key signaling pathways, suggesting that RSV infection alters the functional characteristics of BMSCs exosomes, shifting their role from anti-inflammatory and repair mechanisms to a pro-inflammatory function. This transformation may be mediated by changes in the miRNA expression profile.
Similar content being viewed by others
Introduction
Respiratory syncytial virus (RSV) is a common virus that primarily infects infants and young children. Several studies have reported that RSV-induced bronchiolitis is a major risk factor for the onset and development of asthma1,2,3. Elucidating the pathogenic mechanisms of RSV-induced asthma and reducing its occurrence and progression have become popular topics in paediatric research3,4,5.
Viral infections may alter the differentiation and proliferation of stem cells, and the immunoregulatory function of stem cells may affect the host antiviral immune response, thereby affecting the persistence of infections6,7,8.
Bone marrow mesenchymal stromal cells (BMSCs) are multipotent stem cells with potential antiviral and immunomodulatory effects9. BMSCs are multipotent stem cells with potential antiviral and immunomodulatory effects.Previous studies have confirmed that BMSCs are target cells for RSV, which can infect BMSCs and replicate within them. Following RSV infection, BMSCs undergo a series of responses, including apoptosis, cytokine release, and immune responses, which affect their functions and immunoregulatory roles10.
However, there is currently limited research regarding their roles during RSV infection7,8,10. The mechanisms of action of RSV on host cell immune dysregulation, airway inflammation, and injury remain unclear. Exosomes, the carriers of substances and information between cells, have been found to play an important role in the interaction between viruses and host cells. Exosomes are a class of heterogeneous, vesicle-like, small bodies with diameters of approximately 30–150 nm that are secreted by living cells. They are widely distributed in various body fluids. Exosomes mediate intercellular communication by transporting functional proteins, lipids, and bioactive substances such as microRNAs (miRNAs)11,12.
Extracellular vesicles secreted by BMSCs can affect a wide range of immune cells and play an important role in repairing tissue damage, suppressing local inflammation and regulating immunity13,14,15. Exosomes can be released into the extracellular space by cytosis or fusion pathway, which contain a variety of bioactive molecules, such as miRNAs, proteins, and DNA. Exosome contents also play an important role in virus research16,17. Viral infection alters the miRNA expression profile of host cells, leading to changes in the content and type of miRNAs in exosomes. These changes may affect the host cell’s immune response, metabolic pathways, etc., thereby influencing viral replication and transmission18,19. Therefore, studying the effects of exosome contents on viral infection can help to reveal the mechanism of interaction between the virus and host cells. RSV infection can also regulate the expression of a variety of miRNAs, which are associated with viral replication, immune response, and the extent of viral damage. miRNAs may be mediators of RSV-induced inflammatory responses and immune dysfunction20,21. miRNA expression profile differences play an important regulatory role in viral infections, not only affecting the immune response and physiological processes of host cells, but also can directly influence viral replication and life cycle. Viruses can evade and inhibit the host immune response through information exchange and function transfer of exosomal miRNAs between cells to ensure that the virus infiltrates into host cells for replication and release22,23.
Owing to differences in pathogens and source cells, exosomes can play an immunostimulatory role after viral infection, promoting the host antiviral immune response and assisting the virus in intercellular spread, dissemination, and immune evasion24. The role of RSV-induced BMSC-derived exosomes (BMSCs-Exo-RSV) after infection is unknown.
Therefore, this study aimed to better understand the pathogenic mechanism of viral infection in an RSV infection mouse model using BMSCs-Exo-RSV and non-induced exosomes. The identification of the differentially expressed miRNA profiles provides new insights into the mechanisms of interaction between respiratory syncytial virus infection and the microenvironment of host immune cells, as well as intercellular signaling25,26.
Materials and methods
In vitro identification of BMSC characteristics
Culture and identification of primary mouse BMSCs
BMSCs were purified from C57BL/6 mice and expanded using whole bone marrow in vitro isolation combined with the differential apposition method. Cell growth was observed morphologically (Axio observer3, Zeiss, Jena, Germany).
