Abstract
The development of advanced wound dressings has seen a significant leap with the integration of biodegradable nanofibers. This study introduces an innovative approach by designing polylactic acid (PLA)-curcumin nanofiber wound dressings enhanced with carbon nanotubes (CNTs). Using the electrospinning method, various formulations were crafted, incorporating diverse weight percentages of curcumin and CNTs. Comprehensive analyses, including FT-IR and SEM, confirmed the structural and physical integrity of the nanofibers, while tensile testing revealed a notable enhancement in mechanical strength with the addition of CNTs. Drug release evaluations highlighted a controlled and predictable release pattern of curcumin across all samples. Water absorption tests demonstrated the ability of PLA nanofibers to absorb up to 364%, with PLA-Cur-0.03%CNT samples absorbing 163%, showcasing their adaptability to wound exudates. Importantly, cytotoxicity assessments confirmed the biocompatibility of all samples, with high cell viability observed after 3 and 7 days. Antibacterial tests underscored the efficacy of CNT-incorporated samples, with PLA-Cur-0.05%CNT achieving the highest antibacterial activity at 78.95%. Additionally, using Density Functional Theory (DFT) calculations, the transition state, HOMO-LUMO energy, and equilibrium constant were explored, revealing higher equilibrium constants for keto-enol transformations compared to enol-keto in various solvents. Tautomeric conversion is easier in polar solvents due to the stability of charged species. HOMO-LUMO energy analysis revealed the stability and chemical activity of curcumin in solvents. This comprehensive research not only highlighted the mechanical, antibacterial, and drug delivery capabilities of the wound dressing but also provided an innovative approach for designing and optimizing pharmaceutical compounds under challenging chemical environments through advanced modeling and computational techniques.
Similar content being viewed by others
Introduction
The skin is the largest organ of the human body, serving as a protective barrier against external threats such as bacteria, viruses, and environmental pollutants while regulating body temperature, storing water and fat, and sensing the external environment through touch, heat, and pain receptors. The skin’s functions are essential in preventing diseases and maintaining overall health. Its role in providing a physical barrier and housing immune cells helps in warding off pathogens and reducing the risk of infections1. The body’s natural healing process is capable of repairing injuries to the skin. However, in cases of severe wounds, additional treatment methods may be necessary to facilitate the healing process2,3. Traditional wound dressings, made from natural materials like cotton or linen, have been used for centuries to promote healing and protect wounds from infection. They provide benefits such as absorbency, breathability, cost-effectiveness, and biocompatibility4,5 but they have notable drawbacks, including insufficient protection against pathogens, poor moisture regulation, discomfort during changes, and inadequate conformity to wound sites that can hinder healing and patient compliance. These challenges underscore the pressing need for innovative and advanced wound dressing solutions that can effectively address these limitations6,7,8,9,10,11,12. In recent years, modern wound dressings, especially those based on nanofibers, have gained attention for their unique properties13,14. These nanoscale fibers provide a high surface area to volume ratio, allowing for better exudate absorption from the wound and creating a barrier against bacteria and other pathogens with their small pore size. Moreover, they promote cell adhesion and proliferation, supporting the formation of new tissue and ultimately accelerating the healing process15,16,17,18.
Today, the use of biodegradable polymer nanofibers is widely used in the production of wound dressings19,20. These polymers, derived from renewable sources such as polyethylene glycol (PEG), polyethylene oxide (PEO), polyacrylic acid (PAA), polylactic acid (PLA), poly(glycolic acid), corn starch, cellulose, or chitosan, offer a sustainable alternative to traditional petroleum-based polymers21,22. The use of biodegradable polymers in wound dressings provides several advantages, including non-toxicity and biocompatibility, minimizing the risk of adverse reactions, and promoting wound healing. These polymers can be engineered to have specific properties such as moisture retention, antimicrobial activity, or controlled drug release, enhancing their effectiveness in promoting wound healing23,24,25,26,27,28,29. These nanofibers are utilized for the encapsulation and controlled release of various pharmaceutical agents, including drugs, growth factors, and antimicrobial agents30. Recent studies have demonstrated the compatibility of carbon nanotubes with biodegradable polymers for nanofiber production31. Carbon nanotubes can be used in wound dressings as carriers for drugs to enhance wound healing32,33. By loading therapeutic agents onto their surface, these nanotubes enable effective delivery to the injury site, allowing for sustained release and improved therapeutic effects34,35.
Numerous studies have explored the application of CNTs in creating advanced wound dressings. Yang et al. studied the electrospinning of PLA with CNTs, examining the solution, solvent environment, and CNT loading. The results showed that the morphology of electrospun PLA and PLA/CNT fibers strongly depended on the solution concentration and solvent type. At low CNT loading, CNTs were well integrated into the PLA matrix, while high loading resulted in clustered CNTs and poor fiber morphology36. In a study, Chi-Hui Tsou et al. successfully developed innovative multi-walled carbon nanotubes coated with nanosilver (MWCNT-Ag). These nanotubes were integrated into PLA to produce nanocomposites that are both biocompatible and antimicrobial. Experimental results demonstrated that incorporating just 0.3 phr of MWCNT-Ag significantly enhanced the material’s mechanical strength, thermal stability, and antibacterial effectiveness36. Rui Cui et al. created a controlled-release antibacterial food packaging film using PLA, PCL, CNTs, and CIN via the solvent evaporation method. The resulting film exhibited enhanced UV resistance, improved flexibility, and a sustained release of CIN over 21 days. Furthermore, the film demonstrated an antibacterial effect against Staphylococcus aureus and Escherichia coli lasting for 21 days, which was 7 days longer than films without CNTs37.
