Abstract
Our study aimed to develop a nomogram to predict overall survival (OS) at 1, 3, and 5 years for patients with primary renal neuroendocrine tumor (PRNET). The Surveillance, Epidemiology, and End Results database (2000–2021) was utilized to gather cases and extract data. We performed a multivariate analysis using a Cox proportional-hazards model to identify prognostic factors independently affecting OS. Based on these predictors, a nomogram was constructed and validated internally via a bootstrap resampling method. Finally, we included 266 PRNET patients. The multivariate analysis demonstrated that age, Fuhrman grade, surgery, summary stage, N stage, and histology were prognostic factors independently affecting OS (all P < 0.05). A nomogram was then constructed using the abovementioned predictors, except for the N stage. The bootstrap-corrected concordance index (C-index) of the nomogram was 0.820 (95% CI 0.805–0.835), surpassing the C-index of the TNM stage (0.571, 95% CI 0.550–0.592, P < 0.001). Based on time-dependent C-index results, the nomogram demonstrated a better discriminative ability compared to the TNM staging system. There was a good consistency between the observed values and predicted probabilities indicated by the calibration curves. The nomogram’s clinical utility was supported by the decision curve analysis. Additionally, the nomogram can classify PRNET patients into low-risk and high-risk subgroups, with high-risk patients having poorer OS (P < 0.0001). The prognostic nomogram, based on individualized clinicopathological information, may be helpful in predicting survival outcomes for PRNET patients more accurately. Further external validation is required in future studies to confirm our developed nomogram’s prognostic accuracy and clinical applicability.
Similar content being viewed by others
Introduction
The incidence of kidney cancer is rising and currently accounts for 2–3% of all cancers1. Globally, there were approximately 434,419 new kidney cancer cases and 155,702 deaths in 20221. Approximately 90% of kidney cancer cases are classified as renal cell carcinomas (RCCs), which is the primary histological type2. By contrast, in normal renal parenchyma, neuroendocrine cells are absent, making primary renal neuroendocrine tumors (PRNETs) extremely rare3. Approximately 100–200 clinical cases have been reported worldwide4,5. Generally, PRNETs can be categorized as well-differentiated or poorly-differentiated tumors6,7. There are typical and atypical carcinoid tumors within well-differentiated neuroendocrine tumors, whereas small-cell and large-cell neuroendocrine carcinomas (NECs) are found in poorly differentiated neuroendocrine tumors. According to Yi et al.8, patients with carcinoid tumors had a lower grade, a smaller tumor size, and an earlier stage than those with renal NECs. Due to the diverse clinicopathological characteristics of patients with PRNET, predicting their prognosis is essential for selecting more personalized treatment strategies and optimizing clinical management.
Although the TNM staging system is currently regarded as the gold standard for predicting tumor prognosis, it does have certain limitations. A primary disadvantage of this approach is that other variables, such as age, tumor grade, surgery, chemotherapy, summary stage, and histological type, cannot be incorporated to predict prognosis9. As a result of disregarding these factors, the TNM staging system failed to provide accurate predictions of survival outcomes10,11. Thus, it is essential to construct a robust prognostic tool incorporating these potential predictors. The nomogram provides a reliable and sophisticated tool for assessing individual survival probabilities12. It incorporates factors based on the comprehensive characteristics of both the patient and the disease. It has been widely reported that a nomogram can estimate recurrence13, cancer-specific survival (CSS)14,15, and overall survival (OS)16,17 of a patient with a tumor. As far as we know, no prognostic nomogram has been developed specifically for PRNET patients.
Our study aimed to construct a prognostic nomogram to predict the OS at 1, 3, and 5 years for PRNET patients based on clinicopathological characteristics. A bootstrap resampling method was applied to evaluate the nomogram’s performance with internal validation. Due to the rarity of PRNETs, detailed clinicopathological and prognostic information is lacking. Thus, the Surveillance, Epidemiology, and End Results (SEER) database was utilized in our study to gather cases and extract data.
