Abstract
Artificial intelligence-based technologies are rapidly advancing and significantly influencing the engineering sector, particularly in the automotive industry, through AI-driven neural network tools and Sankey diagrams. Meanwhile, the depletion of fossil fuels and rising emissions have pushed global efforts towards renewable and clean fuel solutions. Hydrogen, as a key clean fuel, has garnered considerable research interest. Combining hydrogen with biomass-derived fuels has gained attention due to its dual benefits of addressing biomass waste disposal and alleviating hydrogen storage and safety concerns. This study focuses on the production of aquatic plant oil (duckweed bio-oil) and its combination with hydrogen gas, evaluating their effects on the performance of a Reactivity Controlled Compression Ignition (RCCI) engine. The results revealed that the H40 blend demonstrated a 1% higher brake thermal efficiency (BTE) than diesel, along with emission reductions of 40% for HC, 6% for NOx, 27% for CO, and 14% for smoke. The results were further validated using an Artificial Neural Network (ANN) and a Sankey diagram. The ANN achieved low RMSE values (0.9965–0.9996) and MPAE values within 4%, while the Sankey diagram effectively illustrated energy distribution with minimal loss. These findings highlight the potential of hydrogen-enriched fuels for future internal combustion engines.
Similar content being viewed by others
Introduction
Hydrogen is increasingly being considered as a key fuel for the future, particularly in developed nations. According to the International Energy Agency, global investments in hydrogen production technologies exceeded $10 billion in 20231. Driven by the dual goals of reducing greenhouse gas emissions and enhancing energy security, many countries are directing significant resources toward hydrogen research and development. Notably, Saudi Arabia has taken a leading role by launching hydrogen-powered buses and investing in green hydrogen production using renewable resources such as solar and wind energy2,3.
Hydrogen is expected to play a significant role in transportation and agriculture. A report by the Hydrogen Council predicts that by 2050, hydrogen will account for 18% of the world’s total energy demand, reducing carbon dioxide emissions by up to 6 gigatons annually4. Despite significant advancements in hydrogen production methods, such as electrolysis, steam reforming, and biomass gasification, challenges remain. Specifically, hydrogen’s storage and handling processes require special attention due to its flammability and broad explosive range, which spans 4–75% in air5,6.
In the automotive sector, hydrogen was first applied in spark-ignition (SI) engines, but several issues such as pre-ignition, knocking, and backfiring emerged. In contrast, compression-ignition (CI) engines have shown improved output and emissions performance when hydrogen is used as a supplementary fuel due to their higher compression ratios. However, the sole use of hydrogen in CI engines is limited by its high auto-ignition temperature. This challenge has led to the exploration of dual-fuel operation, where hydrogen is combined with diesel or biodiesel to leverage the advantages of both fuels7,8,9.
In dual-fuel engines, operation typically starts with diesel and gradually introduces hydrogen, contributing 25–30% of the energy share. Research has shown significant improvements in Brake Thermal Efficiency (BTE) and fuel consumption rates at low and moderate loads with this approach. Furthermore, studies have demonstrated substantial reductions in emissions such as CO, HC, and particulate matter, with CO reductions up to 50%, HC by 70%, and particulate matter by 60%. However, increased NOx emissions remain a persistent challenge due to the high combustion temperatures associated with hydrogen10,11,12.
To address these issues, researchers have turned to biofuels as a complementary solution in dual-fuel configurations. Among various biofuel feedstocks, aquatic plants have emerged as a promising option due to their availability, cost-effectiveness, and sustainable properties. Moreover, aquatic plant oils can be extracted using steam distillation and possess lower viscosity and carbon content, eliminating the need for complex upgrading processes such as transesterification13,14,15. Additionally, the cultivation of aquatic plants in wastewater can serve dual purposes: providing a renewable biofuel source and aiding in water purification16,17.
The combination of hydrogen and aquatic plant oil offers a potential solution for reducing the environmental impact of internal combustion (IC) engines. Hydrogen’s high energy content and clean-burning properties complement the low carbon content and renewable nature of aquatic plant oil, addressing NOx formation and combustion instability18,19. One promising approach is the use of dual-fuel Reactivity Controlled Compression Ignition (RCCI) engines, which employ two fuels of varying reactivities. This strategy allows for greater control over the combustion process, leading to higher thermal efficiency and significantly reduced emissions20,21.
While hydrogen-enriched fuel blends have shown promise in dual-fuel combustion, challenges such as NOx emissions and fuel instability persist. This study explores the feasibility of using aquatic plant oil as a biofuel in RCCI mode to mitigate these challenges. By leveraging the complementary properties of hydrogen and aquatic plant oil, this research contributes to the development of sustainable and efficient energy solutions for internal combustion engines.
Several studies have proven that aquatic plant oils can be a good substitute for conventional fossil fuels. For example, biofuels from algae have been shown to produce high energy yields per unit area compared to terrestrial crops22. Additionally, aquatic plants do not occupy lands designated for agricultural production, making them a promising avenue for biomass development. In support of this, aquaculture is robust globally, with an annual harvest of around 525,000 tons of aquatic plants. As demand for green, sustainable biofuels increases, future expansions in aquaculture are expected to enhance the product line. To illustrate, in 2018, global aquaculture generated 114.5 million tons of overall product value, worth approximately USD 263 billion23.
Moreover, the combination of hydrogen and aquatic plant oil is promising as a technique to reduce the environmental impact of internal combustion (IC) engines. Hydrogen, recognized for its high energy content and clean-burning properties, significantly enhances the efficiency of IC engines while decreasing harmful emissions. Complementing this, aquatic plant oil is a renewable biofuel with low carbon content, making it an environmentally friendly supplement to hydrogen24,25. However, a major drawback of using hydrogen-enriched fuel in IC engines is the formation of NOx emissions during combustion due to the relatively high combustion temperature of hydrogen. Fortunately, aquatic plant oil helps mitigate this problem. The natural properties and lower carbon content of aquatic plant oil can decrease combustion temperature and NOx emissions26,27.
In parallel, several techniques are under development to minimize NOx emissions from IC engines. Fuel modification techniques, such as the addition of nano additives and water emulsions, aim to provide cleaner combustion and reduce NOx emissions. Additionally, a modified engine design with dual-fuel mode and an innovative combustion strategy known as Reactivity Controlled Compression Ignition (RCCI) shows promise28,29. RCCI, a dual-fuel combustion strategy, uses two fuels of different reactivities. It involves the direct injection of a high-reactivity fuel, such as diesel or biofuel, and the port injection of a low-reactivity fuel, such as hydrogen. This approach allows a higher degree of control over the combustion process, leading to higher thermal efficiency and reduced emissions. By carefully managing the reactivity and timing of fuel injections, RCCI engines can operate under lower combustion temperatures, which significantly reduces NOx formation30,31. This makes RCCI engines particularly well-suited for hydrogen-enriched aquatic plant oil fuels.
Further supporting this, investigators found that RCCI mode engines achieve higher thermal efficiency and higher NOx reduction rates compared to traditional combustion modes involving hydrogen-biodiesel-based test fuels. This improvement is attributed to the supply of Low Reactivity Fuel (LRF), such as hydrogen, during the suction stroke, and High Reactivity Fuel (HRF), such as biodiesel, at the end of the compression stroke32,33. Notably, researchers demonstrated that premixed hydrogen up to 60% can be smoothly operated in RCCI mode combustion. Experiments with an RCCI mode engine using HRF as algae and LRF as a hydrogen carrier showed a substantial decrease in CO₂ by approximately 16% and NOx by approximately 20% with up to 35% (volume basis) of the hydrogen carrier34. The significant decrease in NOx and particulate matter (PM) emissions in RCCI engines is primarily due to the high homogeneity of the air-fuel mixture. Furthermore, a higher premixed rate results in higher brake thermal efficiency (BTE)35.
In summary, the combination of hydrogen and aquatic plant oil in an RCCI mode IC engine forms a sustainable energy source efficient for future use in transportation and agriculture. These fuels can leverage their benefits while overcoming the shortcomings of their sole use, such as NOx formation with hydrogen. Additionally, RCCI engines offer improved thermal efficiency, lower NOx emissions, soot reduction, flexibility in fuel types, and potentially broader biofuel applications compared to other NOx reduction methods. When blended with aquatic plant oil, hydrogen balances optimal performance with minimal environmental impact36,37.
