Abstract
Parenteral nutrition (PN) is lifesaving for patients with short bowel syndrome and other gastrointestinal disorders, however long-term use may lead to complications including hepatosteatosis and sepsis. We have previously demonstrated the anti-steatotic, -fibrotic, and -inflammatory properties of SEFA-6179, an engineered medium-chain fatty acid analogue. We hypothesized that SEFA-6179 treatment would protect against endotoxin-induced liver injury in a murine model of PN-induced hepatosteatosis. C57Bl/6J mice were administered a high-carbohydrate liquid diet plus intravenous lipid emulsion (Intralipid, 4 g fat/kg/d) or intravenous saline for 19 days to induce hepatosteatosis. SEFA-6179 (100 mg/kg) or vehicle (MCT/medium-chain triglyceride) was administered via oral gavage for four days leading up to intraperitoneal challenge with lipopolysaccharide (15 mg/kg) or saline on day 19. Age-matched, chow-fed controls received the same treatments. The primary outcome was liver biomarkers: alanine aminotransferase and aspartate aminotransferase. Pro-inflammatory cytokines, IL-6, TNF-alpha, and monocyte chemoattractant protein (MCP1), were analyzed. Liver immunofluorescence staining was performed to evaluate macrophage phenotypes. In endotoxin-challenged mice, pre-treatment with SEFA-6179 lowered liver enzymes and pro-inflammatory cytokine levels compared to vehicle. On liver histology, SEFA-6179 pre-treatment led to greater polarization of M1/pro-inflammatory macrophages to an M2/anti-inflammatory phenotype compared to vehicle. SEFA-6179 is currently in Phase II clinical trials. These findings support the potential application of SEFA-6179 in high-risk, PN-dependent patients.
Similar content being viewed by others
Introduction
Short bowel syndrome (SBS) is a malabsorptive state that results from insufficient bowel length and can occur secondary to acquired or congenital conditions. Four million people live with SBS globally, with the number of SBS-related hospitalizations increasing by 55% between 2005 and 2014 in the United States1,2. Patients with SBS are unable to absorb adequate nutrition through their gastrointestinal tract and may require intravenous i.e. parenteral nutrition (PN). While PN is lifesaving for SBS patients, long-term use is associated with significant complications including intestinal failure-associated liver disease (IFALD) and central line-associated bloodstream infections (CLABSIs). IFALD pathophysiology is multifactorial, involving nutrient imbalance-related steatosis, disruption of the gut-liver axis, and inflammation from a variety of causes including endotoxin-driven sepsis3. Limited treatment options exist for patients with IFALD. In patients with IFALD, switching from soybean oil-based to fish oil-based lipid emulsion improved histologic liver injury, improved survival, and reduced rates of organ transplantation4. Sepsis is an additional modifiable risk factor associated with liver disease and death in some PN-dependent patients; new treatments that protect the liver can be lifesaving5.
Among patients with malabsorptive syndromes, medium-chain fatty acids (MCFAs) are commonly used in nutritional supplementation and intestinal rehabilitation6. Unlike long-chain fatty acids which must first be packaged into chylomicrons and transported via the lymphatic system, MCFAs are quickly and passively absorbed from the gastrointestinal tract directly into portal circulation, enabling liver targeting7. MCFAs are bioactive molecules that play a role in numerous physiologic mechanisms related to inflammation, fibrogenesis, and metabolism8,9,10. Interestingly, the ten-carbon MCFA decanoic acid has previously been shown to bind peroxisome proliferator-activated receptor (PPAR)-gamma without inducing adipogenesis11. However, the therapeutic potential of MCFAs in vivo are likely limited by their rapid utilization as an energy source.
Structurally-engineered fatty acid (SEFA)-6179 is an engineered MCFA analogue that, as for unmodified MCFAs, acts on a variety of receptors in vitro, including PPAR-alpha and -gamma which help to downregulate inflammatory signaling12. However, while structurally similar to decanoic acid, SEFA-6179 has moieties that confer resistance to beta-oxidation, with the intention of maximizing availability of the compound for receptor engagement13. Our lab has previously demonstrated the anti-fibrotic, anti-steatotic, and anti-inflammatory properties of SEFA-6179 in a preterm piglet model of intestinal failure and murine model of PN-induced hepatosteatosis13,14. These animal models resulted in a type of liver injury similar to that of patients with IFALD15.
PN-dependent patients universally have indwelling foreign bodies as a means of central venous access for the provision of PN. These patients are not only at risk of developing hepatosteatosis from long-term PN administration, but are additionally susceptible to line-related bacteremia. Murine models of sepsis have long employed the use of intraperitoneally-delivered lipopolysaccharide (LPS), a Gram-negative bacterial cell wall component that drives pro-inflammatory signaling, leading to organ failure and sepsis16. Since PN dependence places patients at risk of developing both liver disease and inflammation, we aimed to investigate the potential therapeutic effects of SEFA-6179 in an animal model that encapsulates both of these elements. Recurrent sepsis contributes to the pathophysiology of IFALD17,18. We sought to investigate whether SEFA-6179 would be protective against liver injury in a murine model of PN-associated hepatosteatosis and endotoxin-driven inflammation.
