Abstract
The Phase Angle (PhA) and water cell distribution ratios are essential indicators of inflammation linked to serum inflammatory markers. We aim to investigate the relationship between PhA and extracellular water to total body water (ECW/TBW) ratio with hematological inflammatory markers. The study utilized data from the PERSIAN Organizational Cohort Study in Mashhad (POCM), incorporating information from bioelectrical impedance analysis (BIA) and blood tests. It examined two groups: individuals of normal weight (N = 1654) and those categorized as overweight or obese (N = 3651). In addition to Hemoglobin (Hgb), hematocrit (Hct), Platelet (Plt), White blood cell (WBC) and its components, the study also computed various hematologic inflammatory markers, such as platelet to HDL ratio (PHR), platelet to lymphocyte ratio (PLR), monocyte to HDL ratio (MHR), lymphocyte to HDL ratio (LHR), RDW to lymphocyte ratio (RLR), RDW to platelet ratio (RPR), and granulocyte to lymphocyte ratio (GLR). In the adjusted model, an increase in PhA is linked to increases in Hgb (Normal-weight: 17.1%, BMI > 25: 12.3%) and Hct (Normal-weight: 15.3%, BMI > 25: 13.6%), WBC (Normal-weight: 9%, BMI > 25: 6.8%), and RPR (Normal-weight: 17.1%, BMI > 25: 12.3%). Additionally, in the BMI > 25 categories, an increase in PhA is associated with increases in Plt (16.7%), Lymphocytes (5.6%), and PHR (5.2%). Furthermore, there is a decrease in RPR (Normal-weight: 7.4%, BMI > 25: 6.6%) with a unit increase in PhA. A unit increase in ECW/TBW is associated with a decrease in Hgb (Normal-weight: 19.9%, BMI > 25: 17.3%) and Hct (Normal-weight: 17.8%, BMI > 25: 17.6%). This is also linked to decreased WBC (9.6%) in the normal-weight category. Furthermore, in the BMI > 25 category, there are decreases in Plt (5.5%), RLR (6.5%), RPR (6.9%), GLR (5.9%), Granulocytes (7.5%), and Lymphocytes (8.3%) with an increase in ECW/TBW. The findings revealed that ECW/TBW demonstrates a stronger correlation with hematological markers than PhA, with these associations being particularly significant among normal-weight individuals. These results highlight the need for further research to explore the underlying mechanisms driving these relationships.
Similar content being viewed by others
Introduction
Bioelectrical Impedance Analysis (BIA) assesses body composition by measuring the relationship between impedance and body water content. Phase angle (PhA) is an essential indicator of inflammation. It is calculated by taking the ratio of the raw measures of resistance (R) and reactance (Xc) obtained from BIA at a frequency of 50 kHz1. PhA is inversely related to the extracellular water to total body water ratio (ECW/TBW) derived from BIA2. Both PhA and water cell distribution ratios are used to evaluate cell integrity and how it is affected by body fat. Higher body fat leads to a reduction in PhA and an increase in ECW/TBW3. Multiple studies have confirmed that PhA is a crucial indicator of inflammation in the body4 and is linked to various health outcomes. For instance, in patients with cardiovascular diseases, PhA was negatively associated with C-reactive protein (CRP) and tumor necrosis factor alpha (TNF-α) in 80% and 100% of the related studies, respectively5.
Inflammatory markers are essential for diagnosing and monitoring various medical conditions. They fall into two main categories: serum and hematological inflammatory markers, each serving distinct roles in clinical practice. Serum inflammatory markers are substances found in the blood that indicate inflammation. Common examples include cytokines, CRP, Procalcitonin (PCT), Interleukin-6 (IL-6), and Serum amyloid A (SAA)6. On the other hand, measuring peripheral blood cells, the source of some of these cytokines, by complete blood count (CBC) is a practical, cost-effective, and rapid procedure that can provide valuable insights into the body’s inflammatory status7. Hematological inflammatory markers, derived from the CBC, include white blood cell (WBC) count, platelet counts, and some ratios. These markers are often used for acute conditions8.