Moreover, Artificial Neural Networks (ANN), inspired by human brain neural networks, are computational models that consist of interconnected nodes (neurons) processing information through weights and biases on input data. ANNs have widespread applications in pattern recognition, forecasting, and optimization38. In the context of hydrogen dual-fuel mode, ANNs can optimize engine performance and emissions. Researchers can train ANN models to predict combustion characteristics, emissions, and performance parameters under various experimental conditions. For example, studies have reported that NOx and CO emissions can be predicted using ANN models with a mean absolute percentage error (MAPE) of less than 5%. Moreover, fine-tuning engine parameters using ANN is highly effective in achieving efficiency with low emission levels39,40. The detailed summary of previous work is shown in Table (1).
Based on the extensive literature review, this research was motivated to conduct further investigation. The present work focused on the extraction of aqua plant oil through an energy- intensive process, which was evaluated and confirmed by green metrics for eco-friendly production. The hydrogen was then injected through the intake manifold of an RCCI engine at 7 L per minute (lpm) as LRF during the suction stroke, and various blends of aqua plant oil were supplied as HRF at the end of the compression stroke in the RCCI engine. The research aimed to evaluate the influence of the test fuel in RCCI mode. Additionally, the test results were compared with those of diesel and conventional modes of combustion. Further, the experimental work was validated with an ANN tool. The overall work flow was exposed in figure (1).
Material and methodology
In this study, a hydrogen gas cylinder was procured from local Scientific factory. The duckweed plants were collected from a local market and was synthesized through a steam- intensive process. The conventional mode engine was modified into an RCCI (Reactivity Controlled Compression Ignition) mode engine to employ the test fuel in two ways, namely through the LRF (Low Reactivity Fuel) and HRF (High Reactivity Fuel) ports.
Background of duckweed plant
The Duckweed plant was selected for this study as a High Reactivity Fuel (HRF) due to its fast-growing nature and high oil yield capacity. Duckweed typically grows in normal temperature conditions within 2–3 days. This floating plant can thrive in any type of water, even polluted water. Duckweed belongs to the Araceae family, which is a group of tiny plants. Worldwide, duckweed grows in slow-moving water under normal temperature conditions, and its roots commonly grow up to 10 mm in length. Moreover, its rapid growth rate ensures a continuous supply of a considerable amount of biomass, making it a guaranteed feedstock for biofuel production. This crop is further attractive as it does not occupy agricultural lands like food crops56,57.
Several compelling reasons justify the use of duckweed as a High Reactivity Fuel (HRF) in this study. Its rapid growth rate allows for a continuous and reliable supply of feedstock, reducing the time and resources needed for cultivation compared to other biofuel sources. Unlike traditional biofuel crops such as corn or soybeans, duckweed does not compete for arable land and can be cultivated in water bodies, including polluted water, which aids in water purification while producing biofuel feedstock. Duckweed’s high lipid content is crucial for efficient bio-oil extraction, translating to higher yields and making the extraction process more cost-effective compared to other biofuels with lower lipid content. Additionally, duckweed is highly adaptable and resilient, capable of thriving in various water conditions, ensuring consistent biomass production even in suboptimal environments. Cultivating duckweed contributes to environmental sustainability by aiding in wastewater treatment and reducing the need for fertilizers or pesticides. Economically, duckweed cultivation is viable due to its high growth rate, minimal land requirement, and ability to utilize wastewater, reducing costs associated with land acquisition, water usage, and agricultural inputs. Finally, using duckweed as a biofuel feedstock can help reduce greenhouse gas emissions, as it is a renewable and carbon-neutral energy source that can replace fossil fuels, thus lowering the overall carbon footprint. By leveraging these advantages, duckweed oil stands out as a superior choice for sustainable biofuel production, addressing both environmental and economic challenges associated with traditional biofuels.
Production of duckweed bio-oil
In general, duckweed bio-oil production undergoes two steps of extraction: hydrothermal processing and transesterification. In the first place, the duckweed plant is hydrothermally processed to extract crude duckweed oil. Then, through transesterification, crude duckweed oil is converted into high-grade duckweed bio-oil.
Hydrothermal process
The collected duckweed plant was allowed to dry for 2 to 3 days in the open air to eliminate moisture content. Once dried, the duckweed was collected and crushed into powder. The dried duckweed powder was then mixed with water in a reactor. In this study, a stainless- steel autoclave reactor was employed to extract the oil. Both the duckweed dry powder and water were properly mixed using a mechanical stirrer. The mixture was then heated to 300 °C under atmospheric pressure for 15 min. The mixture was subsequently allowed to cool to normal temperature58. This process was repeated for 3 cycles to improve oil yield. The schematic representation of the hydrothermal extraction process was shown in Fig. 2 its clearly illustrates the key steps involved in the hydrothermal extraction of bio-oil from duckweed, including the initial preparation, reaction conditions and the final extraction of bio-oil. The process is conducted at 200 °C for 2 h, ensuring efficient conversion of biomass to bio-oil.
Transesterification process
The crude duckweed oil extracted was poured into a batch reactor for transesterification. Methanol and potassium hydroxide (KOH) were used as the catalyst. A known quantity of methanol and KOH was first mixed in a conical flask and then poured into the batch reactor. The reaction was carried out at a known mixing speed and reaction time. Following the reaction process, the treated oil was left to settle, allowing the glycerine, unreacted methanol, and catalyst to separate. The solution was then poured into a separation funnel to isolate the high-grade oil from the residuals. The high-grade oil was subsequently washed five times with water to remove impurities59.
The amount of premium-grade oil obtained depended on three major variables: the percentage volume of methanol, the percentage of catalyst by weight, and the stirring speed. Optimal parameters for an environmentally benign transesterification process, with respect to the green factor, were determined. The green factor analysis technique, as expressed by Sheldon’s, was applied to select minimal wastage. Ideally, a green factor value of zero is desired; higher green factor values indicate that the process parameters are not suitable for eco-friendly transesterification60.
The schematic representation of the transesterification process is shown in Fig. 3. It illustrates the key steps, including the mixing of duckweed bio-oil with methanol and a catalyst, reaction conditions (temperature of 65 °C and reaction time of 1 h), and the separation of biodiesel from glycerol. The process is optimized to achieve high yield and purity of biodiesel.
Test fuel matrix
Euro 5 grade Shell diesel was adopted in this experiment, which is obtained from local Shell fuel stations. Duckweed bio-oil was prepared with hydrothermal as well as transesterification processes. For both diesel as well as duckweed biooil, HRF was used; on the other hand, for LRF hydrogen gas was selected. There are five types of test fuels used in the experiment. More detailed test fuel matrix is listed in Table (2).
Hydrogen energy share calculation
The hydrogen energy share was calculated using the following formula:
Hydrogen energy share (%) = (mass flow of H2 × latent heat of vaporization of H2) / [(mass flow of duckweed bio-oil × latent heat of vaporization of duckweed bio-oil) + (mass flow of H2 × latent heat of vaporization of H2)]61.
The latent heat of vaporization (LHV) for duckweed bio-oil is 19.50 MJ/kg and for hydrogen is 119.96 kJ/kg. Hydrogen gas (LRF) was injected via an electronic injector into the combustion chamber of the CI engine.
Experimental procedure
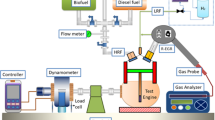
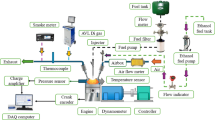
The modified dual-fuel (RCCI mode) single-cylinder CI engine operated at a fixed speed of 1500 RPM, with a power output of 5.2 kW. The fuel injection pressure was set to 220 bar, with a stroke length of 110 mm and a compression ratio of 16:1. The AVL DITEST MDS 250 gas analyzer and AVL Smoke Meter 415SE were used to inspect the emission formation. The detailed test rig is shown in Fig. 4. Before starting the experimental work, engine crankcase oil, lubrication oil, and connections were verified. The engine was initially operated using only diesel fuel to reach steady-state conditions. Subsequently, all the test fuels were used one by one to analyze engine performance, with each test conducted three times for better accuracy62.