Results
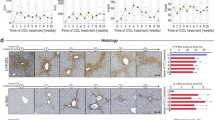
Body and organ weights
In a murine model of PN-associated hepatosteatosis and endotoxin-induced inflammation (Fig. 1), there were no inter-group differences in overall body weight, amount of PN consumption, or normalized organ weights (liver, spleen, and kidney) (Fig. 2). PN-dependent mice experienced an initial period of mild weight loss (1.7 g ± 0.1) prior to steady weight gain until the onset of oral gavage treatments with drug or vehicle on day 15, when PN consumption and weight (2.6 g ± 0.04) decreased, followed by recovery to near-baseline weight at euthanasia on day 19. Chow-fed control mice experienced gradual weight gain over time (3.2 g ± 0.2) without an initial weight loss period.
Experimental design investigating an engineered medium-chain fatty acid analogue, SEFA-6179, in a murine model of PN-associated liver disease and endotoxin challenge. Mice were randomized to parenteral nutrition-equivalent intake or standard rodent chow. Mice received various intravenous (saline or soybean oil-based lipid emulsion i.e. Intralipid), oral gavage (MCT or SEFA-6179), and intraperitoneal (saline or LPS) treatments. A total of n = 70 C57Bl/6J mice were utilized (n = 5 per group, total of 14 groups). MCT, medium-chain triglyceride; SEFA-6179, structurally engineered fatty acid-6179; LPS, lipopolysaccharide (E. coli).
(A) Body weights, (B) Parenteral nutrition consumption, and (C) Normalized organ weights of liver, spleen, and kidney by treatment group over the course of a 19-day experiment involving a murine model of parenteral nutrition-associated liver disease. For (A) and (B), treatment groups are named in the following order: ad libitum oral intake (PN vs. chow), intravenous injection (saline vs. Intralipid), oral gavage (MCT vs. SEFA), and intraperitoneal treatment (saline vs. LPS). Mean ± Standard Error of Mean. PN, parenteral nutrition; SEFA, structurally engineered fatty acid (i.e. SEFA-6179); MCT, medium-chain triglyceride; LPS, lipopolysaccharide.
Liver histology
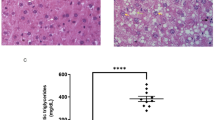
PN-dependent mice developed hepatosteatosis compared to chow-fed controls, as evidenced by macrovascular steatosis on hematoxylin and eosin (H&E) stain and excessive intracellular fat deposition on Oil Red O stain on representative liver histology (Fig. 3). SEFA-6179 treatment led to the reduction of liver steatosis compared to MCT in PN-sustained groups. Chow-fed mice did not develop steatosis in either the MCT or SEFA-6179-treated groups.
Representative liver histology in a murine model of PN-associated liver disease. (A) Standard H&E stain demonstrates evidence of steatosis such as hepatocyte ballooning. (B) Oil Red O stain demonstrates areas of fat and triglyceride deposition as orange-red in color. 40X magnification, Bar = 60uM. MCT, medium-chain triglyceride; PN, parenteral nutrition; SEFA, structurally engineered fatty acid (i.e. SEFA-6179).
Liver enzymes and pro-inflammatory cytokines
LPS challenge increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations compared to saline controls in all groups, including mice provided with PN solution (ALT 167.3 ± 14.6 vs 62.3 ± 7.3 U/L, P = 0.001; AST 233.8 ± 25.7 vs 130.5 ± 12.9 U/L, P = 0.0007) and chow-fed cohorts (ALT 158.8 ± 3.0 vs 22.8 ± 5.9 U/L, P < 0.0001; AST 120.8 ± 8.0 vs 44.2 ± 6.2 U/L, P = 0.007) (Fig. 4). In PN-sustained animals challenged with endotoxin, pre-treatment with SEFA-6179 normalized ALT (42.5 ± 16.2 vs 160.0 ± 12.0 U/L, P = 0.001) and AST (56.3 ± 10.1 vs 159.3 ± 7.8 U/L, P = 0.0002) compared to vehicle-treated controls. Alkaline phosphatase and gamma-glutamyl transferase levels were additionally assessed, however there were no statistically significant differences in these values between MCT and SEFA-6179 treated groups in the present study. SEFA-6179 pretreatment normalized serum levels of pro-inflammatory cytokines IL-6 (interleukin-6) and TNF-alpha (tumor necrosis factor-alpha) (Fig. 5). In endotoxin-challenged chow-fed mice, IL-6 was lower in SEFA-6179-pretreated animals (95.7 ± 3.2 pg/mL) compared to MCT (775.0 ± 103.7 pg/mL, P = 0.003) and saline (1627 ± 175 pg/mL, P = 0.0009). TNF-alpha levels were lower in the SEFA-6179-treated group compared to vehicle-treated controls (2556 ± 65 vs 3375 ± 57 pg/mL, P < 0.0001) among LPS-challenged mice with PN-induced hepatosteatosis. MCP1 (monocyte chemoattractant protein 1) concentration was greater in LPS-exposed animals compared to saline controls (5209 ± 568 vs 34.33 ± 7.89 pg/mL, P < 0.001). Trends in MCP1 generally followed that of IL-6. The mean MCP1 levels trended lower for SEFA-6179-pretreated mice compared to MCT-pretreated mice in the PN + saline (3954 ± 11742 vs 7665 ± 2191 pg/mL, P = 0.210), PN + Intralipid (3138 ± 300 vs 4016 ± 595 pg/mL, P = 0.236), and chow (2388 ± 342 vs 3417 ± 1056 pg/mL, P = 0.381) groups in the setting of LPS exposure, although this did not reach statistical significance. In chow-fed, LPS-exposed animals, SEFA-6179-treated mice had lower MCP1 levels compared to saline-treated mice (2388 ± 342 vs 5209 ± 568 pg/mL, P = 0.003).