The cell suspension was collected and centrifuged at 1000 rpm for 5 min. The cell pellet was washed with PBS, and after centrifugation at 1000 rpm for another 5 min, the cell pellet was collected. The cells were resuspended in 500 μl of the basic culture medium and divided into 4 tubes, to which CD34 (11-0341-81, eBioscience, San Diego, California, USA), CD44 (12-0441-81, eBioscience), and CD90 (12-0900-81, eBioscience) antibodies were added. They were incubated in the dark for 30 min, while a negative control tube was set up at the same time. The tubes were centrifuged again at 1000 rpm for 5 min, and the cell pellet was collected. The cells were then resuspended in 200 μl of PBS for detection using a flow cytometer (A00-1-1102, Beckman, Brea, California, USA).
Extraction and identification of BMSCs-Exo-RSV
The purified P4-generation BMSCs were divided into model and control groups. The model group was inoculated with RSV (MOI = 1). After adsorption at 35 °C for 1 h, the solution was maintained at 37 °C. The control group did not undergo treatment. After 24 h, The cell culture fluid was collected, centrifuged at 4 °C, 300 × g, 2000 × g for 10 min each, and 10,000 g for 30 min (the supernatant after centrifugation was taken for the next centrifugation operation), and the resulting supernatant was ultracentrifuged at 140,000 × g for 90 min, the supernatant was discarded, and a small amount of PBS was added to dissolve the precipitate, and the centrifugation was repeated once, and the resultant precipitate was purified RSV-treated BMSCs exosomes (BMSCs-Exo-RSV). The resulting precipitate was purified BMSCs-Exo-RSV, and the precipitate was resuspended with 100 μL of PBS and frozen at − 80 °C. Simultaneous extraction of BMSCs exosomes without RSV treatment (BMSCs-Exo). A small amount of supernatant with cell debris and impurities removed and cell precipitate obtained by trypsin digestion followed by centrifugation were retained to be used as a control for exosome marker protein identification.
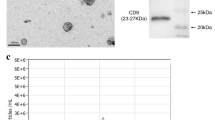
Transmission electron microscopy (JEM-1200 EX, JEOL, Tokyo, Japan) was used to observe the morphology of exosomes; nanoparticle tracking analysis (ZetaView PMX 110, Particle Metrix, Melbusch, Germany) was used to detect the size and distribution of exosome particles; Western blot was used to detect the expression of the exosome membrane marker proteins CD63 and CD81.Cellular protein was extracted and quantified (BCA1-1KT, Sigma, St. Louis, USA) by western blotting. The remaining film was transferred according to the protein quantification results. The CD81 (1:1000; ab219209, Abcam, Cambridge, UK), CD63 (1:1000; 25682-1-AP; Proteintech, Wuhan, China), and calnexin (1 ug/mL; ab22595, Abcam) antibodies were added after blocking. Then, the membranes were incubated overnight with the secondary antibody (1:5000; AWS0002, Abiowell, Changsha, China). After incubation, 1.5 h colour development/exposure of protein bands was performed.
Enzyme-linked immunosorbent assay analysis
Interleukin-4 (IL-4), IL-6, IL-12, and interferon γ(IFN-γ) levels of the supernatant were detected using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (#KE10010, KE10007, KE10001, and KE10014, Proteintech).
Realtime polymerase chain reaction
The Trizol method was used to extract the exosomal RNA (15,596,026, Thermo Fischer Scientific, MA Waltham, USA). The RNA was reverse transcribed into cDNA, and the sequence of the target gene was determined using the National Center for Biotechnology Information (NCBI) database. Primers were synthesised by Shanghai Biosynthesis (Shanghai, China). Two-step real time polymerase chain reaction (RT-PCR; PIKOREAL96, Thermo Fischer Scientific) was conducted. Dissolution and amplification curves were created, and the 2-ΔΔ Ct method was used to analyse the results. The amplification procedure and primer sequences are listed in the Supporting Information.
Exosome miRNA sequencing and differential miRNA expression
RNA was extracted from the control and model cells according to the manufacturer’s instructions (331,505, QIAGEN, Shanghai, China). The miRNA library was constructed and sequenced by Shanghai Burho Biotechnology Co., Ltd. (Shanghai, China) to detect the differential miRNA expression profiles of the exosomes from control and model cells. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were conducted on the differentially expressed genes. The two common differentially expressed miRNAs between exosome sequencing were validated. The two groups of exosomal RNAs were reverse transcribed using primers synthesised by Beijing Kengke (Beijing, China). Quantitative reverse transcription PCR (qRT-PCR) was conducted to detect differential miRNA expression levels.