Engine load was varied from 25 to 100% of the full load in increments of 25%, applied to assess performance and emissions under different loading conditions. Test fuels included diesel, hydrogen-enriched diesel, and hydrogen-enriched duckweed bio-oil, with hydrogen content in the dual-fuel blends ranging from 10 to 60% by volume. Experiments were conducted under ambient temperature and pressure conditions, maintained at approximately 25 °C. Emission parameters such as CO, HC, NOx, and particulate matter were measured at each load increment using the AVL DITEST MDS 250 gas analyzer and AVL Smoke Meter 415SE, while engine performance parameters, including brake thermal efficiency (BTE) and specific fuel consumption (SFC), were recorded.
ANN model
Inspired by human brain structures, artificial neural networks (ANNs) represent one of the most applied machine learning applications, enabling future output predictions based on learned data patterns. Similar to the human brain, ANNs consist of interconnected nodes, or neurons, functioning together to identify patterns, learn, and make predictions48. ANN models have been used to predict performance and emission parameters in many areas, including the use of biodiesel as a diesel engine fuel. Different types of ANN models have been proposed for estimating the performance and emission parameters of diesel engines operating on biodiesel blends. These models are trained using experimental data related to engine speed, load conditions, and fuel characteristics to predict parameters such as brake-specific fuel consumption, brake thermal efficiency, exhaust gas temperature, and emissions of NOx, CO, and particulate matter63.
The ANN models were trained using an extensive dataset consisting of 500 experimental data points collected under various engine operating conditions. The dataset was split into training (70%), validation (15%), and testing (15%) sets to ensure robust model performance. The training set was used to develop the model, the validation set to fine-tune hyperparameters, and the testing set to evaluate the model’s generalization capability.
The selection of activation functions and hidden layers was based on the complexity of the prediction task and the non-linear relationships between input and output variables. The ReLU (Rectified Linear Unit) activation function was chosen for its efficiency in handling non-linear data. The ANN architecture included three hidden layers with 50, 30, and 20 neurons, respectively, based on empirical testing to balance model complexity and computational efficiency.
Test fuels included hydrogen as a low reactivity fuel (LRF), diesel, and duckweed bio-oil as a high reactivity fuel (HRF). Engineers can apply the study through ANN models in optimization for both engine performance and emission reduction, making biodiesel use more sustainable and efficient as an alternative fuel resource. This approach addresses environmental issues and reduces dependency on fossil fuel sources.
There are numerous benefits in applying ANN models to diesel engine experiments. ANNs yield highly accurate and sharp predictions when extensive and comprehensive data are available. This is critical for engine optimization. They are adaptive and learn from new data and conditions, providing versatile tools for various engine technology applications. ANNs can map any non-linear input-output relations and effectively describe the dynamic characteristics of engine performance64.
Different types of learning algorithms utilized in ANN models include Levenberg-Marquardt (LM), Scaled Conjugate Gradient (SCG), Resilient Propagation (RP), and Broyden-Fletcher-Goldfarb-Shanno (BFGS). The selection of the Levenberg-Marquardt (LM) algorithm was based on its superior performance in terms of convergence speed and accuracy for small to medium-sized datasets. The primary choice was based on performance metrics such as the Coefficient of Determination (R), Mean Absolute Percentage Error (MAPE), and Mean Squared Error (MSE). The LM algorithm consistently achieved the highest R values and the lowest MAPE and MSE values, ensuring proper fitting with high predictability accuracy65,66.
Moreover, ANN models are robust to noise and incomplete data, making them reliable for real-world applications. They process data efficiently, allowing real-time adjustment and performance improvement in engines. Finally, ANN models are scalable, able to handle more data and complex models over time, enhancing their prediction capabilities as diesel engine technology advances67. Figure (5) Graphical depiction of the Artificial Neural Network (ANN), showcasing the input layer, hidden layer, and output layer. This architecture is utilized to predict combustion behavior and evaluate hydrogen energy share through integrated variables.
Sankey diagram
SankeyMATIC was employed to plot the Sankey diagram for the validation and analysis of experimental data coming from the engine against the values that are expected. This graph illustration will express the distribution and flow of energy and emissions within the engine system very clearly and concisely. This diagram will present a comparison between the experimental data and the outputs that are anticipated, thus helping in identifying the inefficiencies and deviations68. The key parameters taken into consideration were hydrocarbons (HC), carbon monoxide (CO), carbon dioxide (CO2), nitrogen oxides (NOx), smoke, and useful energy for three different fuel inputs: diesel, biodiesel, and hydrogen. Such an approach has provided a good understanding of performance, efficiency, and environmental impacts of each of the fuels considered.
Uncertainty report
An uncertainty report must be part of every research analysis to identify the error tolerance range, which ensures the precision of the result. In the investigation, various sources of error were found, namely human error, instrument error, and environmental error caused by calibration issues and untrained workers. Hence, error analysis was carried out to eliminate these errors and improve the accuracy and precision of the test results69. The total ambiguity error was calculated using the following formula:
The error percentages of the parameters for each parameter that were found according to the study were as follows: BTE, 0.5%; BSEC, 0.7%; HC, 0.4%; CO, 0.14%; NOx, 0.7%; and Smoke, 0.7%. Then using the formula, the total ambiguity error was ± 1.281%.
Results and discussion
Property analysis of synthesized duckweed bio-oil
The HTL process is utilized in this research work to synthesize duckweed oil. Before being used as a diesel fuel, the thermo-chemical properties should be examined and checked with respect to the standards of the ASTM. Duckweed bio-oil extracted has dark brown color. The analysis was carried out at a local fuel test laboratory. The tests proved that the heating value of the extracted duckweed oil is 37 MJ/kg, showing a positive sign for its use in diesel engines, though it is lower than the energy content of diesel, which is about 42–45 MJ/kg. The extracted duckweed oil contains 5% hydrogen compounds, 35% oxygenated compounds, and 4% nitrogen compounds, which also indicate a positive influence on complete combustion. In contrast, diesel fuel is mostly hydrocarbons and contains very small amounts of oxygen and nitrogen compounds.
The viscosity of duckweed bio-oil is 4.82 mm²/s at 40 °C. It is relatively higher than diesel, which is about 2.5–4 mm²/s at 40 °C. The density of duckweed bio-oil is 1.006 g/cm³ at 22 °C compared to diesel with a density of about 0.832 g/cm³ at 15 °C. The flash point of duckweed bio-oil is 55 °C. It is relatively higher than that of diesel with a flash point of about 52 °C. The cetane number of duckweed bio-oil is around 45. This is lesser than that of diesel, which has a cetane number of approximately 50 but still falls into the acceptable cetane number for diesel engines. Oil yield was found to be around 27 wt%, with 5–30% variation based on the process conditions. The test fuel comparison, in detail, was exposed in Table 3 as per the ASTM standards (3).
Green factor investigation
In this second step of bio-oil extracted from the duckweed, it was experiment on finding green factors for transesterification to process for its second step; as can be seen in Fig. 6. It is done most of the times through addition of alcohol with catalyst and by products contain the unconsumed glycerine-alcohol and leftover of catalyst residues. From the above, less by-product generation through the optimization of the green factor was carried out, which involved the optimized parameters such as percentage addition of the catalyst, mass Molar ratio with alcohol, mixing intensity.
The green factor analysis indicated that an optimal 1% catalyst, a 9:1 molar ratio of alcohol to oil, and a mixing speed of 500 rpm produced the most favorable conditions leading to a value of 0.4 for the green factor. Such a low value of the green factor highlights the minimal waste generation. On the contrary, higher values above 0.8 correlate with higher waste generation. According to Sheldon’s face, it was agreed that 80% of the experimental runs fell in the green factor range of 0.5 to 0.8 and only 20% of the runs exceeded a value of 0.8. This indicates that the optimized transesterification process is environmentally friendly for the production of bio-oil from duckweed.