Plasma liver enzyme concentrations including (A) alanine aminotransferase and (B) aspartate aminotransferase in a murine model of parenteral nutrition-associated liver disease for PN + Saline, PN + Intralipid, and Chow-Fed Groups. Values expressed are as the mean ± standard error of the mean. ALT, alanine aminotransferase; AST, aspartate aminotransferase; PN, parenteral nutrition; MCT, medium-chain triglyceride; LPS, lipopolysaccharide; SEFA, structurally engineered fatty acid (i.e. SEFA-6179); OG, oral gavage; IP, intraperitoneal; whereby * indicates P < 0.05; ** indicates P < 0.01, *** indicates P < 0.001 on unpaired Student t-test.
Pro-inflammatory cytokine levels in a murine model of parenteral nutrition-associated liver disease for PN + Saline, PN + Intralipid, and Chow-Fed Groups. (A) Serum IL-6 Levels, (B) Liver Lysate TNF-alpha Levels, (C) Liver Lysate MCP1 Levels. Mean ± Standard Error of Mean. PN, parenteral nutrition; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MCT, medium-chain triglyceride; LPS, lipopolysaccharide; SEFA, structurally engineered fatty acid (i.e. SEFA-6179); OG, oral gavage; IP, intraperitoneal; IL-6, interleukin-6; TNF-alpha, tumor necrosis factor-alpha, MCP1, monocyte chemoattractant protein-1, whereby * indicates P < 0.05; ** indicates P < 0.01, *** indicates P < 0.001 on unpaired Student t-test.
Macrophage phenotypes on immunofluorescence
Mice that underwent endotoxin challenge had greater hepatic M1/pro-inflammatory macrophage populations compared to animals that received saline control as measured by mean fluorescent signal intensity on immunofluorescence histology (Fig. 6). In LPS-challenged mice, pre-treatment with SEFA-6179 led to a greater relative population of M2/anti-inflammatory to M1/pro-inflammatory macrophages in the liver compared to MCT on immunofluorescence. The ratio of M1 to M2 macrophages was highest for mice administered saline (5.411 ± 1.263), followed by MCT (3.676 ± 1.619), and lowest for SEFA-6179 (0.7851 ± 0.1701). While the difference in M1:M2 macrophage ratios between SEFA-6179 and MCT trended towards but did not reach significance (P = 0.13), the difference between SEFA-6179 and saline control was statistically significant (P = 0.01).
(A) Representative immunofluorescence stains of liver specimens in a murine model of PN-induced hepatosteatosis and endotoxin-driven inflammation. M1/pro-inflammatory macrophages were stained with anti-CD80 (pink, 1:100), M2/anti-inflammatory macrophages were stained with anti-CD206 (green, 1:100), nuclear material was stained with DAPI as a control (blue). Bright field microscopy at 40X magnification, Bar = 100 uM. (B) Ratio of M1 to M2 macrophages quantified using ImageJ software according to published protocol. MCT, medium-chain triglyceride; LPS, lipopolysaccharide; SEFA, structurally engineered fatty acid (i.e. SEFA-6179); OG, oral gavage; IP, intraperitoneal, whereby * indicates P < 0.05 on unpaired Student t-test.
Discussion
Patients with PN dependence experience a pro-inflammatory state partly due to repeated infections and endotoxin translocation in the setting of a compromised gut barrier19. Additionally, the very composition of lipid emulsions administered as part of the PN can lead to the development of hepatosteatosis, which can disrupt physiologic liver-dependent processes20. Commercially available soybean oil-based lipid emulsions contain high levels of pro-inflammatory omega-6 fatty acids and phytosterols, which have been linked to IFALD with long-term use21,22. Efforts aimed at reducing liver injury resulting from intestinal failure and/or PN administration include changing the lipid composition, restricting the amount of soybean oil-based lipid emulsion administered, increasing enteral feeding, preventing and treating infections, and preserving bowel length during surgical procedures. However, these interventions may not always be clinically feasible. Current therapeutic options for IFALD are limited, leading to the development of SEFA-6179 as a potential treatment.
Our lab has previously demonstrated the anti-inflammatory, -steatotic, and -fibrotic effects of SEFA-6179, a fully synthetic medium-chain fatty acid (MCFA) analogue, in a piglet model of intestinal failure and a murine model of PN-induced hepatosteatosis13,14. Based on this knowledge, the present study aimed to evaluate SEFA-6179 in a murine model that combines the hepatotoxic effects from PN provision and bacterial endotoxin. SEFA-6179 did not produce statistically significant differences in body or organ weights relative to MCT vehicle. SEFA-6179 had demonstrable safety and tolerability in Phase I clinical trials, and is currently under investigation in Phase II in adult patients23. The efficacy of SEFA-6179 is likely associated with structural modifications that, in contrast to unmodified MCFAs, prevent its rapid oxidation as an energy source, permitting activation of key receptors in the liver and elsewhere regulating inflammation and metabolism23,24. Pharmacokinetic studies in a piglet model of SBS with intestinal failure demonstrated that SEFA-6179 has superior gastrointestinal absorption when dissolved in MCT compared to saline25. For the present study, MCT was chosen as the vehicle not only due to this enhanced absorption, but also to isolate the effect of SEFA-6179 from that of MCT which also has anti-inflammatory effects26,27. We induced steatotic liver injury in previously healthy, wild-type mice by providing a high-carbohydrate liquid diet and injecting a pro-inflammatory, soybean oil-based lipid emulsion used in clinical practice. The provision of a high-carbohydrate diet promotes de novo lipogenesis with resultant liver injury. This experiment corroborates previous work which demonstrated that treatment with SEFA-6179 maintained normal liver histology in a murine model of PN-induced hepatosteatosis14.