This research aims to fabricate and evaluate a wound dressing composed of polylactic acid-curcumin-carbon nanotubes by using the electrospinning method. Polylactic acid possesses suitable thermal, rheological, and biodegradable properties, making it a favorable polymer for wound dressings. To further enhance the physical, thermal, and antibacterial properties of polymer matrix wound dressings while also achieving a controlled release of curcumin, carbon nanotubes were incorporated as fillers. Curcumin, a compound present in medicinal plants like turmeric, exhibits various beneficial properties, including anti-cancer, anti-viral, anti-arthritic, antioxidant, and anti-inflammatory effects38,39,40,41,42,43. Combining these three components creates an ideal platform for curcumin release, in addition to wound dressing, to minimize the adverse effects of chemical drugs. This innovative approach holds great promise for developing advanced drug delivery systems with enhanced biocompatibility and therapeutic efficacy.
Experimental
Materials and methods
Polylactic acid with a molecular mass of 203,000 g/mol and carbon nanotubes was obtained from the Sigma-Aldrich company. Curcumin (C21H20O6), dimethylformamide (C3H7NO), Tween 80 (C64H124O26), and absolute ethanol were prepared from Merck Company. Dichloromethane was purchased from CDH, and phosphate-buffered saline was obtained from DNAbiotech.
Infrared Fourier transform spectra of the compounds were performed by the Nicolet 800 device. Ultraviolet-visible spectroscopy was measured on a Shimadzu UV-1800. The SANTAM-STM20 device performed the tensile mechanical properties. The diameter and surface morphology of the samples were determined using a field emission scanning electron microscope (FE-SEM) model ZEISS-SIGMA VP. The optical microscope was measured with NIKON-E100 (magnification 40-1000). The TGA analyses were performed by the STA-1500 device at a heating rate of 10 ℃/min from 25 to 600 °C. The TGA analyses were performed under an argon atmosphere.
General method of Preparation of polylactic acid-curcumin-carbon nanotube wound dressing by electrospinning method
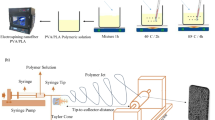
To prepare the wound dressing, polylactic acid-curcumin-carbon nanotubes were prepared according to the previously reported protocol with specified ratios, as outlined in Table 1. Briefly, first, polylactic acid was dried in an oven at 70 °C and 60 rpm for 2 h. Subsequently, 1.5 g of polylactic acid were dissolved in 7 ml of dichloromethane and stirred for 45 min at room temperature until complete dissolution. Concurrently, 1% by weight of curcumin was dissolved in 3 ml of dichloromethane at room temperature, and the solution was added to the polylactic acid solution and stirred for 30 min until homogeneity was achieved. The resulting mixture was subjected to ultrasonication for 2 min and further stirred for 5 min to ensure complete homogenization. The prepared sample was then electrospun at a distance of 15 cm and a flow rate of 0.5 ml/min, facilitating the complete electrospinning of the syringe’s contents. Following the process, polylactic acid fibers of suitable thickness were obtained and examined using an optical microscope (The viscosity of the polymer solution sample was 201 mPa·s at 25 °C(.
Results and discussions
Chemical and structural characterization
In this study, the electrospinning method was used to fabricate wound dressing fibers containing different ratios of polylactic acid, curcumin, and carbon nanotubes. For the first time, the impact of incorporating CNTs into PLA-curcumin fibers was investigated. The chemical structure and mechanical properties of these fibers were examined using FT-IR and tensile tests. The FT-IR spectra of samples including PLA, PLA-Cur, PLA-Cur-0.05%CNTs, PLA-Cur-0.03%CNTs, and PLA-Cur-0.01%CNTs are presented in Fig. 1. Notably, the peak at 1760 cm− 1 confirms the presence of the carbonyl group, while multiple peaks in 2855 cm− 1 to 2998 cm− 1 correspond to the stretching vibrations of -CH and -CH3 groups. Additionally, peaks at 1386 cm− 1 and 1459 cm− 1 are attributed to the bending vibrations of C-H, and peaks of 1088 cm− 1 and 1183 cm− 1 confirm the stretching vibrations of C-O groups.
The FT-IR spectra comparison between PLA and PLA-Cur samples, as depicted in Fig. 2, highlights key structural differences. The peak at 1602 cm− 1 corresponds to the stretching vibration of the aromatic ring of curcumin, and at 1514 cm− 1 confirming the presence of C = O and C = C groups. A peak at 1630 cm− 1 is observed, attributed to the C = C aromatic group. Following the addition of carbon nanotubes, the FT-IR spectra exhibit pronounced broadening and intensification of relevant peaks. Notably, the peak at 1630 cm− 1, corresponding to C = C double bond stretching, shows increased intensity. Additionally, the peak at 1386 cm− 1 associated with C-C stretching vibrations and the peak at 1086 cm− 1, related to C-O stretching, both demonstrate heightened intensities44.
In the FTIR spectrum analysis, the sample PLA-Cur-0.05%CNTs exhibits characteristic peaks located at the same positions as those observed for pure PLA. However, the intensity of these peaks shows slight variations. This indicates that while the addition of carbon nanotubes (CNTs) and curcumin influences the spectral intensity, it does not alter the fundamental molecular bonding or surface interactions within the PLA matrix. The structural integrity and chemical framework of the PLA remain unchanged despite the incorporation of these additives.