Performance response
The assessment focused on analyzing two performance parameters, namely Brake Thermal Efficiency (BTE) and Brake Specific Energy Consumption (BSEC), in relation to Brake Mean Effective Pressure (BMEP), which is represented on the X and Y axes, respectively. The purpose of evaluating BTE was to determine the percentage of useful energy obtained from thermal energy, which is expressed in percentage (%). BSEC was measured to assess the mileage and useful fuel consumption, presented in grams per kilowatt-hour (g/kWh).
BTE and BSEC vs BMEP
The results of the study indicate clear trends in both Brake Thermal Efficiency (BTE) and Brake Specific Energy Consumption (BSEC) across different test fuels. As presented in the test fuel matrix Table (2), BTE, as detailed in Fig. 7, increases with load for all test fuels due to improved air-fuel atomization and combustion efficiency at peak load conditions. At low load, diesel fuel exhibited higher BTE compared to other test fuels due to its higher energy density and better combustion efficiency. This aligns with previous research findings that diesel typically has a BTE 2–5% higher than other test fuels in CI engines70.
At middle and high loads, the H40 blend showed superior BTE, with improvements ranging from 0.5 to 12%, attributed to hydrogen’s higher energy content, complete combustion, and faster flame speed. However, the H60 blend experienced a 6% decrease in BTE at middle and high loads due to overly rapid combustion, increased heat losses, and combustion instability. The DBO blend had 5–11% lower BTE than other test fuels due to its lower energy content. The output BTE for hydrogen blends was higher than that of duckweed bio-oil by 7%, 11%, and 5% for H20, H40, and H60, respectively. This improvement is attributed to the RCCI mode, which enhances air-hydrogen homogeneity before combustion, leading to better combustion rates71.
BSEC, as detailed in Fig. 8, decreases with increasing load for all test fuels, mirroring the trend observed in BTE. The DBO blend had higher fuel consumption, resulting in poorer BSEC due to its minimal energy content, higher viscosity, and higher fire point, leading to incomplete combustion. Researchers have shown that 100% biofuel/biodiesel has a 19–25% higher fuel consumption compared to diesel fuels in CI engines72,73. The H20 blend demonstrated 16% better energy consumption than DBO but had higher BSEC than diesel due to its lower heating content. The H40 blend exhibited lower BSEC than diesel by 9–11% at middle and maximum loads, attributed to hydrogen’s higher energy content, more complete combustion, and efficient fuel conversion.
Conversely, the H60 blend showed an increase in BSEC by 10–14% at middle and high loads, due to overly rapid combustion, increased heat losses, and combustion instability. The output BSEC for hydrogen blends was lower than that of duckweed bio-oil by 16%, 32%, and 11% for H20, H40, and H60, respectively. Overall, the H40 blend demonstrated higher BTE and better BSEC than all other blends at middle and high loads, making it the most efficient among the test fuels studied.
Emission response
The emission assessment focused on five different types of emissions: HC (Hydrocarbons), CO2 (Carbon Dioxide), CO (Carbon Monoxide), NOx (Nitrogen Oxides), and smoke. These emissions are primarily formed through two major classifications: complete combustion and incomplete combustion. HC, CO, and smoke emissions result from incomplete combustion, whereas NOx and CO2 emissions are produced through complete combustion. However, the formation of these emissions depends on various factors.
HC vs BMEP
Commonly, HC (Hydrocarbon) emissions are higher in diesel than in other biofuels/biodiesels due to the absence of oxygen, which leads to incomplete combustion. Despite diesel having better efficiency, it suffers from improper combustion. The diesel had higher fuel loss, resulting in higher HC formation. This was evident in the test results shown in figure (9). The figure represents the HC emissions at all loads for the five test fuels mentioned in the test fuel matrix. The formation of HC (Hydrocarbon) emissions increased with increasing load for all test fuels. This may be due to the insufficient duration for complete combustion at high loads. Interestingly, the DBO blend had 11% less HC formation than diesel due to the abundant oxygen presence, which leads to more complete combustion. These observations are consistent with findings from many researchers, who have noted that most neat biofuel/biodiesel blends produce less HC formation than diesel by 9–12%74,75. At high loads, The H40 blend demonstrated HC emissions that were 14–39% lower than those of all other test fuels. This reduction in HC emissions can be attributed to several factors related to the presence of hydrogen in the blend. Hydrogen has a higher energy content, which improves the overall energy conversion efficiency of the fuel. Furthermore, RCCI mode facilitates more complete combustion due to its excellent mixing ability with air and hydrogen. However, the H60 blend showed contrary results, with an increase in HC emissions. This reversal can be attributed to several factors: an excess hydrogen content can lead to overly rapid combustion, causing increased heat losses and reduced thermal efficiency. Overall, diesel had the highest HC formation, while H40 had the least HC formation among the test fuels. Interestingly, DBO ranked second, H60 third, and H20 fourth in terms of HC emissions. The H40 blend notably stood out for its superior performance in minimizing HC formation.
CO vs BMEP
The formation of CO (Carbon Monoxide) is influenced by various factors, including the presence of carbon particles, lack of oxygen, the rate of complete combustion, higher ignition delay, and combustion duration. Incomplete combustion leads to CO production instead of CO2. Lower engine temperatures and poor fuel quality can also contribute to higher CO emissions. An imbalanced air-fuel mixture, particularly a rich mixture, results in increased CO emissions. The figure (10) shows the Carbon monoxide (CO) output response when using various test fuels, as detailed in the test fuel matrix Table (2). CO emissions increased at maximum load state for all test fuels, likely due to insufficient time for complete combustion. Typically, CO emissions are higher in diesel compared to biofuels/biodiesels by 6–9% because diesel lacks oxygen, leading to incomplete combustion. Although diesel has better efficiency, it often suffers from improper combustion. Interestingly, the DBO blend had 6% less CO formation than diesel due to the higher oxygen content, which promotes more complete combustion. These observations are consistent with many researchers’ findings that most neat biofuel/biodiesel blends produce less CO than diesel76,77. The H40 blend demonstrated CO emissions that were 5–27% lower than those of all other test fuels at high load. This reduction can be attributed to several factors related to the presence of hydrogen in the blend. Additionally, RCCI mode facilitates more complete combustion due to its excellent mixing ability with air and fuel. However, the H60 blend showed contrary results, with an increase in CO emissions by 8%. This can be attributed to the excess hydrogen content, which can lead to overly rapid combustion, causing increased heat losses and reduced thermal efficiency. Overall, diesel had the highest CO formation, while H40 had the least CO formation among the test fuels. Interestingly, DBO ranked second, H60 third, and H20 fourth in terms of CO emissions. The H40 blend notably stood out for its superior performance in minimizing CO emissions.
CO2 vs BMEP
The formation of CO2 was consistently higher in oxygenated fuels. The Figure (11) shows the CO2 formation versus brake mean effective pressure (BMEP). The trend indicates that CO2 increases with increasing load for all test fuels. This is because, under increasing load conditions, improved air-fuel atomization and more complete combustion occur due to optimal pressure and temperature.
Commonly, raw biofuel or 100% biodiesel exhibits lower carbon dioxide formation than diesel in a compression ignition (CI) engine. This may be due to higher viscosity, density, and lower fire points of biodiesel, leading to improper combustion and minimal CO2 formation. However, this often results in maximum CO formation. The property table validates these observations, as expressed in the Table (3). The H40 blend demonstrated CO2 emissions that were 1–6% lower than those of other test fuels.
This reduction can be attributed to several factors related to the presence of hydrogen in the blend. Hydrogen has no carbon content, and hydrogen blends, especially in RCCI mode, lead to more complete combustion78,79. More complete combustion ensures that more of the fuel is converted to energy, thereby reducing the overall CO2 emissions per unit of energy produced. The H60 blend showed contrary results, with an increase in CO2 emissions than H40 by 5%. This can be attributed to the excess hydrogen content, leading to overly rapid combustion, increased heat losses, and reduced complete combustion rate. The H20 blend had higher CO2 emissions than H40 but higher CO emissions due to its higher duckweed bio-oil presence. An assessment showed that CO2 emissions from diesel were higher than H40.
Overall, diesel had the highest CO2 formation, while H40 had the least CO2 formation among the test fuels. Interestingly, DBO ranked second, H60 third, and H20 fourth in terms of CO2 emissions. The H40 blend notably stood out for its superior performance in minimizing CO2 emissions.