In the current study, mice were additionally challenged with bacterial endotoxin to mimic the acute inflammation and sepsis that may affect PN-dependent patients in the clinical setting. Commonly used blood biomarkers including ALT and AST increase in response to liver injury28. In the PNALD group that received endotoxin challenge, mice pre-treated with SEFA-6179 had significant reductions in liver enzymes compared to those treated with MCT. The liver enzyme levels of SEFA-6179-treated, LPS-challenged mice were comparable to those of non-LPS challenged mice. These data suggest that SEFA-6179, when delivered as a pre-treatment before acute inflammatory injury, may confer broadly anti-inflammatory effects in other sepsis models. Absolute values of AST were greater for the PN plus intravenous saline group compared to the lipid emulsion group, possibly due to the absence of an exogenous fat source which can lead to essential fatty acid deficiency and related liver dysfunction29.
A murine intestinal injury model demonstrated that bacterial endotoxin-mediated activation of toll-like receptor 4 (TLR-4) signaling led to hepatocyte damage30. The same group of investigators demonstrated that mice with aberrant TLR-4 expression had reduced macrophage activation and improved plasma chemistry, thus elucidating a potential mechanism by which liver injury may be attenuated. SEFA-6179 acts on a variety of receptors in vitro, including PPAR-alpha and PPAR-gamma, which belong to a class of nuclear receptors involved in the regulation of inflammation and glucose and lipid metabolism13,31. With respect to liver disease, PPAR-targeted therapies are currently under development for patients with metabolic dysfunction-associated steatohepatitis and are approved for the treatment of primary biliary cholangitis32,33. In the present study, we demonstrated that SEFA-6179 treatment not only prevented the development of steatosis in a well-established murine model of PN-associated hepatosteatosis, but additionally had hepatoprotective effects in the presence of endotoxin administration. LPS classically activates pro-inflammatory M1 macrophages via a TLR4-dependent pathway that leads to the production of acute phase cytokines, such as IL-6 and TNF-alpha, that recruit downstream mediators of inflammation34. MCP1, also called chemokine ligand 2 (CCL2), is a chemoattractant factor that recruits monocytes; MCP1 levels correlate with the degree of liver injury and in fact, MCP1 deficiency has been found to protect mice against alcoholic liver disease35,36. Our results demonstrate that liver MCP1 levels are elevated in the presence of LPS exposure, compared to saline control. Additionally, mean MCP1 levels were lower in SEFA-6179-treated mice compared to MCT-treated mice in all treatment groups, though this did not reach statistical significance and may be a testament to the known anti-inflammatory and liver-protective properties of MCT alone37,38. PPAR-gamma activation aids in the polarization of pro-inflammatory M1 macrophages to an anti-inflammatory M2 phenotype39,40. The liver is the primary organ responsible for the activation of the initial cytokine storm, thus the early anti-inflammatory effects of SEFA-6179 may have led to the attenuated liver injury as measured by transaminases.
This study has several limitations. IFALD is a complex, multifactorial disease that is difficult to replicate in the laboratory setting. Unlike in human subjects, mice in this investigation did not have central venous catheters for the provision of PN. Although other investigators have conducted total parenteral nutrition experiments in mice, our 19-day PN-induced hepatosteatosis model has the benefit of avoiding catheter-related animal stress, which often result in animal welfare concerns and early death14,41. The present study involved mice with intact gastrointestinal tracts capable of normal absorption, thus it is not a short bowel syndrome model. Previous work from our lab has demonstrated that orally-administered SEFA-6179 is readily absorbed in a SBS model of Yucatan minipigs, albeit with reduced absorption compared to intestinally-intact animals25. The current findings may have limited translatability in human patients with malabsorption related to altered gut anatomy. At the time of LPS injection, mice did not have histologic evidence of steatosis which is attributable to pre-treatment with SEFA-6179. However, our study was designed to approximate the pathophysiology of patients with IFALD who experience acute inflammatory episodes such as infection. In chow-fed, LPS-challenged mice, SEFA-6179 conferred a reduction in AST levels compared to MCT. No statistically significant difference was observed in ALT between MCT and SEFA-6179 groups, although this may be related to limited power within our sample size. Stool was not collected from experimental animals for analysis, although the gut microbiome has proven to have an increasingly important role in modulating nutrient imbalance-related liver disease42. As in any small animal disease model, further dose–response studies involving large animals with greater physiological similarities to human subjects should be conducted.