The SEM images in Fig. 3 illustrate the structural features of the fiber samples. The morphology of both PLA and PLA-Cur samples shows disordered arrangement with visible electrospun voids. The incorporation of carbon nanotubes (CNTs) into the fibers led to a notable change in the morphology of the wound dressings, resulting in a denser and more compact structure. Samples containing 0.01 wt% CNTs were electrospun with high density and without bead formation. However, samples with higher CNT concentrations (0.03 and 0.05 wt%) displayed nanofiber agglomeration, forming distinct fiber clusters. The fiber diameter remained consistent across all samples except for the sample containing 0.05 wt% CNTs. This deviation can be attributed to van der Waals forces between the carbon nanotubes, causing the nanofibers of the 0.05 wt% CNTs sample to adhere and increase in diameter. Based on the experimental results and analyses performed in this study, the weight% of carbon nanotubes (CNTs) was optimized. This observation suggests that the sample with 0.03 wt% CNTs represents the most suitable and optimal composition.
Mechanical and thermal characterization
To investigate the mechanical properties of the samples, including their stress and strain behavior, a tensile test was performed. Figure 4 illustrates the results of the tensile strength analysis performed on different fiber specimens. As shown, the PLA sample exhibited the lowest tensile strength, with a value of 0.87 MPa. The addition of 2% by weight of curcumin resulted in a notable increase in tensile strength to approximately 1.75 MPa. This increase is likely due to the formation of hydrogen bonds between the carbonyl groups of curcumin and the acidic groups of PLA. The highest tensile strength is related to the PLA-Cur-0.05%CNT sample with 2.88 MPa The incorporation of carbon nanotubes (CNTs) into the samples led to an improvement in tensile strength, with a general trend of increasing strength as the CNT concentration increased. Specifically, samples containing 0.01% and 0.03% by weight of CNTs achieved tensile strengths of 2.62 and 2.66 MPa, respectively45.
Thermal analysis serves as a crucial method for studying polymers, particularly in assessing their thermal decomposition behavior. Figure 5 illustrates the thermal curves for PLA, PLA-Cur, and PLA-Cur-0.03%CNTs within the temperature range of 25 to 600 °C. For pure PLA, the TGA curve reveals a single weight loss step initiating at 305 °C and concluding around 381 °C, with a total weight loss of 97.6%. In contrast, the thermal decomposition of PLA-Cur and PLA-Cur-0.03%CNTs begins at 298 °C and ends at 375 °C, indicating a reduction in thermal stability compared to pure PLA. The inclusion of 0.03% CNTs in the PLA-Cur sample results in a very slight improvement in the decomposition temperature; however, the thermal behavior remains similar to that of PLA-Cur due to the minimal CNT content. The observed weight loss for PLA-Cur-0.03% CNT and PLA-Cur is 96.7% and 96.85%, respectively.
Evaluation of Curcumin drug release from electrospun samples
The controlled and sustained release of curcumin from the wound dressing at the wound site promotes healing, reduces inflammation, and maximizes its therapeutic effects. In this study, the release rate of curcumin from electrospun samples was investigated using an ultraviolet spectrophotometer. The curcumin exhibited maximum absorption at a wavelength of 425 nm in its release medium (buffer phosphate + Tween 80). A calibration curve for curcumin was constructed based on this wavelength at various concentrations, allowing the determination of the relationship between absorption and drug concentration from the resulting equation, as illustrated in Fig. 6.
According to Fig. 7, a drug release test was performed on all samples, using PLA as the control, and each sample exhibited a unique drug release behavior. Initially, all the samples showed a burst release within the first 6 h, followed by a sustained and controlled release that continued up to 168 h. The percentage of curcumin release from different samples was as follows: PLA-Cur > PLA-Cur-0.01%CNTs > PLA-Cur-0.03%CNTs > PLA-Cur-0.05%CNTs. The PLA-Cur sample presented the highest release, while the release decreased in the PLA-Cur-0.05%CNTs sample. This decrease in release can be attributed to the hydrophobic nature of carbon nanotubes, which inhibits the release of curcumin, a hydrophobic compound itself. Curcumin can be loaded onto carbon nanotubes through π-π stacking interactions46 potentially reducing the initial burst release. As a result, using carbon nanotubes can lead to a decrease in side effects and enhance drug resistance.
Measuring the contact angle of nanofiber samples with water
The contact angle measurement method is used to investigate the wettability and water repellency of wound dressings. In this analysis, the contact angle of nanofibers with water was measured for PLA, PLA-Cur, PLA-Cur-0.05%CNTs, PLA-Cur-0.03%CNTs, and PLA-Cur-0.01%CNTs. As illustrated in Fig. 8 and detailed in Table 2, the samples containing curcumin exhibited higher water absorption, while those with added carbon nanotubes became more water-repellent. Overall, the presence of curcumin increased the wettability of the samples, while the addition of carbon nanotubes made them more hydrophobic. The surface tension of pure PLA is measured to be 30.7 mN·m− 1, which is lower compared to PLA samples that CNTs. This reduction in surface tension for pure PLA indicates that the addition of CNTs alters the material’s interfacial properties, likely due to the interaction between the polymer matrix and the nanotubes. Such modifications can significantly influence the overall behavior of the nanofibers, including its wettability, adhesion, and compatibility with other substances.
Antibacterial test on wound dressings
The antibacterial test was used to measure the efficacy of wound dressings against microorganisms. Staphylococcus aureus bacteria were cultured on electrospun nanofibers, and after 24 h, the bacteria in the culture dish were counted.
Based on the antibacterial results and the colony images on the samples (Table 3; Fig. 9), it is revealed that samples containing carbon nanotubes are antibacterial. Furthermore, with a higher concentration of carbon nanotubes, the antibacterial properties of the wound dressings are increased. Additionally, curcumin did not exhibit antibacterial properties due to its low concentration in the PLA-Cur sample.