Effects of RSV in vivo
Experimental animals
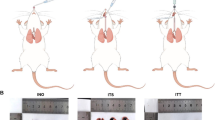
Eighteen SPF-grade C57 mice (3 weeks old; 15–18 g) were purchased from Hunan Slaughter Jingda Laboratory Animal Co., Ltd. (animal qualification certificate no. SCXK (Xiang) 2019-0004; Changsha, China). All animal experiments were performed in a sterile environment at the Animal Experiment Centre of the Hunan University of Traditional Chinese Medicine. All mice had access to food and water ad libitum and housed in a standardised environment (22 ± 2 °C; 50 ± 10% relative humidity; and automatic 12 h light/dark cycle). The experimental protocol was approved by the Animal Experimentation Ethics Committee of the First Affiliated Hospital of Hunan University of Traditional Chinese Medicine (approval number zyfy20220820-04). All parts of the study followed the relevant national and international guidelines for the treatment of animals, and complied with the ARRIVE guidelines. We performed the operation using 1% sodium pentobarbital (50 mg/kg) anesthetized mice by intraperitoneal injection, and at the end of the pulmonary function and other tests the mice were executed under anesthesia using the decapitation method in compliance with the Animal Welfare Act.
Animal grouping and modelling
The mice were divided into control (N = 6) and model (N = 12) groups after five days of adaptive feeding of the 3-week-old SPF-grade male C57 mice. Mice in the model group were nasally inoculated with 5 × 106 pfu (100 μL) of RSV. An equal volume of saline nasal drops was administered to the mice in the control group. Seven days after inoculation, the mice in the model group were randomly divided into BMSCs-Exo-RSV and BMSCs-Exo groups. BMSCs-Exo-RSV group received 100 μg of bone marrow mesenchymal stem cell exosomes extracted after RSV infection via intranasal inoculation and BMSCs-Exo group were inoculated with an equal amount of bone marrow mesenchymal stem cell exosomes.Control group was subjected to the same intervention with an equal amount of PBS.The above treatment was administered once daily for a week.
Animal lung function test
At the end of the nasal drip intervention, a small-animal respiratory anaesthesia machine (R550, Shenzhen Reward, Shenzhen, China) was used to anaesthetise the mice. A small-animal respiratory lung function measurement system (PFT, DATA SCIENCES INTERNATIONAL, INC, U.S.A.) was used to analyse the maximal expiratory flow (PEF), lung resistance (RL), dynamic lung compliance (Cdyn), forced vital capacity (FVC), and pulmonary function.
Enzyme-linked immunosorbent assay analysis
Blood samples were collected from the control mice and each subgroup of model mice. The serum was centrifuged and analysed using an ELISA assay (KE10010, KE10007, KE10001, and KE10014, Proteintech).
Histopathological staining
After the experiments, The left lung was fixed in 4% paraformaldehyde, dehydrated, and sectioned with a thickness of about 4 µm, stained by conventional HE and Masson.then the histopathological changes of the lung were observed under the microscope.Then sections were subjected to hematoxylin and eosin, and Masson staining. Then, sections were dehydrated and rinsed, observed microscopically, and photographed after sealing.The stained samples were scored for inflammatory infiltration and collagen deposition27.
Statistical analysis
All statistical analyses were conducted using SPSS version 25.0 (SPSS, Inc). Measurement data are expressed as mean and standard deviation. The normality and variance alignment of the data were determined. Independent samples t-tests were used to compare normally distributed data. A one-way analysis of variance and multiple comparisons were used to compare data between the groups. The rank-sum test and Kruskal–Wallis H-test were used to compare data that was not distributed normally. Statistical significance was set at P < 0.05.
Results
Identification of BMSCs and exosomes
Over 95% of the P4-generation BMSCs were negative for CD34 and 90% were positive for CD90 and CD44 (Fig. 1A). BMSCs-Exo-RSV were round or oval vesicles of uniform size that were intact (Fig. 1B). No obvious differences in morphology were noted between BMSCs-Exo-RSV and BMSCs-Exo. The diameters of the extracted exosomes were within 150 nm. Both BMSCs-Exo-RSV and BMSCs-Exo were positive for CD63 and CD81 and negative for calnexin (Fig. 1C).