The evidence suggests that PhA is significantly inversely associated with various serum inflammatory markers, including IL-1β, CRP, IL-6, albumin, IL-10, TNF-α, and multisystem inflammatory syndrome (MIS)9. There have been fewer studies on the association between PhA and CBC parameters than serum inflammatory markers. Hori et al. found that hemoglobin (Hgb) and hematocrit (Hct) increased with higher PhA in diabetic adults. However, ECW/TBW had an inverse association with Hgb and Hct10. While one study demonstrated a higher lymphocyte count in individuals with higher PhA11, another study did not find an association between WBC count and PhA12. Our study aimed to investigate the relationship between PhA and ECW/TBW, which serve as indicators of cellular health and hematological inflammatory markers. We also sought to determine if this relationship varies among individuals with different BMI classifications, including normal-weight and over-weight participants.
Methods
Study population
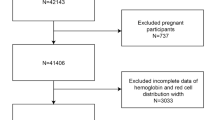
In our current research, we conducted an analysis using data from the PERSIAN Organizational Cohort Study in Mashhad (POCM), which involved a significant study population of 12,000 employees from Mashhad University of Medical Sciences across 35 organizational categories, including 14 hospitals, seven faculties, seven vice-chancellors, five health centers, three research institutes, and the central administrative department13. The study population comprised 5305 participants (2939 females and 2366 males). Among them, 1654 individuals had a normal weight (18.5 < body mass index (BMI) ≤ 24.99) with an average age of 44.13 ± 8.80, while 3651 individuals were classified as overweight or obese (BMI ≥ 25) with an average age of 46.31 ± 9.09. Inclusion criteria required participants to have complete biochemical and body composition data and a normal-weight, overweight, or obese BMI, excluding those underweight.
All methods were conducted in accordance with the Declaration of Helsinki. Ethical approval was secured from the Ethics Committee of Mashhad University of Medical Sciences (MUMS) (4031222), thereby ensuring the prioritization of participants’ rights and welfare throughout the research process.
Assessment of phase angle and water cell distribution ratios
We collected bioelectrical impedance analysis data using the Inbody 770 device from Inbody Corporation, Seoul, Korea. This device applies a low-level alternating current (50 to 1000 kHz) that encounters resistance from the body’s tissues. Participants stood barefoot on the footplate, held hand electrodes, and extended their arms. The key body composition measurements obtained included intracellular and extracellular water, total body water, body fat percentage, BMI, and PhA. Additionally, weight, measured in kilograms, height in centimeters, and waist circumferences (WC) were recorded following the protocols established by the US National Institutes of Health14, with all values recorded to the nearest decimal point.
Assessment of hematological markers
In the Mashhad Persian cohort study, participants were required to fast for 10 to 12 h and refrain from dyeing or cutting their hair and nails for at least two weeks. Approximately 25 ml of blood was drawn using one 9 ml clot aspirator and three 6 ml EDTA aspirators (Greiner Bio-One International GmbH, Kremsmünster, Austria). The blood samples were then processed to obtain whole blood, plasma, and serum samples. Cell count tests (NK Alpha, Nihon Kohden, Tokyo, Japan) and biochemical analyses (plate_number_1 automatic analyzer, Biotecnica Instruments, Rome, Italy) were conducted to evaluate the lipid profile, including total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides. The following hematological inflammatory markers were calculated using the formulas provided:
Statistical analysis
All statistical analyses were conducted precisely and accurately using the SPSS statistical package (version 26.0 for Windows). Continuous data were presented as mean ± standard deviation, and overall differences were assessed using either the Student’s t-test or analysis of variance (ANOVA). We checked the normality of the variables using the Kolmogorov–Smirnov test. We conducted a nonparametric analysis using the Mann–Whitney test for variables not showing a normal distribution. We used linear regression to examine the relationship between PhA and ECW/TBW with hematological markers in crude and adjusted models. We controlled for factors such as age, sex, energy intake, smoking habits, drug use, and alcohol consumption, as well as the presence of diabetes and hypertension, to identify any significant differences among the groups. The degree of variation or similarity between and within groups was determined based on the p-value, with a significance threshold set at p < 0.05. Additionally, scatterplots were generated using SPSS for the indicators that showed statistical significance in the linear regression analysis. PhA, or ECW/TBW, was plotted as an independent variable on the X-axis, while the dependent variables were represented on the Y-axis.