Curcumin, despite its well-known antibacterial properties, may exhibit limited activity in the PLA-Cur composite due to its restricted release or poor dispersion within the polymer matrix. In such a configuration, the curcumin molecules may become trapped or immobilized, reducing their ability to interact effectively with bacterial cells. However, when CNTs are incorporated into the PLA-Cur composite, the antibacterial activity significantly increases. This enhancement can be attributed to the synergistic effects of the CNTs, which improve the dispersion of curcumin and facilitate its release. Additionally, CNTs themselves possess antimicrobial properties and can disrupt bacterial membranes, further amplifying the overall antibacterial performance of the composite. The combination of these factors likely accounts for the observed improvement in activity47,48,49.
Investigation of water absorption of electrospun fibers
The water absorption capacity of wound dressings was evaluated using a water absorption test, with the results summarized in Table 4. The results show that the PLA sample had the highest water absorption, followed by the sample containing curcumin. However, samples containing carbon nanotubes demonstrated lower water absorption compared to other samples. Generally, the presence of curcumin and carbon nanotubes in the nanofibers reduces the percentage of water absorption.
Figure 10 illustrates a comparison of the properties of PLA and its composites containing curcumin, along with varying concentrations of CNTs. The provided data compares the properties of PLA and its composites with curcumin and varying concentrations of CNTs. Pure PLA exhibits the lowest tensile strength and moderate elongation at break (26.94%), while the addition of curcumin enhances tensile strength and slightly increases elongation (27.59%). Introducing CNTs further improves tensile strength, with PLA-Cur-0.05%CNTs achieving the highest value (2.8857 MPa). However, elongation at break decreases significantly as CNT content increases, indicating reduced flexibility. The weight% of thermal decomposition in PLA exhibits the highest value. However, with the incorporation of curcumin and CNTs into PLA, the thermal decomposition slightly decreases. Additionally, the release of curcumin diminishes as the concentration of CNTs increases. The contact angle and surface tension exhibit minimal variation, indicating comparable hydrophobicity across samples. Antibacterial efficacy improves notably with CNT incorporation, peaking at 78.95% for PLA-Cur-0.05%CNTs. Water absorption decreases with CNT addition. Overall, the inclusion of CNTs enhances mechanical strength, antibacterial properties, and thermal stability But it reduces the flexibility and release of curcumin.
Cytotoxicity and cell viability
Cytotoxicity of wound dressings and cell proliferation of L929 cells cultured on fibers are shown in Fig. 11. The toxicity of nanofibers was evaluated by comparing the number of remaining cells with the control sample on days 3 and 7. The sample, without nanofibers in the cell culture medium, served as a control. As shown in Fig. 12, all fiber samples exhibited no toxicity and demonstrated a high survival rate. These findings indicate that the presence of curcumin and carbon nanotubes at low concentrations does not have an adverse effect on cell viability. Furthermore, the non-toxic nature of the samples indicates that potentially harmful solvents, such as DCM and DMF, were effectively removed during the sterilization process50.
Cell adhesion
To evaluate the culture’s morphology and adhesion, the cells were cultured on nanofiber wound dressings and examined by SEM analysis. According to Fig. 13, by the second day, the cultured cells had attached to the surface of the wound dressings. Since the components of the wound dressings are biocompatible, acceptable cellular attachment was expected. Cells were widely distributed in all fibers and grew well. The SEM images exhibited polygonal shapes with filopodial extensions, indicating cell spreading. The cell culture structure showed no specific orientation, and cell dispersion in all directions was observable. Prominent cellular features were visible on the sample surfaces. Notably, no morphological differences were observed between cells on the surfaces of polylactic acid fibers and curcumin-loaded nanofibers or carbon nanotubes. Therefore, it can be concluded that adding curcumin and carbon nanotubes did not adversely affect cellular adhesion51.
Theoretical calculation of the equilibrium constant of the keto-enol form of Curcumin
The impact of tautomerism in drugs is significant. Tautomerism refers to the phenomenon where a molecule can exist in multiple forms due to the migration of a hydrogen atom. This can affect the drug’s stability, solubility, and biological activity52. In pharmaceuticals, understanding tautomerism is crucial for drug design and optimization to ensure the desired therapeutic effects. Additionally, tautomerism can influence the pharmacokinetics and pharmacodynamics of a drug, impacting its overall efficacy and safety profile53. Curcumin can exist in two forms, keto and enol. Both forms have different chemical properties and can be drug targets for various therapeutic applications. In keto form, curcumin exerts antioxidant activity52. The enol form is prone to degradation54. Hence, Gaussian calculations were employed to investigate the transition state and equilibrium constant for the keto-enol transformations of curcumin in various solvents. In this research, the G09 program was utilized for conducting all quantum chemical computations. The ground state geometries were optimized using the DFT functional with the 6-311G(d, p) + basis set55. The minimum energy of compounds, transition states, equilibrium constant, and HOMO-LUMO energy of keto-enol forms of curcumin were determined in various solvents, including water, ethanol, acetone, toluene, chloroform solvents, and solvent-free. According to Fig. 14, the enol form can exist as two stereoisomers of enol-NHB and enol-HB. The isomer that forms an intramolecular hydrogen bond is more stable than the other enol form. Additionally, based on energy levels, the presence of the enol-HB compound with hydrogen bonding is more favorable compared to the enol-NHB. Also, in a compound with a longer bond length, it shows that hydrogen bonding has taken place.
The presence of hydrogen bonding can be detected by examining the covalent bond length between the oxygen and hydrogen carbon in the curcumin forms of enol-HB and enol-NHB. Table 5 shows the bond lengths and energies of the enol-NHB and enol-HB compounds of curcumin.