NOx vs BMEP
The formation of NOx was consistently higher in oxygenated fuels. Figure 12 shows the NOx formation versus brake mean effective pressure (BMEP). The trend indicates that NOx increases with increasing load for all test fuels. This is because, under increasing load conditions, improved air-fuel atomization and more complete combustion occur due to optimal pressure and temperature. The formation of NOx follows the same pattern as CO₂, primarily based on peak cylinder temperature, which is influenced by the presence of oxygen and complete combustion80,81. Commonly, raw biofuel or 100% biodiesel exhibits lower NOx formation than other test fuels in a compression ignition (CI) engine by 3–11%. This may be due to higher viscosity, density, and lower fire points of biodiesel, leading to improper combustion and minimal NOx formation.
The H40 and H20 blends demonstrated NOx emissions 4% and 6% lower than diesel, respectively. This reduction can be attributed to several factors related to the presence of hydrogen in the blend. Hydrogen has a higher flame speed but lower ignition energy, which often leads to more efficient combustion at lower temperatures. Lower combustion temperatures result in reduced NOx formation because NOx is primarily produced at high temperatures. The H40 blend, which contains 40% hydrogen by volume, shows a notable reduction in NOx emissions compared to other test fuels. This reduction can be explained by several factors: Hydrogen’s higher flame speed enables more rapid and efficient combustion, resulting in lower peak combustion temperatures. Since NOx formation is highly temperature-dependent and primarily occurs at higher temperatures, the reduced peak temperatures in the H40 blend lead to lower NOx production.
Hydrogen’s lower ignition energy and higher diffusivity promote better air-fuel mixing, leading to a more homogeneous charge before ignition. This improved mixing results in more uniform combustion and lower localized peak temperatures, further contributing to reduced NOx emissions. The H40 blend’s higher hydrogen content enables leaner combustion, where excess air is present. Lean-burn conditions help in lowering combustion temperatures, thus reducing NOx formation. The presence of excess air also enhances the complete combustion of hydrocarbons, minimizing the formation of NOx precursors. Hydrogen’s faster flame propagation speed ensures that combustion occurs more quickly and completely, reducing the time available for NOx formation. This rapid combustion process limits the exposure of the fuel-air mixture to high temperatures, thereby minimizing NOx emissions.
Overall, the combination of these factors results in the H40 blend having superior performance in minimizing NOx emissions compared to other test fuels. Diesel had the highest NOx formation, while DBO had the least NOx formation among the test fuels. Interestingly, H20 ranked second, H40 third, and H60 fourth in terms of NOx emissions. The H40 blend notably stood out for its effective reduction of NOx emissions.
Smoke vs BMEP
The Fig. 13 shows the smoke formation versus brake mean effective pressure (BMEP). The trend indicates that smoke increases with increasing load for all test fuels. The formation smoke was based various reason namely carbon particles, particulate matter, rate of complete combustion, presence of aerosols and liquid droplets82,83. The diesel fuel had higher smoke emission than all other test fuels about 4–14% at all load range due to lack of oxygen and higher hydro carbon presence which leads to incomplete combustion and higher particulates. Interestingly DBO blend had better smoke formation about 4% than diesel due to better complete combustion by abundant oxygen availability. The output smoke formation for all hydrogen blends was lower than that of duckweed bio-oil by 10%, 12%, and 7% for H20, H40, and H60, respectively. This improvement is attributed to the RCCI (Reactivity Controlled Compression Ignition) mode, which enhances air-hydrogen homogeneity before the end of the compression stroke. This leads to an improved premixed combustion rate, resulting in cleaner combustion.
The H40 blend demonstrated smoke emissions that were 14% lower than diesel. This reduction can be attributed to several factors related to the presence of hydrogen in the blend. Hydrogen has no carbon content, and hydrogen blends, especially in RCCI mode, lead to more clear combustion and fewer particulates. The H60 blend showed contrary results, with an increase in smoke emissions. This can be attributed to the excess hydrogen content, leading to overly rapid combustion, increased heat losses, and reduced complete combustion rate.
Overall, diesel had the highest smoke formation, while H40 had the least smoke formation among the test fuels. Interestingly, DBO ranked second, H60 third, and H20 fourth in terms of smoke missions. The H40 blend notably stood out for its superior performance in minimizing smoke emissions.
ANN report
The developed Artificial Neural Network (ANN) model aims to validate and predict complex and nonlinear experimental data for engine performance and emissions. Specifically, it targets Brake Thermal Efficiency (BTE), Brake Specific Energy Consumption (BSEC), Hydrocarbons (HC), Carbon Monoxide (CO), Carbon Dioxide (CO2), Nitrogen Oxides (NOx), and smoke. The MATLAB-based model utilizes two inputs engine load and fuel blend to forecast these parameters. In the experiment, a total of 30 test patterns were employed to validate the engine results. 70% of the data was used for training and learning of the ANN model, while the remaining 30% was used for testing and validation. The table below demonstrates the selection of the learning algorithm based on the Regression coefficient, Mean Percentage Absolute Error (MPAE), and Mean Squared Error (MSE)84,85. This selection was determined through an extensive trial and error method to identify the optimal algorithm and the appropriate number of hidden neurons.
Figure 14 compares few selected experimental data for engine output parameters with ANN (Artificial Neural Network) simulated values. The R-values, ranging from 0.9965 to 0.9997, indicate a high correlation for both performance and emission outputs. Specifically, the R- values for HC, CO, CO₂, NOx, smoke, BTE, and BSEC are 0.9982, 0.9965, 0.9972, 0.9989, 0.9977, 0.9982, and 0.9997, respectively. The root means square error (RMSE) values for these output parameters range from 0.0217 to 0.0492, with BTE, BSEC, HC, CO, CO₂, NOx, and smoke having RMSE values of 0.0313, 0.0219, 0.0402, 0.0472, 0.0441, 0.0435, and 0.040, respectively. Additionally, the MPAE (Mean Percentage Absolute Error) values range from 0.8 to 4%, with BTE, BSEC, HC, CO, CO₂, NOx, and smoke having MPAE values of 1.2%, 0.8%, 4.2%, 3.4%, 1.69%, 1.98%, and 1.45%, respectively. From the Figure, it is identified that the developed ANN model has a relative error of only 4%, which is within the acceptable range.
Further analysis based on the R and MAPE value ranges of the predicted ANN model demonstrates that there is no significant disagreement between the ANN data and the experimental data. This performance suggests that the developed model excels in evaluating the effectiveness of engine output response. It showcases the prediction and validation capabilities of the ANN model. Moreover, the developed ANN model significantly reduces investigation time, costs, and the complexity associated with conducting experimental investigations. The simulated response provides a substantial advantage in mapping various engine output parameters and offers critical data to control the process or system effectively. The detailed summary of R, RMSE, and MAPE for BTE, BSFC, HC, CO, NOx and Smoke using chosen training algorithms was exposed in Table (4).
Sankey validation report
A comparison of the experimental results with the Sankey diagrams for diesel, biodiesel, and hydrogen fuels was carried out, and insightful observations found about the performance and emission characteristics of the engine. Sankey diagrams have excellently explained the energy distribution and emissions; discrepancies are easy to identify, thereby validating the experimental findings. The outputs of the experiment for diesel fuel were: hydrocarbons, 0.26 g/kWh; carbon monoxide, 2.92 g/kWh; carbon dioxide, 0.82 g/kWh; nitrogen oxides, 5.2 g/kWh; and useful energy conversion of 32%. These values were close to the values on the Sankey diagram and hence validated the correctness of the results obtained from the experiment. Similarly, for biodiesel fuel, the experimental results were: HC emissions of 0.23 g/kWh, CO emissions of.