Methods
Study population
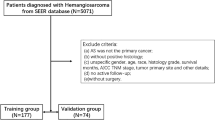
We utilized SEER*Stat version 8.4.4 for data extraction, variable definition, and case selection. The clinicopathological and prognostic data of patients diagnosed with primary kidney cancer (primary site: C64.9) were extracted from the “Incidence-SEER Research Data, 17 Registries, Nov 2023 Sub (2000–2021)”. We collected the following clinicopathological and prognostic data: age, gender, race, Fuhrman grade, marital status, T stage, N stage, M stage, summary stage, histological type, tumor size, therapeutic modes (chemotherapy, surgery, and radiotherapy), cause of death, vital status, and survival months. The nuclear grade of RCC is a pathological assessment that describes the morphological characteristics of the nucleus of a cancer cell (such as size, shape, staining depth, and nucleolus prominence) to reflect the degree of malignancy and aggressiveness of the tumor. The higher the nuclear grade, the worse the differentiation of the tumor, the faster the growth, and the worse the prognosis18. The nuclear grade of RCC was mainly based on the Fuhrman grade. Moreover, the SEER database utilized Fuhrman grading systems19,20. These were the inclusion criteria for the study (n = 271): (1) Year of diagnosis: 2000–2021; (2) Diagnostic confirmation: positive histology; (3) Histology types: primary renal NEC (ICD-O-3 code:8041/3, 8013/3, 8246/3) and carcinoid tumor (ICD-O-3 code: 8240/3). The following criteria were used to exclude cases: the survival month was 0 (n = 5). Finally, our study included 266 participants with PRNET, including 176 primary renal NEC and 90 carcinoid tumor patients.
Population classification
In terms of gender, patients were categorized as male or female. Patients are classified as White, Black, and Others (including American Indian/Alaska Native and Asian or Pacific Islander) based on their racial background. The patients were divided into five subgroups based on Fuhrman nuclear grade: grade I (well differentiated), grade II (moderately differentiated), grade III (poorly differentiated), grade IV (undifferentiated), and unknown. There were three types of marital status: married, not-married, and unknown. In terms of tumor size, three groups were created: < 6 cm, ≥ 6 cm, and unknown. T stages include T1, T2, T3, T4, and unknown. N stages include N0, N1, N2, and unknown. M stages include M0, M1, and unknown. Regarding the summary stage, the patients were divided into four subgroups: localized, regional, distant, and unknown. Other variables included: (1) Surgical status (yes, no/unknown); (2) Chemotherapy record (yes, no/unknown); (3) Radiotherapy record (yes, no/unknown). The study did not require ethics approval or patient informed consent because the data from the US SEER database is publicly accessible.
Model construction and assessment
To screen OS-related variables, we first conducted univariate Cox regression analyses. Subsequently, we included variables with a P-value less than 0.05 from the univariate analysis into the multivariate analysis. We performed a multivariate analysis using a Cox proportional-hazards model to evaluate prognostic factors independently related to PRNETs. We could calculate hazard ratios (HRs) based on univariate or multivariate analyses alongside 95% confidence intervals (CIs). Subsequently, we conducted the restricted cubic splines (RCS) regression using three knots to investigate further the potential nonlinear relationship between age and all-cause mortality. According to the results of the multivariate analysis, we constructed a nomogram to predict the OS at 1, 3, and 5 years for PRNET patients. Using the concordance index (C-index), the discriminative ability of the nomogram was evaluated in comparison to the TNM stage and several prognostic factors. We conducted a calibration curve analysis to evaluate the accuracy of the nomogram and performed a decision curve analysis to estimate its clinical usefulness. A 1000-resampling bootstrapping method was used for all internal validations. Additionally, we computed the total points for each patient using the established nomogram. We then determined the optimal cutoff for the risk score of the nomogram model to stratify patients into low-risk and high-risk groups (cutpoint function; Survminer package in R). We performed Kaplan-Meier survival curves with a log-rank test to compare the OS differences between the two groups.
Statistical analyses
We applied R version 4.2.2 and SPSS version 25.0 to perform all statistical analyses. Moreover, we conducted all statistical analyses of this study with age as a continuous variable. First, we utilized the ‘rms’ package in R to plot the RCS, nomogram, calibration, and decision curve analysis curves. Second, we applied the ‘pec’ package to plot the time-dependent C-index curves. Subsequently, we employed the ‘survminer’ and ‘survival’ packages to perform Kaplan-Meier survival curves with a log-rank test. A P-value less than 0.05 was considered statistically significant.