Identification of BMSCs and their exosomes. (A) Expression of surface markers in BMSCs; more than 95% of the cells were negative for CD34, and 90% of the cells were positive for CD90 and CD44, consistent with the characteristics of BMSCs. (B) Photo of exosomes for TEM, shown as classic tea holder; scale bar is 200 nm. The particle size range of both groups was within 150 nm. (C) The expression of exosomal marker proteins in BMSCs-Exo-RSV and BMSCs-Exo was consistent with that of exosome surface proteins, and both CD81 and CD63 proteins were positive. Negative for Calnexin.
levels of IL-4, IL-6, IL-12, and IFN-γ
Level of IL-4 in the cell supernatants significantly decreased after treatment with RSV (Fig. 2A). The levels of IFN-γ, IL-12, and IL-6 significantly increased after treatment with RSV (Fig. 2A).
Effect of RSV on supernatant and exosomes of BMSCs in vitro. (A) BMSCs supernatant concentration of interleukin (IL)-4, IL-6, IL-12, and interferonγ (IFN-γ). Control is the supernatant of blank BMSCs and Model is the supernatant of BMSCs after RSV intervention. (B) Expression of F protein, G protein, and N genes in exosomes. The internal reference gene was M-GAPDH, and the sequence and expression are shown in the supplementary file.
Exosomal expression of RSV F protein, G protein, and N gene
RSV F protein, G protein, and N gene were not expressed in the control group samples (Fig. 2B). In contrast, they were detected in BMSCs-Exo-RSV samples (Fig. 2B).
Expression of differentially expressed miRNAs in exosomes and their target gene GO and pathway enrichment analysis
A total of 122 differentially expressed miRNAs were screened, including 33 upregulated and 89 downregulated miRNAs. The list of specific differential genes was detailed in the Supplementary Information. Then, based on metrics such as COUNTS and TPM, 20 significantly differentially expressed miRNAs were identified. Upregulated miRNAs include: miR-31-5p, miR-16-5p, miR-34c-5p, miR-155-5p, miR-872-5p, miR-25-3p, miR-182-5p, miR-130a-3p, miR-322-5p, and let-7i-5p. Downregulated miRNAs include: miR-31-5p, miR-142a-3p, miR-126a-3p, miR-10a-5p, miR-101a-3p, miR-145a-5p, miR-34a-5p, miR-122-5p, miR-29b-3p, miR-125b-5p, and let-7a-5p. Two differential miRNAs, let-7i-5p an d let-7a-5p, underwent RT-PCR validation . The let-7i-5p and let-7a-5p expression was lower in the BMSCs-Exo-RSV group than in the BMSCs-Exo group (Fig. 3).
The target genes were mainly enriched in MAP-kinase scaffold activity, transmembrane-ephrin receptor activity, ADP-ribosylation factor binding, and biological processes: phosphatidylethanolamine biosynthetic process, positive regulation of epithelial cell proliferation, smoothened signalling pathway, G protein coupled receptor signalling pathway, cellular components are cortical endoplasmic reticulum and the forkhead box F1–F3 (FHF) complex (Fig. 4). Enriched KEGG is closely associated with intra- and extracellular substance transport and catabolism, signal transduction, signalling molecules and interactions, such as soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SANRE) interactions in vesicular transport, glycerophospholipid metabolism, and the phosphatidylinositol signalling system. KEGG-enriched pathways included the vascular endothelial growth factor (VEGF), T cell receptor, sphingolipid, and gonadotropin releasing hormone (GnRH) signalling pathways (Fig. 5).
Top 30 most significantly up-regulated enriched KEGG signalling pathways between BMSCs-Exo-RSV and BMSCs-Exo. (KEGG bubble chart is depicted, where the X-axis represents the enrichment degree, and the Y-axis denotes the enriched pathways. Larger dots on the graph indicate a higher number of genes in each pathway, with the colour of the bubbles transitioning from purple to blue, green, and finally red. A smaller enrichment p-value indicates greater significance.)