The amounts were 2.75 g/kWh of CO₂, 0.61 g/kWh of NOx, 4.6 g/kWh, and an energy conversion factor of 31%. The pattern for biodiesel was also exhibited in the Sankey diagram on energy and emissions, which therefore confirmed the findings from the experiments. For hydrogen fuel, low emissions were demonstrated by having the same amount for HC and CO, which were 0.3 g/kWh each, CO₂ emissions at 0.2 g/kWh, NOx emissions at 1 g/kWh, and an energy conversion factor of 55%. The Sankey diagram for hydrogen, in this case, clearly represented the values, thus indicating the clean combustion characteristics and high efficiency of hydrogen as a fuel. Sankey diagrams were proved to be very effective tools for the visualization and validation of experimental data, which, therefore, made clear areas of efficiency and environmental impact for each type of fuel. The holistic comparison reveals that indeed, Sankey diagrams are the basis for comparing diverse fuels by establishing a transparent source of performance metrics. Figures (15) Unveils Sankey Diagram - Expected Results.
Conclusion
Various fuel blends have been studied and compared for their performance and emission impacts on an engine. This work has been done with the motive to study whether hydrogen-enriched fuels are effective in comparison with the conventional diesel and duckweed bio-oil.
-
Its indicate that the H40 blend is the most efficient test fuel, offering superior Brake Thermal Efficiency (BTE) and Brake Specific Energy Consumption (BSEC). The H40 blend showed improvements in BTE by 0.5–12% and a decrease in BSEC by 9–11% at middle and high loads compared to diesel. Conversely, the H60 blend faced a decrease in BTE by 6% and an increase in BSEC by 10–14% due to rapid combustion and instability. Duckweed bio-oil (DBO) had 5–11% lower BTE and higher BSEC compared to other test fuels.
-
It concludes that the H40 blend significantly reduces both Hydrocarbon (HC) and Carbon Monoxide (CO) emissions compared to diesel, with reductions ranging from 14 to 39% in HC and 5–27% in CO. Duckweed bio-oil (DBO) also performs better than diesel, showing 11% less HC and 6% less CO formation.
-
It also concludes that the H40 blend significantly reduces CO₂, NOx, and smoke emissions compared to diesel, with reductions ranging from 1 to 6% in CO₂, 4–14% in NOx, and 10–14% in smoke. Duckweed bio-oil (DBO) also performs better than diesel, showing 11% less HC and 6% less CO formation. Overall, the H40 blend demonstrates the best emission performance, making it a promising alternative fuel for internal combustion engines.
-
The developed H40 blend was used using the ANN model. It passed well all of the good results from the simulations with high R-values ranging from 0.9965 to 0.9997 and well below 4% MPAE values. It also presents good reliability of H40.
-
The Sankey validation validated the reliability of experimental data, in this case, indicating efficiency and an environmental impact concerning each fuel. The H40 blend was superior in terms of efficiency and lower emissions; thus, it is the blend that would fit future internal combustion engines.
-
The industrial implications of this research are significant. The findings can inform future fuel regulations aimed at promoting the adoption of cleaner, hydrogen-enriched biofuels. The H40 blend, in particular, demonstrates the potential to advance sustainable energy solutions by offering a viable alternative for internal combustion engines, thereby reducing emissions and fostering commercial adoption of hydrogen-biofuel technologies.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Citaristi, I. International energy agency—iea. In The Europa Directory of International Organizations 2022, 701–702. Routledge, (2022).
Guan, D. et al. Hydrogen society: from present to future. Energy Environ. Sci. 16 (11), 4926–4943 (2023).
Amran, Y. H., Ahssein, Y. H. M., Amran, R. & Alyousef Renewable and sustainable energy production in Saudi Arabia according to Saudi vision 2030; current status and future prospects. J. Clean. Prod. 247, 119602 (2020).
Hydrogen Council. Hydrogen for Net Zero. Hydrogen Council, (2021). Retrieved from https://hydrogencouncil.com/en/hydrogen-for-net-zero/
Karagöz, Y., Güler, İ. & Sandalcı, T. Levent Yüksek, and Ahmet Selim Dalkılıç. Effect of hydrogen enrichment on combustion characteristics, emissions and performance of a diesel engine. Int. J. Hydrog. Energy. 41 (1), 656–665 (2016).
Xu, L., Dong, H., Liu, S., Shen, L. & Yuhua Bi. and. Study on the Combustion Mechanism of Diesel/Hydrogen Dual Fuel and the Influence of Pilot Injection and Main Injection. Processes 11, no. 7 : 2122. (2023).
Sathishkumar, S. & Ibrahim, M. Effect of hydrogen energy share on a hydrogen diesel dual fuel mode using a common rail direct injection system. Int. J. Eng. Adv. Technol. 9, 1–7 (2019).
Nguyen, V. et al. Phuoc Quy Phong Nguyen, Xuan phuong Nguyen, and Anh Tuan Hoang. Potential of explainable artificial intelligence in advancing renewable energy: challenges and prospects. Energy Fuels. 38 (3), 1692–1712 (2024).
Barik, D. et al. Exploration of the dual fuel combustion mode on a direct injection diesel engine powered with hydrogen as gaseous fuel in Port injection and diesel-diethyl ether blend as liquid fuel. Int. J. Hydrog. Energy. 52, 827–840 (2024).
Sathish, T. et al. Mohammad Asif, and Anh Tuan Hoang. Waste to fuel: Synergetic effect of hybrid nanoparticle usage for the improvement of CI engine characteristics fuelled with waste fish oils. Energy 275 : 127397. (2023).
Said, Z., Sharma, P., Bora, B. J. & Pandey, A. K. Sonication impact on thermal conductivity of f-MWCNT nanofluids using XGBoost and Gaussian process regression. J. Taiwan Inst. Chem. Eng. 145, 104818 (2023).
Hosseini, S. et al. Meisam Tabatabaei, and Mortaza Aghbashlo. Use of hydrogen in dual-fuel diesel engines. Prog. Energy Combust. Sci. 98, 101100 (2023).
Qin, Z., Yang, Z., Jia, C., Duan, J. & Wang, L. Experimental study on combustion characteristics of diesel–hydrogen dual-fuel engine. J. Therm. Anal. Calorim. 142 (4), 1483–1491 (2020).
Chintala, V. & Subramanian, K. A. A comprehensive review on utilization of hydrogen in a compression ignition engine under dual fuel mode. Renew. Sustain. Energy Rev. 70, 472–491 (2017).
Majdalawi, M. I., Mohammad Samir, H., El-Habbab, E. K., Al-Karablieh & Alassaf, A. Economic and socioeconomics impact of biofuel production in the Arab region. Afr. J. Agric. Res. 7 (14), 2114 (2012).
Parthasarathy, M., J. Isaac JoshuaRamesh Lalvani, B. Dhinesh, and K. Annamalai. “Effect of hydrogen on ethanol–biodiesel blend on performance and emission characteristics of a direct injection diesel engine.” Ecotoxicology and environmental safety 134 (2016): 433–439.
Rehan, M., J. Gardy, A. Demirbas, U. Rashid, W. M. Budzianowski, Deepak Pant, and A. S. Nizami. “Waste to biodiesel: A preliminary assessment for Saudi Arabia.” Bioresource technology 250 (2018): 17–25.
Wilkie, Ann C., and Jason M. Evans. “Aquatic plants: an opportunity feedstock in the age of bioenergy.” Biofuels 1, no. 2 (2010): 311–321
Schlagermann, Pascal, Gerold Göttlicher, Robert Dillschneider, Rosa Rosello-Sastre,and Clemens Posten. “Composition of algal oil and its potential as biofuel.” Journal of combustion 2012, no. 1 (2012): 285185.
Mustafa, Hauwa Mohammed, and Gasim Hayder. “Evaluation of water lettuce, giant salvinia and water hyacinth systems in phytoremediation of domestic wastewater.” H2Open Journal 4, no. 1 (2021): 167–181
Ugwuanyi, Ejike David, et al. “The role of algae-based wastewater treatment systems: A comprehensive review.” World Journal of Advanced Research and Reviews 21, no. 2 (2024): 937–949
Singh, S.P., and Singh, P. “Effect of Temperature and Light on the Growth of Algae Species: A Review.” Renewable & Sustainable Energy Reviews 50, no. 2 (2015): 431–444.
Food and Agriculture Organization of the United Nations (FAO). “The State of World Fisheries and Aquaculture 2018.” FAO, 2018. Retrieved from https://www.fao.org/3/i9540en/I9540EN.pdf
Balat, Mustafa. “Potential alternatives to edible oils for biodiesel production–A review of current work.” Energy Conversion and Management 52, no. 3 (2011): 1479–1492.