Results
Patient characteristics
As shown in Fig. 1, we included 266 patients with PRNET, including 176 primary renal NEC and 90 carcinoid tumor patients, from the SEER database between 2000 and 2021. Patients ranged in age from 16 to 90, with an average age of 63.14 ± 16.65 and a median age of 65. There were 143 male patients (53.8%) and 123 female patients (46.2%). It was predominantly White in terms of racial distribution (75.9%). The majority of patients received surgical treatment (53.0%), while a minority underwent chemotherapy (27.4%) and radiotherapy (12.0%). More than half of the patients were married (53.4%). Forty patients (15.0%) had Fuhrman grade I–II, and forty-six patients (17.3%) had Fuhrman grade III–IV. Twenty-seven patients (10.2%) had the T1/T2 stage, and forty-nine patients (18.4%) had the T3/T4 stage. The number of patients with N0 and N1/N2 stages was 44 (16.5%) for both categories. Forty-eight patients (18.0%) had the M0 stage, and forty-nine patients (18.4%) had the M1 stage. The number of patients in the localized, regional, and distant stages was 60 (22.6%), 76 (28.6%), and 114 (42.9%), respectively. The number of patients in the tumor size groups < 6 cm and ≥ 6 cm was 104 (39.1%) and 117 (44.0%), respectively. The detailed clinicopathological characteristics are presented in Table 1.
Independent prognostic factors in PRNET patients
Based on univariate analyses, age, gender, Fuhrman grade, surgery, radiotherapy, chemotherapy, summary stage, N stage, M stage, tumor size, and histological type were related to OS (all P < 0.05, Table 2). The multivariable results for OS revealed that age was significantly positive with the risk of all-cause mortality (HR = 1.05, 95% CI 1.04–1.06, P < 0.001). Moreover, RCS regression analysis indicated a linear increase in all-cause mortality risk with increasing age (P for nonlinear = 0.208, P for overall < 0.0001; Fig. 2). Compared to patients with grade I/II, those with grade III/IV (HR = 3.17, 95% CI 1.68–5.99, P < 0.001) exhibited worse survival outcomes. Patients who underwent surgery exhibited better OS than those who did not (HR = 0.41, 95% CI 0.25–0.65, P < 0.001). Patients with regional-stage tumors (HR = 1.89, 95% CI 1.05–3.41, P = 0.034) and distant-stage tumors (HR = 2.63, 95% CI 1.44–4.79, P = 0.002) were significantly related to worse survival outcomes than patients with localized-stage tumors. Compared to patients with N0, those with N1/N2 (HR = 1.77, 95% CI 1.07–2.92, P = 0.026) exhibited worse OS. Patients with NEC (HR = 2.72, 95% CI 1.62–4.58, P < 0.001) were significantly related to worse survival outcomes than patients with carcinoid tumors. The multivariate analysis demonstrated that clinicopathological characteristics, including age, Fuhrman grade, surgery, summary stage, N stage, and histological type, were prognostic factors independently affecting OS (all P < 0.05, Table 2). Since the summary stage already incorporates lymph node status into its ‘regional’ designation, adding the N stage in the same nomogram would overlap this information. Thus, we did not include the N stage in the nomogram to avoid information overlap.
Restricted cubic spline of the linear trends between age and all-cause mortality of PRNET patients. The model was adjusted for gender, race, Fuhrman grade, radiotherapy, chemotherapy, surgery, marital status, tumor size, summary stage, T stage, N stage, M stage, and histology type. PRNET, primary renal neuroendocrine tumor.
Construction and validation of the nomogram
We constructed a prognostic nomogram incorporating the above independent predictors, including age, Fuhrman grade, surgery, summary stage, and histological type, to predict the OS at 1, 3, and 5 years for PRNET patients (Fig. 3). The bootstrap-corrected C-index of the nomogram was 0.820 (95% CI 0.805–0.835), surpassing the C-index of the TNM stage (0.571, 95% CI 0.550–0.592, P < 0.001) and other prognostic factors (all P < 0.001, Table 3). Additionally, according to time-dependent C-index results, the nomogram demonstrated a better discriminative ability than the TNM staging system and other prognostic factors (Fig. 4A). When using the bootstrap resampling method, we observed a similar outcome during the internal validation process (Fig. 4B). In order to assess the nomogram’s predictive accuracy and net benefit, we then performed calibration curves and decision curve analysis. The calibration plot will exhibit how closely the risk estimated by the nomogram aligns with the risk that is actually observed. Figure 5 presents the excellent calibration of the nomogram we developed for predicting the OS at 1, 3, and 5 years for PRNET patients. According to the decision curve analysis, the nomogram demonstrated a higher net benefit than the TNM staging system across most reasonable threshold probabilities (Fig. 6). Overall, our nomogram demonstrated better performance in predicting survival outcomes compared to the TNM staging system.