Lung function tests
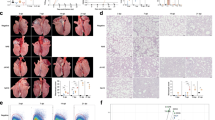
As shown in Fig. 6A, Cdyn significantly decreased in the BMSCs-Exo-RSV group than in the BMSCs-Exo or control groups. The FVC significantly decreased in the BMSCs-Exo-RSV group than in the control group. The FVC was not significantly different between the BMSCs-Exo-RSV and BMSCs-Exo group or the BMSCs-Exo group and control group. The PEF substantially decreased in the BMSCs-Exo-RSV group than in the BMSCs-Exo group. The PEF was not significantly different between the control group and either model group. The RL was not different between the three groups.
Impact of exosome nasal drop on lung function, serum inflammatory factor expression, airway inflammation infiltration, and collagen deposition in mice. (A) The values of lung function indicators CDYN, PEF, FVC, and RL in each group of mice. (B) The concentration of serum IL-4, IL-6, IL-12, and IFN-γ in each group of mice. (C) HE staining and (D) Masson’s trichrome staining of each group of mice, all groups were observed at 200 × . N = 6, **P < 0.01, *P < 0.05.
Serum levels of IL-4, IL-6, IL-12, and IFN-γ
The BMSCs-Exo-RSV group had significantly lower serum IL-4 concentration than the BMSCs-Exo group (Fig. 6B). The concentration of IFN, IL-12, and IL-6 were significantly higher in the BMSCs-Exo-RSV group than in the BMSCs-Exo group (Fig. 6B). IL-4 concentration was lower in the BMSCs-Exo group than in the control group, while the concentration of IFN-γ, IL-12, and IL-6 were higher in the BMSCs-Exo group than in the control group (Fig. 6B).
Histopathological staining
The alveolar epithelial cells were arranged in a single layer, alveolar wall was not thickened, structure of the bronchioles was clear with no obvious abnormalities, blood vessels in the interstitial space did not show obvious siltation, and there was no obvious inflammatory cell infiltration in the lungs of the control mice (Fig. 6C). In the BMSCs-Exo-RSV group, extensive thickening of the alveolar wall was observed along with an unclear alveolar structure, inflammatory cell infiltration within the alveolar wall, alveolar and bronchial haemorrhage, vascular bruising, and focal, perivascular neutrophilic infiltration. In the BMSCs-Exo group, few scattered inflammatory cells were observed infiltrating the alveolar wall and no obvious inflammatory cells were observed in the interstitial blood vessels. No alveolar wall thickening was observed, and the bronchial structure was clear with no obvious abnormalities or interstitial blood vessel bruising. The inflammation score was higher in the BMSCs-Exo-RSV group than in the BMSCs-Exo group and the control group. The inflammation score of the BMSCs-Exo group was higher than that of the control group. The collagen deposition was significantly greater in the BMSCs-Exo-RSV group than in the control group. There was much more collagen deposition in the BMSCs-Exo-RSV group than the BMSCs-Exo group (Fig. 6D).
Discussion
Few studies regarding the role of BMSCs-Exo-RSV have been reported. In this study, BMSCs-Exo-RSV promoted RSV damage to the host, causing cellular inflammatory factor disorders, airway inflammatory infiltration, airway stenosis, and increased airway resistance. However, inflammatory infiltration and airway damage were not observed in BMSCs-Exo mice in this study. Thus, it was speculated that BMSCs-Exo reduces airway inflammatory response in RSV model mice. In contrast, the exosomes derived from BMSCs after RSV intervention exhibit pro-inflammatory effects.
After RSV infection, the imbalance of immune regulatory factor lever is an important cause of airway inflammation and allergic reactions4,28,29,30. In this study, the in vitro IL-4 level decreased and the in vitro IL- 6, IL-12, and IFN-γ levels increased after RSV intervention. The serum IL- 4 levels were decreased, and cytokine levels were increased in both RSV groups, though more significant changes were observed in the BMSCs-Exo-RSV group.
After RSV infection, BMSCs are stimulated to secrete IFN-γ to combat the virus31. Increased IFN-γ level may competitively inhibit the production of IL-431, leading to an enhanced cellular immune response32. The secretion of large amounts of cytokines promotes the differentiation and activation of Th1 cells29 and increases the production of IFN-γ, thus inhibiting the production of IL-429,33.