In the enol-HB form, the lengths of the O5-H28 and C21-O6 bonds increase slightly due to the formation of a hydrogen bond and interaction with another electronegative atom. The C20-O5 bond length in the Enol-HB form is reduced due to the formation of a partial hydrogen bond between O6 and H28. Therefore, due to the stability of the Enol-HB form in the calculations, this form was used.
After choosing the most optimal enol form, the free energy was obtained for all six keto-enol tautomerizations with different solvents. As can be seen in Fig. 15, in general, the enol form is thermodynamically more favorable in all solvents except water, and the energy of the keto form of curcumin is at a higher level.
By comparing the tautomerization transition of enol to keto in solvents, it can be seen that water solvent has the lowest activation energy with 56.03 kcal/mol, and toluene solvent has the highest activation energy with an energy barrier of 58.12 kcal/mol. In nonpolar solvents, curcumin predominantly exists in the enol form due to an intramolecular hydrogen bond. However, in polar solvents, it undergoes a partial transformation into the keto form, while the reason is related to the stabilization of the keto form of curcumin with polar solvent molecules56. In general, the tautomerization energy barrier for solvents is solvent-free > toluene > chloroform > acetone > ethanol > water. The most stable keto form is water solvent, and the most unstable keto form is 10.7 kcal/mol for toluene. Regarding the enol form, the most stable and unstable solvents are water and toluene, respectively, with 0.3 and 8.5 kcal/mol. Based on the activation energy barriers, it can be inferred that the tautomerization of enol-keto occurs more readily in solvents with higher polarity, in contrast, the activation energy barrier is greater in the toluene solvent.
Table 6 shows the equilibrium constant of the tautomerization reaction in solvents. As it is known, the equilibrium constant for the keto-enol tautomerization is higher than that of the enol-keto tautomerization. This indicates that the conversion rate from keto to enol form is much faster than the reverse reaction from enol to keto. Of course, in water solvent, the conversion of the enol form to the keto form is faster due to the stability of the keto form in non-polar compounds. These findings align with the transition states depicted in Fig. 15. The largest equilibrium constant is related to the tautomerization of enol-keto in water solvent with 1.73. According to the equilibrium constant, it is predicted that enol-keto conversions in water solvent will be easier. Conversely, the equilibrium constant in toluene solvent has the lowest value at 0.042. The reason for easier tautomerization in polar solvents is due to the ability of polar solvents to stabilize charged or partially charged species. This stabilization lowers the energy barrier for tautomerization, making the process more favorable in polar solvents than in non-polar solvents. Additionally, polar solvents can facilitate hydrogen bonding interactions and further stabilize tautomeric forms. Therefore, the presence of polar solvents can enhance the tautomerization process by providing a favorable environment for the conversion between tautomeric forms56,57,58. In general, the equilibrium constant for converting enol to keto is water > ethanol > acetone > chloroform > toluene. The rate of tautomerization is generally faster in polar solvents compared to non-polar solvents. This is because polar solvents can stabilize the transition state and intermediates during the tautomerization process through dipole-dipole interactions or hydrogen bonding. Polar solvents, due to their high dipole moment, can interact with the charged or polar species formed during the tautomerization process and make them more stable. These stabilizing effects lower the activation energy required for the reaction, thereby increasing the reaction rate. In contrast, non-polar solvents lack these stabilizing interactions, leading to slower tautomerization57,59,60.
The use of HOMO-LUMO energy in chemistry is to understand and predict the reactivity and stability of chemical compounds. The HOMO-LUMO energy gap is used to understand the reactivity and stability of molecules as well as to predict their electronic and optical properties. This information is valuable in various applications such as drug design, materials science, and catalysis. Therefore, for this purpose, the chemical activity of keto-enol forms in different solvents was investigated. According to Fig. 16, the highest gap energy in keto curcumin forms is related to chloroform solvent with 3.68 eV, which shows the chemical stability of keto curcumin form. A larger HOMO-LUMO gap generally correlates with lower chemical reactivity, as it indicates a greater energy barrier for electronic transitions. The high HOMO-LUMO energy gap in chloroform indicates that the chemical stability is enhanced in this solvent environment. For instance, a higher energy gap might reduce the likelihood of degradation or unwanted side reactions, potentially leading to an extended shelf-life for curcumin-based drugs stored in formulations that mimic or use chloroform-like conditions. Also, keto form in water and solvent-free has the most active possible state with 3.64 and 3.58 eV, respectively. For the enol form, the highest and lowest activities are related to water and solvent-free with 3.02 and 3.18 eV, respectively. In drug design, leveraging such insights could guide the selection of solvents and excipients that preserve the integrity of curcumin61.
The energy levels and distribution of frontier orbitals are critical factors influencing the antioxidant properties of curcumin. As illustrated in Fig. 16, the highest HOMO orbital energies are predominantly distributed the entire molecule. The electron-donating capability of a molecule is directly associated with its HOMO energy levels; Molecules with higher HOMO orbital energies have stronger electron-donating ability. According to the data presented in Table 7, the enol form of curcumin dissolved in toluene exhibits the highest HOMO energy (-5.716 eV) among the examined compounds, thereby demonstrating the greatest electron-donating ability in this solvent environment61.
The DFT computational method is developed to complement experimental findings by offering theoretical insights and aiding in the interpretation of experimental results. Although the experiments and calculations were conducted independently, the DFT outcomes play a crucial role in elucidating mechanisms and validating experimental observations. Furthermore, a comparison of experimental data from prior studies with the computational results of this research reveals that the accuracy of the data derived from DFT calculations aligns closely with experimental data60. While DFT calculations are commonly employed by researchers, this study uniquely investigates curcumin in various solvents using DFT calculations for the first time, providing deeper insight into the keto-enol tautomer transition states.