The ability of nomogram to stratify mortality risk of PRNET patients
Using the cutpoint function in R, we determined that 111.43 was the best cutoff value. Subsequently, the PRNET patients were categorized as low-risk (total points ≤ 111.43) and high-risk (total points > 111.43) subgroups according to the total points of the nomogram. The median OS of patients in the high-risk group was 7 months, while that of patients in the low-risk group was 195 months. According to the Kaplan-Meier survival curve, patients in the high-risk group had a worse OS (P < 0.0001, Fig. 7).
Discussion
The nomogram has become increasingly popular as an essential tool for clinicians to predict the survival outcomes of cancer patients based on individual data21. Compared to the conventional TNM staging system, the nomogram predicts survival outcomes more accurately, resulting in more effective counseling and personalized treatment for patients22,23. As far as we know, no prognostic nomogram has been developed specifically for PRNET patients. Additionally, the rarity of PRNET makes it difficult to collect sufficient samples to draw reliable and robust conclusions from single-center studies. Thus, it is essential to develop a reliable and accurate prognostic model for PRNET patients. Since PRNETs are clinically rare, we gathered clinicopathological and prognostic data from the SEER database. In this study, we established a novel nomogram to predict the OS at 1, 3, and 5 years for PRNET patients, assisting in formulating treatment strategies and optimizing clinical management.
In our study, most (61.3%) of the included population was over 60. Given that the majority of patients were male, white, elderly, married, and had undergone surgery, the sample pool aligns with that of previous research8,24,25. Additionally, our multivariate results demonstrated that age, Fuhrman grade, surgery, histological type, N stage, and summary stage were all prognostic factors independently affecting OS. Our results revealed that age was significantly positive with the risk of all-cause mortality, consistent with the previous study8. The results indicate that PRNET patients of advanced age have worse survival outcomes. Moreover, RCS regression analysis indicated a linear increase in all-cause mortality risk with increasing age. There might be several reasons for the decreasing OS of PRNET patients as they age, including poor nutritional status, more complex underlying diseases, and decreased tolerance to treatment. Moreover, age weakens the immune system, which contributes to tumor deterioration and further reduces OS26. Some studies have demonstrated that the survival probabilities for various cancers were significantly affected by age27,28,29,30,31.
Zeng et al.32 reported that there was a correlation between tumor-related pathological characteristics, such as tumor grade and N stage, and the survival outcomes of cancer patients. Similarly, Wang et al.24 reported that N stage and tumor grade independently affected the OS and CSS of patients with papillary RCC. Research on cancer stem cells has been a focus of recent studies33. A positive correlation was found between the pathological grade of the tumor and the stemness of the cancer cells33. Often, high-grade tumors are associated with high malignancy and strong invasiveness, which adversely affect the prognosis of patients34. In high-grade tumor tissues, CD133 and nestin expression are elevated simultaneously, leading to cell atypia and decreased effectiveness of treatments34. The N stage indicates a regional lymph node involvement. Tumor with lymph node metastasis often indicates an inferior prognosis. Our results revealed that PNRET patients with grade III/IV or N1/N2 stage had a worse prognosis than those with grade I/II or N0 stage, consistent with the previous results. However, Yi et al.8 included 132 PRNET patients and found that tumor grade was not a prognostic factor independently affecting OS (P = 0.13). We consider that the discrepancy in results may be due to their sample size being too small to obtain a robust and reliable conclusion. The more advanced the summary stage, the more likely it is to have distant metastasis, which often indicates a poor prognosis. Our results identified the summary stage as a prognostic factor independently affecting the OS of PRNET patients. Similarly, Yi et al.8 found that PRNET patients with regional and distant stages had worse prognoses than those with localized stages (P = 0.003), further confirming our conclusion.