However, to further explore the mechanism of exosomes, a high-throughput miRNA sequencing method of the exosome miRNA was used, and the nucleic acid information of the RSV virus was detected in the exosomes. Exosomes were found to carry RSV nucleic acid information, such as the F protein, G protein, and N gene, which may be essential for the pro-inflammatory effects of BMSCs-Exo-RSV. The target genes of miRNAs in exosomes are mainly concentrated in the intra-and extracellular signal regulatory pathways and are closely related to airway epithelial homeostasis.
The F and G proteins of RSV are the two major pathogenic proteins proteins on the viral surface that are involved in the binding of the virus to the host cell membrane and transport of the virus into the host cell34. In this study, the F and G proteins of RSV were identified in cells treated with RSV. They may be transferred to the ER, causing stress and damage35. During RSV infection, viral proteins may fuse with the ER membrane, leading to the release of genomic RNA into the cytoplasm, promoting viral replication and the ER stress response, which disrupts ER protein synthesis and folding and interferes with the synthesis of SNARE proteins and phosphatidylethanolamine. These changes result in apoptosis and the release of inflammatory mediators, aggravating the RSV-induced inflammatory response34,36,37.
Sequencing of the exosomal RNA also revealed a differential spectrum of miRNA expression of target genes of the VEGF, T cell receptor, sphingolipid, and GnRH signalling pathways. Exosomes regulate target genes and their pathways through miRNAs to achieve intercellular communication and information transfer. The differential expression of miRNAs was verified via PCR of genetic material from exosomes. let-7 is a microRNA, and down-regulation of let-7 family expression in many viral diseases is thought to be an important factor contributing to the development of viral infections38,39. In turn, the let-7 family has also been closely associated with asthma immune disorders, airway epithelial homeostasis, and latent viral infections40,41. Our study demonstrated low expression of let-7a-5p in RSV-induced exosomes, whereas RSV-induced exosomes had high expression of G and F proteins as well as N genes. RSV has been reported to evade host antiviral defences by suppressing the expression of the let-7 family38.
In this study, the nasal application of BMSCs-Exo-RSV exacerbated RSV airway damage by transferring pro-inflammatory messages and viral nucleic acids, causing airway inflammation and obstruction. The application of BMSCs-Exo attenuated airway inflammation. Therefore, BMSCs-Exo-RSV may exert its pro-inflammatory effects by regulating the expression of miRNAs, including let-7i-5p and let-7a-5p.
Using Illumina sequencing technology and bioinformatics analysis, we conducted a sequencing analysis of miRNAs in exosomes derived from RSV-induced bone marrow mesenchymal stem cells, and we performed a preliminary exploration of the differential expression profile of miRNAs following RSV intervention. Bioinformatics analysis indicated that the differentially expressed miRNAs are mainly involved in intracellular and extracellular signaling pathways, which are closely related to the host’s antiviral immune response and the immune inflammation caused by the virus. Among these, the let-7 family—especially let-7a-5p and let-7i-5p—are key regulatory factors in modulating host immune responses and inflammatory reactions.
However, this study has some limitations. This study explored the differential miRNA expression profile and key pathways through exosome sequencing, but we only selected two differentially expressed miRNAs for in vitro validation, lacking experimental verification of other screened differential miRNAs and key pathways. In the next steps, we plan to conduct in vivo and in vitro validation based on the selected miRNAs and pathways.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Billard, M. N. & Bont, L. J. The link between respiratory syncytial virus infection during infancy and asthma during childhood. Lancet 401(10389), 1632–1633 (2023).
Rosas-Salazar, C. et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): A population-based, prospective birth cohort study. Lancet 401(10389), 1669–1680 (2023).
Talukdar, S. N. et al. RSV-induced expanded ciliated cells contribute to bronchial wall thickening. Virus Res. 327, 199060 (2023).
Hartert, T. V., Wu, P. & Brunwasser, S. M. Respiratory syncytial virus and asthma: Untying the Gordian knot. Lancet Respir. Med. 9(10), 1092–1094 (2021).
Backman, K., Ollikainen, H., Piippo-Savolainen, E., Nuolivirta, K. & Korppi, M. Asthma and lung function in adulthood after a viral wheezing episode in early childhood. Clin. Exp Allergy 48(2), 138–146 (2018).