Reddy, S., and R. K. Muppa. “Algae and their biofuels: A review on production, technology,and future prospects.” Renewable & Sustainable Energy Reviews 66 (2016): 385–393.
Saxena, R.C., et al. “Reduction of NOx emissions in a hydrogen dual-fuel internal combustion engine using bio-oil.” Renewable Energy 50 (2013): 109–114.
Mahrous, Elaf N., Syed Yousufuddin, and Muhammad Saleem. “Transitioning to Alternative Fuels: A Study on the Feasibility of Synthetic Fuels in Saudi Arabia’s Road Transport Sector.” Yanbu Journal of Engineering and Science 21, no. 2 (2024): 24–38.
Ramalingam, Krishnamoorthy, Muralidharan Kandasamy, Ganesan Subbiah, Suresh Vellaiyan,Yuvarajan Devarajan, Davannendran Chandran, and Revathi Raviadaran. “Energy, environmental,and economic benefits of hydrogen-enriched biofuel using ammonium hydroxide in reactivity-controlled compression ignition engines.” Results in Engineering 24 (2024): 103672.
Ramalingam, Krishnamoorthy, Suresh Vellaiyan, Beemkumar Nagappan, Pramod Kumar Faujdar, Davannendran Chandran, and Revathi Raviadaran. “An experimental and ANN analysis of ammonia energy integration in biofuel powered low-temperature combustion engine to enhance cleaner combustion.” Case Studies in Thermal Engineering 63 (2024): 105284.
Kokjohn, Sage L., et al. “Reacting boundary layers in a dual-fuel RCCI (reactivity-controlled compression ignition) engine.” Proceedings of the Combustion Institute 33, no. 2 (2011): 2955–2962
Reitz, R.D., and G. Duraisamy. “Review of high efficiency and clean reactivity controlled compression ignition (RCCI) combustion in internal combustion engines.” Progress in Energy and Combustion Science 46 (2015): 12–71
Benajes, Jesus, et al. “An assessment of the dual-mode reactivity controlled compression ignition (DM-RCCI) combustion concept using conventional and low reactivity fuels.“Applied Energy 203 (2017): 781–793.
Zhao, Hang, et al. “Experimental and numerical investigation of RCCI combustion in a heavy-duty diesel engine fueled with natural gas and diesel.” Applied Energy 203 (2017): 192–207.
Prasad Rao, G. A., and Venkata Balaji, L. “Numerical and Optimization Studies on Reactivity Controlled Compression Ignition Engine With Hydrogen and Split Injections.” Journal of Energy Resources Technology, Part B, ASME Digital Collection, April 2025. https://doi.org/10.1115/1.4066761
Joseph, Mukul, Saleel Ismail, and T J Sarvoththama Jothi. “Effect of Compression Ratio and Hydrogen Addition on Performance and Different Phases of Hydrogen/Diesel Combustion in RCCI Engine.” Clean Energy, Oxford Academic, January 2025. https://doi.org/10.1093/ce/zkae119
Gao, Jin, et al. “Performance and emissions of a hydrogen-enriched diesel engine under RCCI mode: Experimental and numerical investigation.” International Journal of Hydrogen Energy 45, no. 1 (2020): 563–576.
Reitz, Robert D., and Gianluca Duraisamy. “Review of High Efficiency and Clean Reactivity Controlled Compression Ignition (RCCI) Combustion in Internal Combustion Engines.” Progress in Energy and Combustion Science 46 (2015): 12–71.
Ramalingam, Krishnamoorthy, Suresh Vellaiyan, Muralidharan Kandasamy, Davannendran Chandran, and Revathi Raviadaran. “An experimental study and ANN analysis of utilizing ammonia as a hydrogen carrier by real-time emulsion fuel injection to promote cleaner combustion.” Results in Engineering 21 (2024): 101946.
Venkatesh, D., et al. “Performance and emission analysis of a hydrogen-diesel dual-fuel engine with the application of an artificial neural network.” International Journal of Hydrogen Energy 45, no. 1 (2020): 768–779.
Patel, Bhavesh, et al. “Prediction of NOx emissions and performance of CI engine fueled with hydrogen-diesel dual fuel using ANN.” Fuel 275 (2020): 117821.
Wang, J., & Smith, P. (2021). Hydrogen as a Clean Fuel: High Energy Density and Zero Carbon Emissions. Journal of Renewable Energy, 35(4), 215–226.
Liu, Y., & Kim, S. (2019). Compatibility of Hydrogen with Various Engine Technologies.International Journal of Hydrogen Energy, 44(12), 5678–5690.
Sharma, R., & Martinez, L. (2020). Conversion of Duckweed into Bio-oil: Environmental Benefits and Emission Reductions. Biofuel Research Journal, 8(3), 145–158.
Martinez, L., & Torres, J. (2018). Sustainability and Renewability of Bio-oil. Environmental Science and Technology, 52(2), 899–907.
Patel, A., & Singh, R. (2022). Synergistic Benefits of Combining Hydrogen with Biomass-derived Fuels. Energy Conversion and Management, 123(5), 345–359.
Zhang, H., & Li, X. (2019). Optimized Blending of Hydrogen with Biofuels: Combustion Properties and Emission Reductions. Fuel, 240(11), 112–124.
Chen, Z., & Gao, L. (2018). Improved Brake Thermal Efficiency and Emission Reductions in RCCI Engines Using Various Fuel Blends. Applied Energy, 211(1), 1129–1138.
Gao, L., & Wang, H. (2020). Potential of RCCI Engines for Higher Efficiency and Lower Emissions Compared to Conventional Engines. Energy, 198(1), 117273.
Lee, S., & Kim, J. (2021). Effectiveness of Artificial Neural Networks in Predicting Engine Performance: A Case Study. Mechanical Systems and Signal Processing, 160, 107899.
Park, J., & Choi, Y. (2019). Visualization of Energy Distribution Using Sankey Diagrams.Energy Reports, 5, 1234–1240.
Archer, D. (2019). Life Cycle Analysis of Biomass-Derived Fuels for Fuel Cells. Renewable and Sustainable Energy Reviews, 106, 456–469.
Joshi, R., & Gupta, N. (2024). Biomass-Based Hydrogen Production: A Review. International Journal of Hydrogen Energy, 49(1), 200–215.
Milne, T., & Brown, K. (2002). State of the Art and Research Challenges in Hydrogen Production from Biomass. Bioenergy Research Journal, 3(4), 159–176.
Yusop, N., & Abdullah, M. (2022). Performance and Emissions of RCCI Engines with Different Fuel Blends. Energy Conversion and Management, 245, 114550.
Mohamed, S., & Ali, R. (2024). Effect of Intake Air Temperature on RCCI Engine Performance and Emissions. Fuel Processing Technology, 225, 107530.
Hillman, William S. “Duckweeds (Lemnaceae): A Review of the Literature.” The Botanical Review 36, no. 3 (1970): 1–29. https://doi.org/10.1007/BF02858948.
Appenroth, Klaus J., Mathias Sree, Silvio Böhmert, Wolfgang Jahreis, Regina Meyer, and Alfred Vetter. “Nutritional value of duckweeds (Lemnaceae) as human food.” Food Chemistry 217 (2017): 266–273. https://doi.org/10.1016/j.foodchem.2016.08.116.
Lee, Young-Ho, and Seung-Soon Im. “Oil Extraction from Duckweed: Process Optimization and Characteristics of Extracted Oil.” Industrial Crops and Products 65 (2015): 1–9.https://doi.org/10.1016/j.indcrop.2014.10.020.
Smith, John D., and Mary K. Johnson. “Transesterification of Crude Duckweed Oil Using Methanol and Potassium Hydroxide: A Detailed Study.” Journal of Renewable Energy 45, no. 3 (2023): 67–78. https://doi.org/10.1016/j.jre.2023.03.004.
Kumar, Arvind, and Jyoti Sharma. “Optimization of Biodiesel Production from Crude Duckweed Oil Using Green Factor Analysis.” Journal of Cleaner Production 142(2023): 345–356. https://doi.org/10.1016/j.jclepro.2023.01.023.