The prognosis of PRNET is also closely related to its histology. Carcinoid tumors are generally associated with better survival outcomes than NECs. Our results demonstrated that patients with NEC were significantly related to worse survival outcomes than those with carcinoid tumors. We identified histology as a prognostic factor independently affecting the OS of PRNET patients. Yi et al.8 reported that primary renal NEC patients exhibited worse OS than patients with carcinoid tumors (median OS: 27 months vs. 145 months). However, their multivariate results found that histology was not a prognostic factor independent affecting OS (P = 0.23). The discrepancy in findings may be attributed to their small sample size, which could not obtain robust results. Our results also revealed that surgery was a prognostic factor independently affecting OS. However, our multivariate results identified that chemotherapy and radiotherapy were not prognostic factors independent affecting OS (all P > 0.05), consistent with the findings from Yi et al.8. We found that PRNET patients who underwent surgery had better OS than those who did not. Similarly, Jiang et al.35 reported that surgery could independently affect the OS of patients with collecting duct RCC. Wang et al.24 reported that surgery was a prognostic factor independently affecting OS for patients with papillary RCC. However, this does not mean that surgery must be used to improve outcomes, even in patients with distant metastases. The nomogram identifies associations between variables (e.g., surgery) and outcomes, not causal relationships. Patients selected for surgery (even with distant metastasis) often have favorable baseline characteristics (e.g., limited metastatic burden, better performance status) that inherently improve outcomes. For localized renal carcinoid tumors, radical nephrectomy is typically considered the standard treatment36. A follow-up 20 months after surgery revealed that 73.1% of patients exhibited no sign of disease, indicating the possibility that surgery is curative37. For localized renal NEC, surgical resection of all visible diseases is the primary treatment option38. Consequently, we consider surgery to be an early choice for localized PRNET. Surgery for metastatic renal neuroendocrine tumors is uncommon and typically reserved for palliative or cytoreductive purposes (e.g., symptom control, reducing tumor bulk). It is not a standard curative intervention for metastatic disease. For patients with localized PRNET, Yi et al.8 recommend surgery as an early treatment option. Their multivariate results found that surgery was a prognostic factor independently affecting OS, but chemotherapy and radiotherapy were not. Relevant studies recommended that surgery and systemic chemotherapy may be effective treatments for renal NEC5,39,40.
We constructed a prognostic nomogram incorporating the above independent predictors, including age, Fuhrman grade, surgery, summary stage, and histological type, to predict the OS at 1, 3, and 5 years for PRNET patients. Based on the determined independent predictors, it can quantify the OS probabilities for PRNET patients. Moreover, the time-dependent C-index analysis, calibration, and decision curve analysis demonstrated that the nomogram had better predictive performance and overall net benefit compared to the TNM staging system. Although we internally validated the nomogram’s exceptional performance through C-index, calibration curves, and decision curve analysis, the critical question is how to apply the nomogram model to clinical practice, such as patient counseling, risk stratification, and treatment decisions for patients. First, clinicians could provide PRNET patients with accurate 1-year, 3-year, and 5-year OS predictions. For example, an 81-year-old patient with renal NEC who had a grade III tumor and localized stage underwent surgery. The patient would like to inquire about the prognosis after surgery. The total points were 141.25. Accordingly, this patient’s 1-year, 3-year, and 5-year OS probabilities were approximately 52%, 34%, and 22%, respectively. Our developed nomogram, based on individualized clinicopathological information, could help predict survival outcomes and provide patient counseling for PRNET patients more accurately. Second, our developed nomogram could stratify the mortality risk of PRNET patients. Clinicians could categorize the PRNET patients into low-risk (total points ≤ 111.43) and high-risk (total points > 111.43) subgroups according to the total points. In PRNET patients with high risk and a poor prognosis, clinicians could implement additional treatments and shorten the follow-up time to monitor the disease more effectively. Third, for PRNET patients with localized stage, surgery (radical nephrectomy) is recommended. Our results found that surgery was a prognostic factor independently affecting OS. PRNET patients who underwent surgery had better OS than those who did not.