Conclusion
In this research, polylactic acid-curcumin wound dressing containing carbon nanotubes was made by the electrospinning method. FT-IR analysis confirmed the chemical structure, and the addition of CNTs broadened and observed new peaks. SEM images revealed a change in the morphology of the wound dressing, creating a denser structure with the addition of CNTs. Tensile tests demonstrated improved strength with the incorporation of CNTs. The release rate of curcumin decreased due to the hydrophobic nature of CNTs, and the combination of curcumin and carbon nanotubes reduced water absorption by the nanofibers. Additionally, antibacterial properties were enhanced by the presence of CNTs, while curcumin alone did not exhibit antibacterial properties. Cytotoxicity tests indicated that low concentrations of curcumin and carbon nanotubes did not negatively impact cell viability. Investigation of the transition state and equilibrium constant of keto-enol tautomerization was done by DFT calculations. The results showed that the activation energy barrier of the enol to keto form conversion increases from polar to nonpolar solvents, and the equilibrium constant in keto-enol conversions is higher than that in enol-keto. Also, HOMO-LUMO energy studies show that enol and keto forms are most active in water solvent. These findings suggest the potential for utilizing this nanofiber wound dressing in medical applications.
Data availability
All data generated or analyzed during this study are included in this article.
References
Zhang, C., Merana, G. R., Harris-Tryon, T. & Scharschmidt, T. C. Skin immunity: dissecting the complex biology of our body’s outer barrier. Mucosal Immunol. 15 (4), 551–561. https://doi.org/10.1038/S41385-022-00505-Y (2022).
Guo, S. & DiPietro, L. A. Factors affecting wound healing. J. Dent. Res. 89 (3), 219. https://doi.org/10.1177/0022034509359125 (2010).
Boateng, J. S., Matthews, K. H., Stevens, H. N. E. & Eccleston, G. M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 97 (8), 2892–2923. https://doi.org/10.1002/JPS.21210 (2008).
Pilehvar-Soltanahmadi, Y. et al. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. Mini-Reviews Med. Chem. 18 (5), 414–427. https://doi.org/10.2174/1389557517666170308112147 (2017).
Rezvani Ghomi, E., Niazi, M. & Ramakrishna, S. The evolution of wound dressings: from traditional to smart dressings. Polym. Adv. Technol. 34 (2), 520–530. https://doi.org/10.1002/PAT.5929 (2023).
Yousefian, F. et al. Antimicrobial wound dressings: A concise review for clinicians. Antibiot. 2023. 12, Page 1434. (9), 1434. https://doi.org/10.3390/ANTIBIOTICS12091434 (2023).
Farahani, M. & Shafiee, A. Wound healing: from passive to smart dressings. Adv. Healthc. Mater. 10 (16), 2100477. https://doi.org/10.1002/ADHM.202100477 (2021).
Nguyen, H. M., Ngoc Le, T. T., Nguyen, A. T., Thien Le, H. N. & Pham, T. T. Biomedical materials for wound dressing: recent advances and applications. RSC Adv. 13 (8), 5509–5528. https://doi.org/10.1039/D2RA07673J (2023).
Sood, A., Granick, M. S. & Tomaselli, N. L. Wound dressings and comparative effectiveness data. Adv. Wound Care. 3 (8), 511. https://doi.org/10.1089/WOUND.2012.0401 (2014).
Laurano, R., Boffito, M., Ciardelli, G. & Chiono, V. Wound dressing products: A translational investigation from the bench to the market. Eng. Regen. 3 (2), 182–200. https://doi.org/10.1016/J.ENGREG.2022.04.002 (2022).
Tiscar-González, V. et al. Clinical and economic impact of wound care using a polyurethane foam multilayer dressing. Adv. Skin. Wound Care. 34 (1), 23. https://doi.org/10.1097/01.ASW.0000722744.20511.71 (2021).
Dhivya, S., Padma, V. V. & Santhini, E. Wound dressings – a review. BioMedicine 5 (4), 24–28. https://doi.org/10.7603/S40681-015-0022-9 (2015).
Moayedi, M. et al. Preparation and assessment of polylactic acid-curcumin nanofibrous wound dressing containing silver nanoparticles for burn wound treatment. Burns Published Online Febr. 27, 107442. https://doi.org/10.1016/J.BURNS.2025.107442 (2025).
Abrari, H. et al. A study on combination of alkaline treatment and PLA/f-CNTs composite coating on corrosion, biocompatibility and antibacterial activity of Mg alloy. Mater. Today Commun. 40 https://doi.org/10.1016/j.mtcomm.2024.109867 (2024).
Zeng, J. et al. Poly(vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules 6 (3), 1484–1488. https://doi.org/10.1021/BM0492576 (2005).
Venmathi Maran, B. A., Jeyachandran, S. & Kimura, M. A review on the electrospinning of polymer nanofibers and its biomedical applications. J. Compos. Sci. 2024. 8, Page 32. (1), 32. https://doi.org/10.3390/JCS8010032 (2024).
Hiwrale, A., Bharati, S., Pingale, P., Rajput, A. & Nanofibers A current era in drug delivery system. Heliyon 9 (9), e18917. https://doi.org/10.1016/J.HELIYON.2023.E18917 (2023).
Pham, Q. P., Sharma, U. & Mikos, A. G. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 12 (5), 1197–1211. https://doi.org/10.1089/TEN.2006.12.1197 (2006).
Barkhordari, S. et al. Introducing PCL-Based electrospun nanocomposite wound dressings: synergistic effects of Curcumin and reduced graphene oxide. Polym. Technol. Mater. Published Online Dec. 11 https://doi.org/10.1080/25740881.2024.2378096 (2024).