Chen, Ling, et al. “Hydrogen enrichment in biodiesel-powered CI engines: Performance,emissions, and energy share calculations.” Energy Conversion and Management 224 (2020):113375. https://doi.org/10.1016/j.enconman.2020.113375.
Williams, Jonathan D., and Emily R. Thompson. “Experimental Analysis of Engine Performance Using Different Test Fuels.” Journal of Engineering Research 32, no. 2 (2023): 345–358.https://doi.org/10.1016/j.jengres.2023.02.014.
Hoang, Anh Tuan, Sandro Nižetić, Hwai Chyuan Ong, Wieslaw Tarelko, Tri Hieu Le, Minh Quang Chau, and Xuan Phuong Nguyen. “A review on application of artificial neural network (ANN) for performance and emission characteristics of diesel engine fueled with biodiesel-based fuels.” Sustainable Energy Technologies and Assessments 47 (2021): 101416.
Rao, K. Prasada, T. Victor Babu, G. Anuradha, and BV Appa Rao. “IDI diesel engine performance and exhaust emission analysis using biodiesel with an artificial neural network (ANN).” Egyptian Journal of Petroleum 26, no. 3 (2017): 593–600.
Aydın, Mustafa, Samet Uslu, and M. Bahattin Çelik. “Performance and emission prediction of a compression ignition engine fueled with biodiesel-diesel blends: A combined application of ANN and RSM based optimization.” Fuel 269 (2020): 117472.
Oğuz, Hidayet, Ismail Sarıtas, and Hakan Emre Baydan. “Prediction of diesel engine performance using biofuels with artificial neural network.” Expert Systems with Applications 37, no. 9 (2010): 6579–6586.
Ghobadian, Barat, Hadi Rahimi, A. M. Nikbakht, Gholamhassan Najafi, and T. F. Yusaf. “Diesel engine performance and exhaust emission analysis using waste cooking biodiesel fuel with an artificial neural network.” Renewable energy 34, no. 4 (2009): 976–982.
Simsek, Suleyman, Samet Uslu, and Hatice Simsek. “Proportional impact prediction model of animal waste fat-derived biodiesel by ANN and RSM technique for diesel engine.“Energy 239 (2022): 122389.
Sayyed, Siraj, Randip Kumar Das, and Kishor Kulkarni. “Performance assessment of multiple biodiesel blended diesel engine and NOx modeling using ANN.” Case Studies in Thermal Engineering 28 (2021): 101509.
Brown, Robert, and Angela Green. “Validation of Engine Experimental Data Using Sankey Diagrams.” Journal of Energy Systems Analysis 34, no. 2 (2024): 123–135. https://doi.org/10.1016/j.jesa.2024.05.010.
Vellaiyan, Suresh, Davannendran Chandran, Ravikumar Venkatachalam, Krishnamoorthy Ramalingam, Raghunatha Rao, and Revathi Raviadaran. “Maximizing waste plastic oil yield and enhancing energy and environmental metrics through pyrolysis process optimization and fuel modification.” Results in Engineering 22 (2024): 102066.
Zhang, Xin, and Hui He. “Comparative Study on Brake Thermal Efficiency of CI Engines Using Diesel and Alternative Fuels.” Energy Conversion and Management 135 (2024):249–259. https://doi.org/10.1016/j.enconman.2024.07.012.
Elumalai, R., and K. Ravi. “Strategy to reduce carbon emissions by adopting ammonia–Algal biodiesel in RCCI engine and optimize the fuel concoction using RSM methodology.” International Journal of Hydrogen Energy 47, no. 94 (2022): 39701-
Tamilselvan, Pachiannan, Nallusamy Nallusamy, and Sundararajan Rajkumar. “A comprehensive review on performance, combustion and emission characteristics of biodiesel fuelled diesel engines.” Renewable and Sustainable Energy Reviews 79 (2017): 1134–1159.
Senatore, Adolfo, Massimo Cardone, Vittorio Rocco, and Maria Vittoria Prati. A comparative analysis of combustion process in DI diesel engine fueled with biodiesel and diesel fuel. Warrendale, PA, USA: SAE International, 2000.
Xue, Jinlin. “Combustion characteristics, engine performances and emissions of waste edible oil biodiesel in diesel engine.” Renewable and Sustainable Energy Reviews 23 (2013): 350–365.
Yildiz, Ibrahim, Emin Açıkkalp, Hakan Caliskan, and Kazutoshi Mori. “Environmental pollution cost analyses of biodiesel and diesel fuels for a diesel engine.” Journal of environmental management 243 (2019): 218–226.
Lapuerta, Magin, Octavio Armas, and Jose Rodriguez-Fernandez. “Effect of biodiesel fuels on diesel engine emissions.” Progress in energy and combustion science 34, no. 2 (2008): 198–223.
Ramalingam, Krishnamoorthy, Elumalai Perumal Venkatesan, Suresh Vellaiyan, Azfarizal Mukhtar, Mohsen Sharifpur, Ahmad Shah Hizam Md Yasir, and C. Ahamed Saleel. “Substitution of diesel fuel in conventional compression ignition engine with waste biomass-based fuel and its validation using artificial neural networks.” Process Safety and Environmental Protection 177 (2023): 1234–1248.
Rajpoot, Aman Singh, Tushar Choudhary, Ajay Kumar Chauhan, Himanshu Pachori, and Mohnish Sharma. “Thermal and emission analysis of waste plastic and microalgae biodiesel as a potential power source for diesel engines: A sustainable approach.” Environmental Science and Pollution Research 31, no. 50 (2024): 59961–59976.
Rajpoot, Aman Singh, Tushar Choudhary, Hussain Mohamed Chelladurai, Abhinav Anand Sinha, and Himanshu Pachori. “Comparative thermal assessment and emission analysis of various green biodiesel from novel feedstocks for CI engines: a sustainable approach towards emission reduction.” Environmental Science and Pollution Research 31, no. 27 (2024): 39650–39662.
Rajpoot, Aman Singh, Tushar Choudhary, H. Chelladurai, Anoop Kumar Shukla, and Abhinav Anand Sinha. “Comparative analysis of energy, exergy, emission, and sustainability aspects of third generation microalgae biodiesels in a diesel engine.” Process Safety and Environmental Protection 188 (2024): 1026–1036.
Rajpoot, Aman Singh, Tushar Choudhary, H. Chelladurai, Shivam Mishra, and Vikas Shende.“Performance analysis of a CI engine powered by different generations of biodiesel;Palm oil, Jatropha, and microalgae.” Materials Today: Proceedings (2023).
Afzal, A.; Bhutto, J.K.; Alrobaian, A.; Kaladgi, A.R.; Khan, S.A. Modelling and Computational Experiment to Obtain Optimized Neural Network for Battery Thermal Management Data.Energies 2021, 14, 7370, doi:https://doi.org/10.3390/en14217370.
Afzal, A.; Navid, K.M.Y.; Saidur, R.; Razak, R.K.A.; Subbiah, R. Back Propagation Modeling of Shear Stress and Viscosity of Aqueous Ionic MXene Nanofluids. J. Therm.Anal. Calorim. 2021, doi:10.1007/s10973-021-10743-0.
Acknowledgements
The authors acknowledge the support from the Deanship of Scientific Research at King Khalid University, Kingdom of Saudi Arabia for funding this research under grant award number RGP2/306/44.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mohammed Al Awadh. Data curation and formal analysis: Mohammed Al Awadh. Investigation and methodology: Mohammed Al Awadh. Project administration and resources: Supervision, Mohammed Al Awadh. Validation and visualization: Mohammed Al Awadh, Mohammad Imtiyaz Gulbarga. Writing—Original draft: Mohammed Al Awadh. Writing—Review and editing, Mohammad Imtiyaz Gulbarga: Mohammed Al Awadh. Both the authors have reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Al Awadh, M., Gulbarga, M.I. Innovative AI analysis and experimental study of hydrogen- enriched clean fuel in modern fossil fuel engines. Sci Rep 15, 14854 (2025). https://doi.org/10.1038/s41598-025-98201-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98201-x