Results
Characteristics of subjects
A total of 5,305 participants were divided into two groups: those with a normal BMI (N = 1,654) and those classified as overweight or obese (N = 3,651). The physical and laboratory characteristics of the study subjects are detailed in Table 1. In the normal BMI category, 96.4% reported non-smoking status, with an average physical activity level of 39.0 ± 5.0 and a waist circumference of 88.6 ± 7.2 cm. In the overweight or obese group, 95% of participants were non-smokers, with an average physical activity level of 38.4 ± 5.7 and a waist circumference of 100.1 ± 9.1 cm. Comparative analysis showed significantly higher means of indices such as WBC, Hgb, Hct, Plt, RDW, PHR, PLR, MHR, LHR, TBW, and PhA in the BMI ≥ 25 groups. Additionally, the prevalence of diabetes or hypertension was higher in this group compared to the normal weight group. Conversely, the GLR, RPR, and HDL levels were higher in the normal weight group than in their overweight or obese counterparts.
Associations between BIA parameters (PhA, ECW/TBW) and hematological parameters in the crude model
The results in Table 2 present the ECW/TBW ratio and PhA associations across two BMI categories in the crude model. In the BMI ≥ 25 group, PhA exhibited significant correlations with all examined markers. In the normal-weight group, PhA demonstrated significant correlations with all examined factors, except for lymphocytes, RLR, and GLR. Similarly, ECW/TBW was associated with nearly all hematological markers analyzed; however, it did not show significant correlations with Plt and RLR in the normal-weight group. In individuals with a BMI ≥ 25, ECW/TBW was not correlated with monocytes and WBC. Additionally, neither BMI category showed a correlation between ECW/TBW and RPR.
Associations between BIA parameters (PhA, ECW/TBW) and hematological parameters in the adjusted model
In Table 3, the adjusted regression results reveal a positive association between PhA and WBC (normal-weight: β: 0.090; BMI ≥ 25: β: 0.068), Hgb (normal-weight: β: 0.171; BMI ≥ 25: β: 0.123), and Hct (normal-weight: β: 0.153; BMI ≥ 25: β: 0.136) in both groups. Furthermore, there is a positive correlation between PhA and PHR (β: 0.052), Lymphocytes (β: 0.056), and Plt (β: 0.167) observed only in the BMI ≥ 25 group, while an inverse relationship with RPR was noted in both groups (normal-weight: β: −0.074; BMI ≥ 25: β: =−0.066). However, no significant associations were found between PhA and the other markers.
Regarding ECW/TBW associations with hematological indicators in the BMI ≥ 25 groups, elevated ECW/TBW were correlated positively with RLR (β: 0.065), RPR (β: 0.069), GLR (β: 0.059), and Granulocyte (β: 0.075). In both weight groups, an inverse association was found between ECW/TBW and Hgb (normal-weight: β: −0.199; BMI ≥ 25: β: −0.173) as well as Hct (normal-weight: β: −0.178; BMI ≥ 25: β: −0.176). An inverse relationship with Plt (β: − 0.055), and Lymphocytes (β: −0.083) were noted only in the overweight and obese group and with WBC (β: −0.096) only in the normal weight group. No significant correlations were observed between ECW/TBW and the other markers. Finally, Figs. 1 (association between phase angle and A) WBC, B) Hgb, C) Hct, D) Lymphocyte, E) Platelet, and F) PHR) and 2 (association between ECW/TBW and A) WBC, B) Hgb, C) Hct, D) Lymphocyte, E) Platelet, F) RPR, G) Granulocyte, and H) GLR) illustrate the scatter plots depicting the statistically significant relationships identified in our analysis.