Kallmeyer, K., Ryder, M. A. & Pepper, M. S. Mesenchymal stromal cells: A possible reservoir for HIV-1. Stem Cell Rev. Rep. 18(4), 1253–1280 (2022).
Piedimonte, G. Respiratory syncytial virus and asthma: Speed-dating or long-term relationship. Curr. Opin. Pediatr. 25(3), 344–349 (2013).
Amaral, J. K. et al. Pathogenesis of chronic chikungunya arthritis: Resemblances and links with rheumatoid arthritis. Travel Med. Infect. Dis. 52, 102534 (2023).
Cheng, G. et al. Reparative homing of bone mesenchymal stem cells induced by iMSCs via the SDF-1/CXCR4 axis for articular cartilage defect restoration. Biomed. Pharmacother. 181, 117649 (2024).
Rezaee, F., Gibson, L. F., Piktel, D., Othumpangat, S. & Piedimonte, G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am. J. Respir. Cell Mol. Biol. 45(2), 277–286 (2011).
Alashkar Alhamwe, B. et al. Extracellular vesicles and asthma-more than just a co-existence. Int. J. Mol. Sci. 22(9), 4984 (2021).
Hough, K. P. & Deshane, J. S. Exosomes in allergic airway diseases. Curr. Allergy Asthma Rep. 19(5), 26 (2019).
Gu, J. et al. BMSCs-derived exosomes inhibit macrophage/microglia pyroptosis by increasing autophagy through the miR-21a-5p/PELI1 axis in spinal cord injury. Aging (Albany NY). 16(6), 5184 (2024).
Shen, X., Qin, J., Wei, Z. & Liu, F. Bone marrow mesenchymal stem cell exosome-derived lncRNA TUC339 influences the progression of osteoarthritis by regulating synovial macrophage polarization and chondrocyte apoptosis. Biomed. Pharmacother. 167, 115488 (2023).
Ren, J. et al. Intranasal delivery of MSC-derived exosomes attenuates allergic asthma via expanding IL-10 producing lung interstitial macrophages in mice. Int. Immunopharmacol. 91, 107288 (2021).
Schwab, A. et al. Extracellular vesicles from infected cells: Potential for direct pathogenesis. Front. Microbiol. 6, 1132 (2015).
Peng, O. et al. Integrative transcriptomic profiling of mRNA, miRNA, circRNA, and lncRNA in alveolar macrophages isolated from PRRSV-infected porcine. Front. Immunol. 14, 1258778 (2023).
Ostrycharz, E. & Hukowska-Szematowicz, B. Micro-players of great significance-host microRNA signature in viral infections in humans and animals. Int. J. Mol. Sci. 23(18), 10536 (2022).
Ao, J. et al. Real-time dissection of the exosome pathway for influenza virus infection. ACS Nano 18(5), 4507–4519 (2024).
Martinez-Espinoza, I., Banos-Lara, M. & Guerrero-Plata, A. The importance of miRNA identification during respiratory viral infections. J. Cell Immunol. 3(4), 207–214 (2021).
Feng, S., Zeng, D., Zheng, J. & Zhao, D. MicroRNAs: Mediators and therapeutic targets to airway hyper reactivity after respiratory syncytial virus infection. Front. Microbiol. 9, 2177 (2018).
Ahsan, N. A. et al. Presence of viral rna and proteins in exosomes from cellular clones resistant to rift valley fever virus infection. Front. Microbiol. 7, 139 (2016).
Bakre, A. A., Maleki, A. & Tripp, R. A. MicroRNA and nonsense transcripts as putative viral evasion mechanisms. Front. Cell Infect. Microbiol. 9, 152 (2019).
Zhu, Y., Yu, S., Qiu, H. J. & Wang, C. Exosomes: Another arena for the game between viruses and hosts. Sheng Wu Gong Cheng Xue Bao. 36(9), 1732–1740 (2020).
Zhang, X., Huang, F., Yang, D., Peng, T. & Lu, G. Identification of miRNA-mRNA crosstalk in respiratory syncytial virus- (RSV-) associated pediatric pneumonia through integrated miRNAome and transcriptome analysis. Mediators Inflamm. 2020, 8919534 (2020).