Conclusions
Parenteral nutrition is lifesaving for patients with intestinal malabsorption. However, long-term administration of PN may cause liver injury due to the inflammatory nature of frequently used commercially available lipid emulsions, as well as patients’ increased susceptibility to infection. Treatment options in this vulnerable patient population are limited. In the current murine model of PN-induced hepatosteatosis and endotoxin-driven inflammation, treatment with SEFA-6179 normalized liver enzymes and reduced levels of pro-inflammatory cytokines, IL-6 and TNF-alpha, compared to MCT vehicle. The anti-inflammatory properties of SEFA-6179 are partly attributable to its ability to polarize pro-inflammatory M1 macrophages to an anti-inflammatory M2 phenotype. Few treatment options exist for patients with IFALD. SEFA-6179 is currently in a multi-institutional Phase II clinical trial in adults with IFALD. Prophylactic treatment with SEFA-6179 may be valuable in high risk PN-dependent patients, such as those with anticipated long-term PN use and history of recurrent infections. In light of current findings, future studies should also examine the potential effects of SEFA-6179 in non-cholestatic inflammatory/fibrosing liver disease models.
Methods
Animal model
All protocols were approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee (IACUC) per National Institutes of Health (NIH) Animal Research Advisory Committee guidelines. All methods were carried out in accordance with relevant guidelines and regulations, and reported in accordance with ARRIVE guidelines. Hepatosteatosis was induced in otherwise healthy eight-week-old male C57Bl/6J mice (The Jackson Laboratory, Bar Harbor, ME) using a well-established murine model15. Experimental design is summarized in Fig. 1. Briefly, mice received an ad libitum fat-free, high-carbohydrate parenteral nutrition (PN)-equivalent oral solution for 19 days, with every-other-day tail vein injection of a commercial soybean oil-based lipid emulsion (Intralipid, 4 g fat/kg body weight/day, Fresenius Kabi, Sweden) or isovolumetric saline control. The composition of the PN solution is identical to that of PN administered to patients at Boston Children’s Hospital: 20% dextrose, 2% amino acid, 30 mEq/L sodium, 20 mEq/L potassium, 15 mEq/L calcium, 10 mEq/L magnesium, 10 mMol/L phosphate, 36.67 mEq/L chloride, 19.4 mEq/L acetate, multivitamins, and essential trace elements. PN consumption was measured twice daily. Body weights were measured every other day. PN-dependent mice did not receive any other source of nutrition or hydration. Mice were randomized to one of two oral treatments: SEFA-6179 drug (100 mg/kg) (NorthSea Therapeutics, Netherlands) dissolved in medium-chain triglyceride (MCT) vehicle (Nestle, Switzerland) or isovolumetric MCT. Treatments were administered via oral gavage daily on days 15–18. On day 19, a single dose of Escherichia coli O111:B4-derived lipopolysaccharide (LPS, 15 mg/kg body weight, Sigma-Aldrich, St. Louis, MO) or isovolumetric saline vehicle was administered intraperitoneally six hours before planned euthanasia. The dose of SEFA-6179, 100 mg/kg, was based on previous work from our lab that demonstrated its safety and tolerability14. The dose of LPS, 15 mg/kg, was based on published rodent sepsis models which most frequently utilized E. coli-derived LPS (ranging from 10–25 mg/kg) and subsequent lot-specific pilot testing in the lab, as toxicity may vary in a batch-dependent manner16,43,44. Animals were anesthetized using inhaled isoflurane (3% induction and 2% maintenance, VetOne, Boise, ID) prior to euthanasia via terminal blood draw followed by cervical dislocation. Age-matched, chow-fed mice received either SEFA-6179 drug (100 mg/kg), isovolumetric MCT (vehicle control), or normal saline (negative control) daily via oral gavage on days 15–18. Chow-fed controls were similarly treated with intraperitoneal endotoxin (15 mg/kg) or isovolumetric saline and sacrificed on day 19. Mice were housed under the same conditions (12-h light/dark cycle, 21 °C ambient temperature) and underwent concurrent treatment in order to minimize the potential for confounding. Fourteen experimental groups were utilized (n = 5 per group) for a total of 70 animals.
Biochemical assay
The primary outcome was biochemical assessment of liver injury through plasma concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Whole blood was collected from live animals and centrifuged at 1,500 relative centrifugal force (RCF) for 15 min at 4 °C in lithium heparin plasma separator tubes (BD, Franklin Lakes, NJ). Plasma was flash frozen and stored at − 80 °C, then assayed for chemistry using veterinary rotors on the VetScan VS2 device (Zoetis, Parsippany, NJ). Enzyme-linked immunosorbent assays (ELISAs) were used to measure levels of pro-inflammatory cytokines, including mouse-specific plasma IL-6 (Abcam, Waltham, MA), liver TNF-alpha (RayBiotech, Norcross, GA), and liver MCP1/CCL2 (R&D Systems, Minneapolis, MN). For the liver TNF-alpha and MCP1 assays, the same mass of liver tissue (30.0 µg/sample) was digested in identical amounts of lysis buffer (300 µL/sample for TNF-alpha and 250 µL/sample for MCP1) prior to completing the kits according to the manufacturer’s instructions.
Histopathology
The largest (i.e. left) lobe of the liver, weighing approximately 0.4–0.5 g underwent 24 h of formalin fixation, paraffin-embedding, and subsequent hematoxylin and eosin (H&E) staining to assess hepatic architecture. Frozen optimal cutting temperature (OCT) compound-embedded caudate lobes of the liver, weighing approximately 0.2–0.3 g, were additionally stained using Oil Red O to evaluate fatty deposition.