Discussion
The current research was the first to comprehensively investigate the relationship between PhA and ECW/TBW with hematological markers. After accounting for the influence of confounding factors, most of the associations were no longer significant, highlighting the substantial role of these confounders in the levels of these hematological markers. Our findings revealed that PhA is directly associated with WBC, Hgb, and Hct in both BMI categories and with PHR, Platelet, and Lymphocytes only in the overweight group. Unlike PhA, ECW/TBW, in addition to the aforementioned markers, showed a direct relationship with granulocyte, RLR, RPR, and GLR markers (Fig. 3).
Hemoglobin and hematocrit levels
The evidence consistently shows a correlation between the destruction of red blood cells (RBC) and changes in PhA. For example, Varlet-Marie et al. found a link between the flexibility of RBCs and whole-body impedance at 50 kHz15. Similarly, Tran et al. observed a significant correlation between the release of cytoplasm after RBC hemolysis and the changes in the electrical properties of blood cells16. Our current study also discovered a positive connection between PhA and Hgb and Hct and a negative connection between ECW/TBW and Hgb and Hct in both BMI groups. Interestingly, these correlations were more pronounced in individuals with a normal BMI compared to those with a BMI ≥ 25. Similarly, Hori et al. identified a direct relationship between PhA with Hgb and Hct in adults with diabetes10. In a pilot study, Khatun et al. observed an inverse correlation between PhA and glycated hemoglobin (HbA1c), suggesting the potential of PhA as a valuable biomarker for monitoring metabolic health17. While our findings revealed a positive link between PhA and Hgb levels, it is essential to note that a study by Kim et al. indicated that PhA values are associated with the median value of hemoglobin rather than its variability in hemodialysis patients18. This suggests that stable average hemoglobin levels may reflect nutritional status, as indicated by PhA, but PhA may not influence fluctuations in hemoglobin in the same way.
In line with our research, reports indicate a connection between fluid retention and anemia in chronic kidney disease patients19 and those with heart failure19,20. Also, Hori et al. showed that higher ECW/TBW, Hgb, and Hct values decreased in adults with diabetes10. This association is likely linked to hydration and nutritional levels impacting erythropoiesis. Studies on animals have demonstrated that erythropoiesis is disrupted in dehydrated conditions21,22.
Platelet count
Our research indicates that PhA, as well as ECW/TBW, is linked to increased platelet levels. Additionally, we found that PhA is associated with elevated RHR, whereas ECW/TBW is linked to increased PLR. Devarasu et al. also noted a connection between PhA and increased platelets in children with dengue fever23. An increase in PhA indicates improved cellular integrity and overall health, including platelet health24,25. Moreover, a higher PhA may signify better nutritional status, supporting optimal platelet function and production. On the other hand, platelet-activating factor (PAF) is recognized as a potent inflammatory lipid mediator that plays a crucial role in inflammation, thrombosis, and disease pathophysiology15,16,17,18,19,20. PAF is produced by various cells, including endothelial cells, platelets, macrophages, monocytes, neutrophils, and mast cells, and is activated by various triggers26. Notably, PAF levels are inversely associated with PhA27. Furthermore, several studies have indicated an inverse relationship between PAF and platelet count. In certain inflammatory conditions, PAF can cause platelet sequestration in the microcirculation, particularly in organs like the lungs. For instance, a study demonstrated that allergen exposure in asthma patients led to decreased platelet count correlated with increased PAF28.
WBC and its component counts
Our study demonstrated higher WBC counts in higher PhA levels. Conversely, Lee et al. found no link between PhA and WBC in critically ill patients12. Lee et al.‘s study used PhA as a surveillance tool for postoperative infection in 221 patients admitted to the ICU after abdominal surgery12. Therefore, our different results can be attributed to our larger sample size and our target population, which consists of healthy adults. Also, we found a direct association between ECW/TBW and WBC in the normal-weight group. This is expected because elevated ECW/TBW indicates inflammation, and WBC levels typically increase as part of the immune response29. ECW/TBW, as a marker of inflammation, also affects WBC concentration through changes in fluid balance. During inflammation, ECW increases due to fluid leakage from blood vessels into surrounding tissues. This leads to a relative decrease in plasma volume, which can increase the concentration of WBCs in the bloodstream30.