Abrari, H. et al. A study on combination of alkaline treatment and PLA/f-CNTs composite coating on corrosion, biocompatibility and antibacterial activity of Mg alloy. Mater. Today Commun. 40, 109867. https://doi.org/10.1016/J.MTCOMM.2024.109867 (2024).
Samir, A., Ashour, F. H., Hakim, A. A. A. & Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. Npj Mater. Degrad. 2022 61. 6 (1), 1–28. https://doi.org/10.1038/s41529-022-00277-7 (2022).
El Foujji, L., Qaiss, A. & kacem, Bouhfid, R. Advanced biodegradable polymer nanocomposites for rechargeable lithium and sodium-ion battery applications. Biodegrad Biocompatible Polym. Nanocomposites Process. Charact. Appl. Published Online January. 1, 353–395. https://doi.org/10.1016/B978-0-323-91696-7.00014-3 (2023).
Ahmed, S. & Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 10 (1), 27–37. https://doi.org/10.1016/J.ALS.2016.04.001 (2016).
Oliveira, C., Sousa, D., Teixeira, J. A., Ferreira-Santos, P. & Botelho, C. M. Polymeric biomaterials for wound healing. Front. Bioeng. Biotechnol. 11, 1136077. https://doi.org/10.3389/FBIOE.2023.1136077/BIBTEX (2023).
Xu, R. et al. Recent advances in biodegradable and biocompatible synthetic polymers used in skin wound healing. Mater. (Basel Switzerland). 16 (15). https://doi.org/10.3390/MA16155459 (2023).
Sikka, M. P. & Midha, V. K. The role of biopolymers and biodegradable polymeric dressings in managing chronic wounds. Adv text wound care. Published Online January. 1, 463–488. https://doi.org/10.1016/B978-0-08-102192-7.00016-3 (2019).
Xu, R. et al. Recent advances in biodegradable and biocompatible synthetic polymers used in skin wound healing. Mater. (Basel). 16 (15). https://doi.org/10.3390/MA16155459 (2023).
Miguel, S. P., Ribeiro, M. P. & Coutinho, P. Biomedical applications of biodegradable polymers in wound care. Wound heal res curr trends futur dir. Published Online January. 1, 509–597. https://doi.org/10.1007/978-981-16-2677-7_17 (2021).
Zhang, H. et al. Developing natural polymers for skin wound healing. Bioact Mater. 33, 355–376. https://doi.org/10.1016/J.BIOACTMAT.2023.11.012 (2024).
Xie, J. & Wang, C. H. Electrospun micro- and nanofibers for sustained delivery of Paclitaxel to treat C6 glioma in vitro. Pharm. Res. 23 (8), 1817–1826. https://doi.org/10.1007/S11095-006-9036-Z (2006).
Kausar, A. Carbonaceous nanofillers in polymer matrix. Polym nanocomposites with carbonaceous nanofillers Aerosp appl. Published Online January. 1, 23–53. https://doi.org/10.1016/B978-0-323-99657-0.00009-0 (2023).
Ashwini, T. et al. Transforming wound management: nanomaterials and their clinical impact. Pharmaceutics 15 (5). https://doi.org/10.3390/PHARMACEUTICS15051560 (2023).
Shalaby, M. A., Anwar, M. M. & Saeed, H. Nanomaterials for application in wound healing: current state-of-the-art and future perspectives. J. Polym. Res. 2022 293. 29 (3), 1–37. https://doi.org/10.1007/S10965-021-02870-X (2022).
Zare, H. et al. Carbon nanotubes: smart drug/gene delivery carriers. Int. J. Nanomed. 16, 1681. https://doi.org/10.2147/IJN.S299448 (2021).
Ali, A., Rahimian Koloor, S. S., Alshehri, A. H. & Arockiarajan, A. Carbon nanotube characteristics and enhancement effects on the mechanical features of polymer-based materials and structures – A review. J. Mater. Res. Technol. 24, 6495–6521. https://doi.org/10.1016/J.JMRT.2023.04.072 (2023).
Yang, T., Wu, D., Lu, L., Zhou, W. & Zhang, M. Electrospinning of polylactide and its composites with carbon nanotubes. Polym. Compos. 32 (8), 1280–1288. https://doi.org/10.1002/PC.21149 (2011).
Cui, R. et al. Antimicrobial film based on polylactic acid and carbon nanotube for controlled cinnamaldehyde release. J. Mater. Res. Technol. 9 (5), 10130–10138. https://doi.org/10.1016/j.jmrt.2020.07.016 (2020).
Kou, H. et al. Effect of Curcumin on rheumatoid arthritis: a systematic review and meta-analysis. Front. Immunol. 14, 1121655. https://doi.org/10.3389/FIMMU.2023.1121655/BIBTEX (2023).
Wilken, R., Veena, M. S., Wang, M. B., Srivatsan, E. S. & Curcumin A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011 101. 10 (1), 1–19. https://doi.org/10.1186/1476-4598-10-12 (2011).
Fadus, M. C., Lau, C., Bikhchandani, J., Lynch, H. T. & Curcumin An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit Complement. Med. 7 (3), 339–346. https://doi.org/10.1016/J.JTCME.2016.08.002 (2017).
Gupta, S. C., Patchva, S. & Aggarwal, B. B. Therapeutic roles of Curcumin: lessons learned from clinical trials. AAPS J. 15 (1), 195. https://doi.org/10.1208/S12248-012-9432-8 (2013).
Sharma, R. A., Gescher, A. J., Steward, W. P. & Curcumin The story so Far. Eur. J. Cancer. 41 (13), 1955–1968. https://doi.org/10.1016/J.EJCA.2005.05.009 (2005).