However, we must acknowledge some limitations of our research. First, we are prone to selection bias since our study is retrospective. Second, although our study includes the largest number of PRNET patients, the small sample size may limit our analysis. Third, a notable limitation of this study was the substantial proportion of missing data regarding the T, N, and M stages within the SEER database. However, given the rarity of PRNET and the small sample size, we must retain patients who lack TNM staging data for further study. This incomplete data might preclude the inclusion of the T, N, and M stages in multivariable analyses and the final nomogram model, potentially influencing the model’s prognostic accuracy and clinical applicability. Additionally, the incomplete data on T, N, and M stages may limit the direct comparison of our nomogram’s predictive performance against established TNM-based systems, as the absence of complete T, N, and M staging data hinders a fair evaluation of their relative strengths. Fourth, our study lacks external validation. Although internal validation via bootstrap resampling methods demonstrated exceptional model performance, our nomogram requires external validation in independent, heterogeneous cohorts to confirm its generalizability. Internal validation inherently carries the risk of overfitting to the study population, and performance may vary across populations with differing demographics, clinical practices, or disease characteristics. In future studies, further external validation is required to confirm our developed nomogram’s prognostic accuracy and clinical applicability. External validation is critical to verify the reproducibility of our findings, refine risk stratification, and ensure clinical utility in broader settings. Last but not least, another limitation is the lack of granular data for certain variables, such as chemotherapy and radiotherapy. The SEER database does not provide detailed information on the dose, type, or duration of radiotherapy or chemotherapy. Some patients may not receive sufficient doses or the appropriate length of systemic treatment, leading to the illusion that the treatment is useless. Additionally, the SEER database did not include information on comorbidities (e.g., hypertension, diabetes), targeted therapy, surgical margin status, smoking history, drinking history, and other potential prognostic factors, which were not included in the nomogram and could affect the nomogram’s accuracy.
Conclusions
The prognostic nomogram, based on individualized clinicopathological information, may be helpful in predicting survival outcomes for PRNET patients more accurately. In the future, our nomogram may serve as a simple, readily available prognostic tool in clinical practice, provided that large-scale, multicenter prospective validation is accomplished.
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 74, 229–263 (2024).
Novara, G. et al. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: Are further improvements needed?? Eur. Urol. 58, 588–595 (2010).
Rindi, G. et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr. Pathol. 33, 115–154 (2022).
Monaghan, T. F. et al. Primary small cell carcinoma of the kidney: Disease characteristics and treatment outcomes. Medicines 8, 6 (2021).
Majhail, N. S., Elson, P. & Bukowski, R. M. Therapy and outcome of small cell carcinoma of the kidney: Report of two cases and a systematic review of the literature. Cancer 97, 1436–1441 (2003).
Moch, H., Cubilla, A. L., Humphrey, P. A., Reuter, V. E. & Ulbright, T. M. The 2016 WHO classification of tumours of the urinary system and male genital Organs—Part A: Renal, penile, and testicular tumours. Eur. Urol. 70, 93–105 (2016).
Moch, H. et al. The 2022 world health organization classification of tumours of the urinary system and male genital Organs—Part A: Renal, penile, and testicular tumours. Eur. Urol. 82, 458–468 (2022).
Yi, Z. et al. Clinicopathologic features and survival outcomes for primary renal neuroendocrine neoplasms. Clin. Genitourin. Cancer. 19, 155–161 (2021).
Balachandran, V. P., Gonen, M., Smith, J. J. & DeMatteo, R. P. Nomograms in oncology: More than Meets the eye. Lancet Oncol. 16, e173–e180 (2015).
Leibovich, B. C. et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J. Urol. 183, 1309–1315 (2010).
Cairns, P. Renal cell carcinoma. Cancer Biomark. 9, 461–473 (2010).
Iasonos, A., Schrag, D., Raj, G. V. & Panageas, K. S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 26, 1364–1370 (2008).
Gold, J. S. et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary Gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol. 10, 1045–1052 (2009).
Kattan, M. W., Karpeh, M. S., Mazumdar, M. & Brennan, M. F. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J. Clin. Oncol. 21, 3647–3650 (2003).
Kattan, M. W. et al. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann. Surg. 247, 282–287 (2008).
Zivanovic, O. et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer 118, 660–669 (2012).
Yang, L., Takimoto, T. & Fujimoto, J. Prognostic model for predicting overall survival in children and adolescents with rhabdomyosarcoma. BMC Cancer. 14, 654 (2014).
Bretheau, D. et al. Prognostic value of nuclear grade of renal cell carcinoma. Cancer 76, 2543–2549 (1995).
Fuhrman, S. A., Lasky, L. C. & Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 6, 655–663 (1982).
Delahunt, B. et al. Grading of clear cell renal cell carcinoma should be based on nucleolar prominence. Am. J. Surg. Pathol. 35, 1134–1139 (2011).
Freedman, A. N. et al. Cancer risk prediction models: A workshop on development, evaluation, and application. J. Natl. Cancer Inst. 97, 715–723 (2005).
Brateanu, A., Yu, C., Kattan, M. W., Olender, J. & Nielsen, C. A nomogram to predict the probability of passing the American board of internal medicine examination. Med. Educ. Online. 17, 18810 (2012).