Wu, W., Choi, E. J., Lee, I., Lee, Y. S. & Bao, X. Non-coding RNAs and their role in respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) infections. Viruses 12(3), 345 (2020).
Luo, J., Deng, Y., Ding, Y., Tang, C. & Wang, M. Xiebai Zengye decoction improves respiratory function and attenuates inflammation in juvenile rats with postinfection cough via regulating ERK signaling pathway. Cell Biochem. Funct. 41(7), 857–867 (2023).
Shi, T. et al. Th17/Treg cell imbalance plays an important role in respiratory syncytial virus infection compromising asthma tolerance in mice. Microb. Pathog. 156, 104867 (2021).
Cheung, M. B. et al. Respiratory syncytial virus-infected mesenchymal stem cells regulate immunity via interferon beta and indoleamine-2,3-dioxygenase. PLoS ONE 11(10), e0163709 (2016).
Makino, A. et al. RSV infection-elicited high MMP-12-producing macrophages exacerbate allergic airway inflammation with neutrophil infiltration. iScience. 24(10), 103201 (2021).
Rodríguez-Fernández, R. et al. Longitudinal plasma cytokine concentrations and recurrent wheezing after RSV bronchiolitis. Cytokine 140, 155434 (2021).
Luo, J. et al. Virus-like particles containing a prefusion-stabilized F protein induce a balanced immune response and confer protection against respiratory syncytial virus infection in mice. Front. Immunol. 13, 1054005 (2022).
Mori, K. et al. Chemokine/interleukin imbalance aggravates the pathology of respiratory syncytial virus infection. J. Clin. Med. 11(20), 6042 (2022).
Hu, M., Bogoyevitch, M. A. & Jans, D. A. Impact of respiratory syncytial virus infection on host functions: Implications for antiviral strategies. Physiol. Rev. 100(4), 1527–1594 (2020).
Li, Z., Guo, D., Qin, Y. & Chen, M. PI4KB on inclusion bodies formed by ER membrane remodeling facilitates replication of human parainfluenza virus type 3. Cell Rep. 29(8), 2229-2242.e4 (2019).
Macauslane, K. L., Pegg, C. L., Short, K. R. & Schulz, B. L. Modulation of endoplasmic reticulum stress response pathways by respiratory viruses. Crit. Rev. Microbiol. 50(5), 750–768 (2023).
Qiao, D. et al. Paramyxovirus replication induces the hexosamine biosynthetic pathway and mesenchymal transition via the IRE1α-XBP1s arm of the unfolded protein response. Am. J. Physiol. Lung. Cell Mol. Physiol. 321(3), L576–L594 (2021).
You, X. et al. miRNA let-7 family regulated by NEAT1 and ARID3A/NF-κB inhibits PRRSV-2 replication in vitro and in vivo. PLoS Pathog. 18(10), e1010820 (2022).
Wu, W., Wang, C., Xia, C., Liu, S. & Mei, Q. MicroRNA let-7 suppresses influenza A virus infection by targeting RPS16 and enhancing type i interferon response. Front. Cell Infect. Microbiol. 12, 904775 (2022).
Letafati, A. et al. MicroRNA let-7 and viral infections: Focus on mechanisms of action. Cell Mol. Biol. Lett. 27(1), 14 (2022).
Wen, K. et al. MircroRNA Let-7a-5p in airway smooth muscle cells is most responsive to high stretch in association with cell mechanics modulation. Front. Physiol. 13, 830406 (2022).
Funding
This research was funded by the National Natural Science Foundation of China (81774368 and 82174437) and the Natural Science Foundation of Hunan Province (2022JJ30037).The funding bodies had no role in the study design; the collection, analysis, and interpretation of data; or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Bing Yao and Jinglei Liu wrote the main manuscript text and conducted cell experiments, Zexiang Li was responsible for animal experiments, Jing Xie and Yinhe Luo were responsible for the layout of the figures and the organization of the data. Mengqing Wang reviewed and revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yao, B., Liu, J., Li, Z. et al. Exploring the mechanism of bone marrow mesenchymal stromal cell exosomes in respiratory syncytial virus infection based on miRNA sequencing. Sci Rep 15, 13797 (2025). https://doi.org/10.1038/s41598-025-98160-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98160-3