Hewlings, S. J., Kalman, D. S. & Curcumin A review of its’ effects on human health. Foods 6 (10). https://doi.org/10.3390/FOODS6100092 (2017).
Dhurai, B. et al. Electrospinning of Curcumin loaded Chitosan/poly (lactic acid) nanofilm and evaluation of its medicinal characteristics. Front. Mater. Sci. 2013 74. 7 (4), 350–361. https://doi.org/10.1007/S11706-013-0222-8 (2013).
Han, C. et al. Enhanced drug delivery, mechanical properties and antimicrobial activities in poly(lactic acid) nanofiber with mesoporous Fe3O4-COOH nanoparticles. Colloids Surf. Physicochem Eng. Asp. 559, 104–114. https://doi.org/10.1016/J.COLSURFA.2018.09.012 (2018).
Koupaei Malek, S. et al. Adsorption and in vitro release study of Curcumin form polyethyleneglycol functionalized multi walled carbon nanotube: kinetic and isotherm study. Daru 27 (1), 9–20. https://doi.org/10.1007/S40199-018-0232-2 (2019).
Sagadevan, M. M. Enhancing biocompatibility and functionality: carbon nanotube-polymer nanocomposites for improved biomedical applications. J. Drug Deliv Sci. Technol. 99, 105958. https://doi.org/10.1016/J.JDDST.2024.105958 (2024).
Moniruzzaman, M. & Winey, K. I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 39 (16), 5194–5205. https://doi.org/10.1021/MA060733P (2006).
Sonowal, L. & Gautam, S. Advancements and challenges in carbon nanotube-based drug delivery systems. Nano-Structures Nano-Objects. 38, 101117. https://doi.org/10.1016/J.NANOSO.2024.101117 (2024).
Elsayed, R. E., Madkour, T. M. & Azzam, R. A. Tailored-design of electrospun nanofiber cellulose acetate/poly(lactic acid) dressing Mats loaded with a newly synthesized sulfonamide analog exhibiting superior wound healing. Int. J. Biol. Macromol. 164, 1984–1999. https://doi.org/10.1016/J.IJBIOMAC.2020.07.316 (2020).
Ren, Y. et al. Stereocomplexed electrospun nanofibers containing Poly (lactic acid) modified quaternized Chitosan for wound healing. Carbohydr. Polym. 247, 116754. https://doi.org/10.1016/J.CARBPOL.2020.116754 (2020).
Puglisi, A., Giovannini, T., Antonov, L. & Cappelli, C. Interplay between conformational and solvent effects in UV-visible absorption spectra: Curcumin tautomers as a case study. Phys. Chem. Chem. Phys. 21 (28), 15504–15514. https://doi.org/10.1039/C9CP00907H (2019).
Priyadarsini, K. I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Mol Vol 19, Pages 20091–20112. 2014;19(12):20091–20112. (2014). https://doi.org/10.3390/MOLECULES191220091
Yang, F. et al. Curcumin inhibits formation of amyloid Β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 280 (7), 5892–5901. https://doi.org/10.1074/JBC.M404751200 (2005).
Faal, M., Mahyari, M., Hosseini, S. G., Tavangar, S. & Zarei, M. A. Synthesis of HAZPMA-g-PHPGN as a well-defined energetic binder: experimental and computational approaches. Mater. Today Commun. 28, 102601. https://doi.org/10.1016/J.MTCOMM.2021.102601 (2021).
Kazakova, O., Lipkovska, N. & Barvinchenko, V. Keto-enol tautomerism of Curcumin in the Preparation of nanobiocomposites with fumed silica. Spectrochim Acta Part. Mol. Biomol. Spectrosc. 277, 121287. https://doi.org/10.1016/J.SAA.2022.121287 (2022).
Jezuita, A., Wieczorkiewicz, P. A., Szatylowicz, H. & Krygowski, T. M. Effect of the solvent and substituent on tautomeric preferences of Amine-Adenine tautomers. ACS Omega. 6 (29), 18890–18903. https://doi.org/10.1021/ACSOMEGA.1C02118 (2021).
Cook, G. & Feltman, P. M. Determination of solvent effects on Keto—Enol equilibria of 1,3-Dicarbonyl compounds using NMR. J. Chem. Educ. 84 (11), 1827–1829. https://doi.org/10.1021/ED084P1827 (2007).
Ruiz, D. L., Albesa, A. G., Ponzinibbio, A., Allegretti, P. E. & Schiavoni, M. M. Solvent effects on tautomerics equilibria in β-ketonitriles: NMR and theoretical studies. J. Phys. Org. Chem. 23 (10), 985–994. https://doi.org/10.1002/POC.1764 (2010).
Siani, G., Angelini, G., De Maria, P., Fontana, A. & Pierini, M. Solvent effects on the rate of the keto–enol interconversion of 2-nitrocyclohexanone. Org. Biomol. Chem. 6 (22), 4236–4241. https://doi.org/10.1039/B813011F (2008).
Yang, C. et al. The relationships between direct substituents, aromaticity and kinetic stability of pentazole ring. FirePhysChem 3 (4), 350–355. https://doi.org/10.1016/J.FPC.2023.04.003 (2023).
Author information
Authors and Affiliations
Contributions
Masoud Faal: Conceptualization, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing, drafting. editing, modeling.Tahmineh Ahmadi: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, editing.Mahmood Faal: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, editing, modeling, Writing.Fatemeh Dehgan: editing, resources, analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Faal, M., Faal, M., Ahmadi, T. et al. Fabrication and evaluation of polylactic acid-curcumin containing carbon nanotubes (CNTs) wound dressing using electrospinning method with experimental and computational approaches. Sci Rep 15, 13398 (2025). https://doi.org/10.1038/s41598-025-98393-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98393-2