Immunofluorescence
Immunofluorescence staining of liver specimens, specifically the largest (i.e. left) lobe, was performed to assess for M1 and M2 macrophage phenotypes. Slides were deparaffinized and rehydrated as follows: 15 min xylene, 15 min xylene, 5 min 100% alcohol, 5 min 100% alcohol, 5 min 70% alcohol, and water rinse. Slides were transferred to antigen retrieval buffer (Antigen Unmasking Solution, citric acid-based pH = 6, Vector Laboratories, Newark, CA). Antigen retrieval was completed in a pressure cooker at 110 °C for 15 min. Slides were allowed to cool for 20 min before they were brought to room temperature through the addition of water to the retrieval container. Once slides reached room temperature, they were washed three times for 5 min each in TBS (Tris-buffered saline), TBS again, and TBST (Tris-buffered Saline + Tween20). Sections were blocked with a 3% bovine serum albumin (BSA) and incubated at room temperature for 20 min. Following blocking, sections were incubated with the primary antibodies diluted in 3% BSA overnight at 4 °C: anti-CD80 (1:100, Invitrogen, Waltham, MA) for M1 macrophages and CD206 (1:100, R&D Systems, Minneapolis, MN) for M2 macrophages. The following morning, slides were washed as before. Donkey anti-Goat IgG diluted in TBS (1:100, Alexa Fluor Plus 647, Thermo Fisher Scientific, Waltham, MA) was applied to sections and allowed to incubate for 1 h at room temperature. Slides were washed as before. Goat anti-Rabbit IgG diluted in TBS (1:100, Alexa Fluor Plus 488, Thermo Fisher Scientific, Waltham, MA) was applied to sections and allowed to incubate for 1 h at room temperature. Slides were washed as before. Slides were stained with Sudan Black for 20 min to quench background and autofluorescence, then washed under running water for 15 min. Slides were washed three times for 5 min each in TBS, TBS again, and TBST. Slides were counterstained with DAPI diluted in PBS (2 μg/mL, ServiceBio, Wuhan, China) and cover-slipped using Fluoroshield (Sigma-Aldrich, St. Louis, MO). Whole slide-scanning was performed (bright field 40x, MoticEasyScan Infinity, Hong Kong) for analysis. ImageJ software (ImageJ 1.54 for Windows, National Institutes of Health, USA) was used to quantify immunofluorescence signal intensity of M1 and M2 macrophages, normalized against DAPI, according to published protocol45.
Statistical analysis
The Shapiro–Wilk test was used to assess distribution of data. Continuous outcomes were assessed using analysis of variance (ANOVA) and described as the mean plus or minus standard error of the mean (SEM). We chose not to adjust for multiple comparisons due to limited sample sizes. All tests of significance were two-sided with P < 0.05 deemed statistically significant. Analysis was performed in SAS version 9.4 (SAS, Cary, NC). Plots were generated with GraphPad Prism version 10.3.1 (GraphPad Software, Boston, MA).
Data availability
Datasets from the present study are available from the corresponding author upon reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BSA:
-
Bovine serum albumin
- CCL2:
-
Chemokine ligand 2
- CLABSI:
-
Central line-associated bloodstream infections
- ELISA:
-
Enzyme-linked immunosorbent assay
- IFALD:
-
Intestinal failure-associated liver disease
- LPS:
-
Lipopolysaccharide
- MCFA:
-
Medium-chain fatty acid
- MCP1:
-
Monocyte chemoattractant protein 1
- MCT:
-
Medium-chain triglyceride
- PPAR:
-
Peroxisome proliferator-activated receptor
- PN:
-
Parenteral nutrition
- PNALD:
-
Parenteral nutrition-associated liver disease
- RCF:
-
Relative centrifugal force
- SEFA:
-
Structurally-engineered fatty acid
- TBS:
-
Tris-buffered saline
- TBST:
-
Tris-buffered Saline + Tween20
References
Siddiqui, M. T., Al-Yaman, W., Singh, A. & Kirby, D. F. Short-bowel syndrome: Epidemiology, hospitalization trends, in-hospital mortality, and healthcare utilization. JPEN J. Parenter Enteral. Nutr. 45, 1441–1455. https://doi.org/10.1002/jpen.2051 (2021).
Lakkasani, S., Seth, D., Khokhar, I., Touza, M. & Dacosta, T. J. Concise review on short bowel syndrome: Etiology, pathophysiology, and management. World J. Clin. Cases 10, 11273–11282. https://doi.org/10.12998/wjcc.v10.i31.11273 (2022).
Peverill, W., Powell, L. W. & Skoien, R. Evolving concepts in the pathogenesis of NASH: Beyond steatosis and inflammation. Int. J. Mol. Sci. 15, 8591–8638. https://doi.org/10.3390/ijms15058591 (2014).
Puder, M. et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann. Surg. 250, 395–402. https://doi.org/10.1097/SLA.0b013e3181b36657 (2009).
Spencer, A. U. et al. Pediatric short bowel syndrome: redefining predictors of success. Ann. Surg. 242, 403–409. https://doi.org/10.1097/01.sla.0000179647.24046.03 (2005) (discussion 409–412).