Cao, J. et al. Clinical nomogram for predicting survival of esophageal cancer patients after esophagectomy. Sci. Rep. 6, 26684 (2016).
Wang, H. et al. Predictive Nomogram for Midterm to Long-Term Prognosis in Patients with Papillary Renal Cell Carcinoma Based on Data from the Surveillance, Epidemiology, and End Results (SEER) Program. Med Sci Monit 26, e921859 (2020).
Chow, W. H. & Devesa, S. S. Contemporary epidemiology of renal cell cancer. Cancer J. 14, 288–301 (2008).
Zeng, C. et al. Disparities by race, age, and sex in the improvement of survival for major cancers: Results from the National cancer Institute surveillance, epidemiology, and end results (SEER) program in the united States, 1990 to 2010. JAMA Oncol. 1, 88–96 (2015).
Dias-Santos, D., Ferrone, C. R., Zheng, H., Lillemoe, K. D. & Fernandez-Del, C. C. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery 157, 881–887 (2015).
Escudier, B. et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 30, 706–720 (2019).
Zhou, Y. et al. Prognostic nomograms and Aggtrmmns scoring system for predicting overall survival and cancer-specific survival of patients with kidney cancer. Cancer Med. 9, 2710–2722 (2020).
Zhanghuang, C. et al. Development and Validation of a Nomogram to Predict Cancer-Specific Survival in Elderly Patients With Papillary Renal Cell Carcinoma. Front Public Health 10, 874427 (2022).
Tong, Y. et al. Clinical Characteristics, Prognostic Factor and a Novel Dynamic Prediction Model for Overall Survival of Elderly Patients With Chondrosarcoma: A Population-Based Study. Frontiers in Public Health 10, 901680 (2022)
Zeng, Y. et al. A nomogram for predicting cancer-Specific survival of TNM 8th edition stage I Non-small-cell lung cancer. Ann. Surg. Oncol. 26, 2053–2062 (2019).
Zhang, Q., Xu, B., Chen, J., Chen, F. & Chen, Z. Clinical significance of CD133 and Nestin in astrocytic tumor: The correlation with pathological grade and survival. J. Clin. Lab. Anal. 34, e23082 (2020).
Kamamoto, D., Saga, I., Ohara, K., Yoshida, K. & Sasaki, H. Association between CD133, CD44, and nestin expression and prognostic factors in high-grade meningioma. World Neurosurg. S1878-8750(18), 32890-0 (2018).
Jiang, W., Zou, Z. & Wen, L. Establishment of a nomogram to predict the overall survival of patients with collecting duct renal cell carcinoma. Discov. Oncol. 15, 261 (2024).
Korkmaz, T., Seber, S., Yavuzer, D., Gumus, M. & Turhal, N. S. Primary renal carcinoid: Treatment and prognosis. Crit. Rev. Oncol. Hematol. 87, 256–264 (2013).
Omiyale, A. O. & Venyo, A. K. Primary carcinoid tumour of the kidney: A review of the literature. Adv. Urol. 2013, 579396 (2013).
McGarrah, P. W. et al. Renal neuroendocrine neoplasms: A single-center experience. Clin. Genitourin. Cancer 18, e343–e349 (2020).
Posfai, B. et al. The colorful palette of neuroendocrine neoplasms in the genitourinary tract. Anticancer Res. 38, 3243–3254 (2018).
Teegavarapu, P. S. et al. Neuroendocrine tumors of the kidney: A single institution experience. Clin. Genitourin. Cancer. 12, 422–427 (2014).
Acknowledgements
We thank Ruijuan Liu for reviewing and examining this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Study Design: YW, XC, HY, and YZ; Data collection: HY, YZ, and YF; Statistical analysis: WL and QL; Drafting the manuscript: YW, HY, XC, and YZ; Revising and editing the manuscript: YF, WL and QL; Final endorsement of the finished article: YW, XC, YZ, and YF. The final manuscript was read and approved by all writers.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Due to the public availability of data in the US SEER database, it was not necessary for this study to obtain ethics approval and informed consent from patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Yang, H., Zhu, Y. et al. Establishment and validation of a nomogram to predict overall survival for patients with primary renal neuroendocrine tumor. Sci Rep 15, 13861 (2025). https://doi.org/10.1038/s41598-025-98228-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98228-0