Tantibhedhyangkul, P. & Hashim, S. A. Medium-chain triglyceride feeding in premature infants: Effects on fat and nitrogen absorption. Pediatrics 55, 359–370 (1975).
Tessitore, M. et al. Malnutrition in pediatric chronic cholestatic disease: An up-to-date overview. Nutrients 13, 2785. https://doi.org/10.3390/nu13082785 (2021).
Wang, M. E. et al. Increasing dietary medium-chain fatty acid ratio mitigates high-fat diet-induced non-alcoholic steatohepatitis by regulating autophagy. Sci. Rep. 7, 13999. https://doi.org/10.1038/s41598-017-14376-y (2017).
Gao, Y. et al. Coconut oil and medium-chain fatty acids attenuate high-fat diet-induced obesity in mice through increased thermogenesis by activating brown adipose tissue. Front Nutr. 9, 896021. https://doi.org/10.3389/fnut.2022.896021 (2022).
Wang, J., Wu, X., Simonavicius, N., Tian, H. & Ling, L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J. Biol. Chem. 281, 34457–34464. https://doi.org/10.1074/jbc.M608019200 (2006).
Malapaka, R. R. V. et al. Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. J. Biol. Chem. 287, 183–195. https://doi.org/10.1074/jbc.M111.294785 (2012).
Fligor, S. C. et al. Inflammation drives pathogenesis of early intestinal failure-associated liver disease. Sci. Rep. 14, 4240. https://doi.org/10.1038/s41598-024-54675-9 (2024).
Fligor, S. C. et al. A medium-chain fatty acid analogue prevents intestinal failure-associated liver disease in preterm Yorkshire piglets. Gastroenterology 165, 733-745.e739. https://doi.org/10.1053/j.gastro.2023.05.035 (2023).
Cho, B. S. et al. A medium-chain fatty acid analogue prevents hepatosteatosis and decreases inflammatory lipid metabolites in a murine model of parenteral nutrition-induced hepatosteatosis. PLoS ONE 18, e0295244. https://doi.org/10.1371/journal.pone.0295244 (2023).
Meisel, J. A. et al. Comparison of 5 intravenous lipid emulsions and their effects on hepatic steatosis in a murine model. J. Pediatr. Surg. 46, 666–673. https://doi.org/10.1016/j.jpedsurg.2010.08.018 (2011).
Lewis, A. J., Seymour, C. W. & Rosengart, M. R. Current murine models of sepsis. Surg. Infect. (Larchmt) 17, 385–393. https://doi.org/10.1089/sur.2016.021 (2016).
Norsa, L., Nicastro, E., Di Giorgio, A., Lacaille, F. & D’Antiga, L. Prevention and treatment of intestinal failure-associated liver disease in children. Nutrients 10, 664. https://doi.org/10.3390/nu10060664 (2018).
Diamond, I. R. et al. The role of parenteral lipids in the development of advanced intestinal failure-associated liver disease in infants: A multiple-variable analysis. JPEN J. Parenter. Enteral. Nutr. 35, 596–602. https://doi.org/10.1177/0148607111413598 (2011).
Yang, H., Feng, Y., Sun, X. & Teitelbaum, D. H. Enteral versus parenteral nutrition: effect on intestinal barrier function. Ann. N. Y. Acad. Sci. 1165, 338–346. https://doi.org/10.1111/j.1749-6632.2009.04026.x (2009).
Calder, P. C., Waitzberg, D. L., Klek, S. & Martindale, R. G. Lipids in parenteral nutrition: Biological aspects. JPEN J. Parenter. Enteral. Nutr. 44(Suppl 1), S21-s27. https://doi.org/10.1002/jpen.1756 (2020).
Goulet, O. J., Cai, W. & Seo, J. M. Lipid emulsion use in pediatric patients requiring long-term parenteral nutrition. JPEN J. Parenter Enteral. Nutr. 44(Suppl 1), S55-s67. https://doi.org/10.1002/jpen.1762 (2020).
Mihajlovic, M., Rosseel, Z., De Waele, E. & Vinken, M. Parenteral nutrition-associated liver injury: Clinical relevance and mechanistic insights. Toxicol Sci 199, 1–11. https://doi.org/10.1093/toxsci/kfae020 (2024).
Beysen C, F. D., Lasson E, Skjaeret T, MacElrevey C, Israel S, Bush J, Harrison S. In ASPEN 2023 (2023).
Luscombe, V. B., Lucy, D., Bataille, C. J. R., Russell, A. J. & Greaves, D. R. 20 years an orphan: Is GPR84 a plausible medium-chain fatty acid-sensing receptor?. DNA Cell Biol. 39, 1926–1937. https://doi.org/10.1089/dna.2020.5846 (2020).
Fligor, S. C. et al. Absorption of an engineered medium-chain fatty acid analogue in two short bowel syndrome minipig models. JPEN J. Parenter. Enteral. Nutr. 47, 1028–1037. https://doi.org/10.1002/jpen.2563 (2023).
Baker, M. A. et al. Fish oil-based injectable lipid emulsions containing medium-chain triglycerides or added α-tocopherol offer anti-inflammatory benefits in a murine model of parenteral nutrition-induced liver injury. Am. J. Clin. Nutr. 109, 1038–1050. https://doi.org/10.1093/ajcn/nqy370 (2019).
Ohue-Kitano, R. et al. Medium-chain fatty acids suppress lipotoxicity-induced hepatic fibrosis via the immunomodulating receptor GPR84. JCI Insight 8, e165469. https://doi.org/10.1172/jci.insight.165469 (2023).
Kwo, P. Y., Cohen, S. M. & Lim, J. K. ACG clinical guideline: Evaluation of abnormal liver chemistries. Am. J. Gastroenterol. 112, 18–35. https://doi.org/10.1038/ajg.2016.517 (2017).
Le, H. D. et al. Docosahexaenoic acid and arachidonic acid prevent essential fatty acid deficiency and hepatic steatosis. JPEN J. Parenter. Enteral. Nutr. 36, 431–441. https://doi.org/10.1177/0148607111414580 (2012).
El Kasmi, K. C. et al. Toll-like receptor 4-dependent Kupffer cell activation and liver injury in a novel mouse model of parenteral nutrition and intestinal injury. Hepatology 55, 1518–1528. https://doi.org/10.1002/hep.25500 (2012).
Liss, K. H. & Finck, B. N. PPARs and nonalcoholic fatty liver disease. Biochimie 136, 65–74. https://doi.org/10.1016/j.biochi.2016.11.009 (2017).
Francque, S. M. et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N. Engl. J. Med. 385, 1547–1558. https://doi.org/10.1056/NEJMoa2036205 (2021).
Schnabl, B. PPAR agonists in primary biliary cholangitis. N. Engl. J. Med. 390, 855–858. https://doi.org/10.1056/NEJMe2313802 (2024).
Zhu, L., Zhao, Q., Yang, T., Ding, W. & Zhao, Y. Cellular metabolism and macrophage functional polarization. Int. Rev. Immunol. 34, 82–100. https://doi.org/10.3109/08830185.2014.969421 (2015).
Mandrekar, P., Ambade, A., Lim, A., Szabo, G. & Catalano, D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: Regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 54, 2185–2197. https://doi.org/10.1002/hep.24599 (2011).
Queck, A. et al. Systemic MCP-1 levels derive mainly from injured liver and are associated with complications in cirrhosis. Front Immunol. 11, 354. https://doi.org/10.3389/fimmu.2020.00354 (2020).
Zhang, L. et al. Medium-chain triglycerides attenuate liver injury in lipopolysaccharide-challenged pigs by inhibiting necroptotic and inflammatory signaling pathways. Int. J. Mol. Sci. 19, 3697. https://doi.org/10.3390/ijms19113697 (2018).
Kono, H. et al. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Ann. Surg. 237, 246–255. https://doi.org/10.1097/01.Sla.0000048450.44868.B1 (2003).
He, L. et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 37, 109955. https://doi.org/10.1016/j.celrep.2021.109955 (2021).
Bouhlel, M. A. et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143. https://doi.org/10.1016/j.cmet.2007.06.010 (2007).
Lou, P. H. et al. Choice of lipid emulsion determines inflammation of the gut-liver axis, incretin profile, and insulin signaling in a murine model of total parenteral nutrition. Mol. Nutr. Food Res. 65, e2000412. https://doi.org/10.1002/mnfr.202000412 (2021).
Kang, H. et al. Interaction effect between NAFLD severity and high carbohydrate diet on gut microbiome alteration and hepatic de novo lipogenesis. Gut Microbes 14, 2078612. https://doi.org/10.1080/19490976.2022.2078612 (2022).
Thomas, R. C., Bath, M. F., Stover, C. M., Lambert, D. G. & Thompson, J. P. Exploring LPS-induced sepsis in rats and mice as a model to study potential protective effects of the nociceptin/orphanin FQ system. Peptides 61, 56–60. https://doi.org/10.1016/j.peptides.2014.08.009 (2014).
Raduolovic, K., Mak’Anyengo, R., Kaya, B., Steinert, A. & Niess, J. H. Injections of lipopolysaccharide into mice to mimic entrance of microbial-derived products after intestinal barrier breach. J. Vis. Exp. https://doi.org/10.3791/57610 (2018).
Shihan, M. H., Novo, S. G., Le Marchand, S. J., Wang, Y. & Duncan, M. K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 25, 100916. https://doi.org/10.1016/j.bbrep.2021.100916 (2021).
Author information
Authors and Affiliations
Contributions
Conceptualization: SZW, SCF, MP; Methodology: SZW, SCF, TIH, MQ, AP; Analysis: SZW, PDM; Writing—Original Draft: SZW; Writing—Review & Editing: All Authors; Supervision: KMG, MP; Funding Acquisition: SZW, TIH, SCF, MP. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
This investigation was performed under a sponsored research agreement and partially funded by NorthSea Therapeutics, to which MP and KG provided external consultation. A patent application has been filed for SEFA-6179 (MP). Additional funding was received from the National Institutes of Health grants 5T32HL007734 (TIH), 2T32DK007754-22 (STT), the Boston Children’s Hospital Vascular Biology Program, Boston Children’s Hospital Surgical Foundation, the Hannah Lillie Fund, the Maisie Ellis and Friends Fund, and the Luke Raymond Celaya Research Fund.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S.Z., Hirsch, T.I., Fligor, S.C. et al. A medium-chain fatty acid analogue prevents endotoxin liver injury in a murine model. Sci Rep 15, 13645 (2025). https://doi.org/10.1038/s41598-025-98200-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98200-y