The study observed a direct relationship between PhA and Lymphocyte count in overweight participants. To support this finding, Vannini et al. also demonstrated a direct association between PhA and Lymphocytes in Hemodialysis patients11. In the present study, ECW/TBW was directly associated with granulocytes while inversely associated with lymphocyte count in overweight participants. This disparity in the relationship between ECW/TBW and these two WBC components can be attributed to their function. When faced with inflammation (indicated by elevated ECW/TBW), lymphocytes migrate to the inflamed tissue31, potentially leading to a decrease in their presence in the bloodstream. Conversely, granulocytes respond to inflammation by increasing in the bloodstream as the initial reaction to infection or injury. The subsequent decrease in granulocytes triggered by inflammatory stimuli could result from a specific expansion of granulocytic progenitors or a general enhancement of the bone marrow’s ability to support hematopoiesis32.
The relationship between PhA and ECW/TBW in normal-weight individuals was more robust than in individuals with a BMI ≥ 25. It is important to note that obesity is associated with chronic inflammation and changes in PhA33. Moreover, obesity can elevate Hgb, Hct levels34, platelet counts35, WBC, and specific components like neutrophils36. Consequently, this factor may impact the linear relationship between cell health indicators and hematological markers. Notably, we observed that ECW/TBW, compared to PhA, was more strongly linked to changes in hematological markers. ECW and TBW provide more general information about bodily fluid distribution and do not specifically indicate cellular health or membrane integrity as PhA does37. Furthermore, the relationship between PhA or ECW/TBW with WBC components (including lymphocytes and granulocytes) was only identified in individuals with BMI ≥ 25. The higher circulating WBC and lymphocyte counts in obese participants could account for this discovery38. A recent study demonstrated higher values of early activated T and B lymphocytes in obese individuals than those of normal-weight39. These findings have significant implications for understanding the health effects of obesity on cellular health and immune response.
Implication
BIA may be a more valuable tool in clinical settings than traditional hematological laboratory tests due to its simplicity. Traditional tests can be prone to errors, and the blood collection process can encounter complications40. The BIA method, being non-invasive, is particularly advantageous for individuals who experience difficulties with blood draws. Our findings reveal a significant association between PhA and the ECW/ICW ratio with Hgb, Hct, and WBC, suggesting potential for indirect estimation methods using BIA measurements, which could benefit both clinical practice and broader applications41. The study emphasizes monitoring PhA and ECW/TBW ratios across various BMI categories. In overweight and obese individuals, a higher PhA is associated with increased platelets and lymphocytes, indicating its potential in managing obesity-related inflammation. Additionally, lower ECW/TBW ratios within this group correlate with further markers, such as granulocyte levels, PLR, RPR, and GLR, suggesting the estimation potential of water distribution in the leukocyte numbers and some rations.
Strengths and limitations
The study comprehensively analyzed the relationship between PhA and ECW/TBW as essential nutritional and cellular health indicators in conjunction with hematological inflammatory markers for the first time. Additionally, we gained specific insights into this relationship across different BMI categories by categorizing participants into two BMI groups. The study delved into indicators related to RBC, including Hgb and Hct, WBC, and its components and ratios. The substantial sample size enabled us to establish these relationships more reliably. However, the study had some limitations. The lack of data on participants’ drug use prevented us from adjusting for drugs that may have impacted the body’s inflammatory state in the regression model. Additionally, the absence of data on specific components of granulocytes (basophils, neutrophils, and eosinophils) restricted our analysis to the overall amount of granulocytes. As a result, we could not calculate specific ratios, such as the neutrophil-to-lymphocyte ratio (NLR), which has been found to have an inverse association with PhA in hemodialysis patients42. Lastly, the sample size of participants with a BMI of 18.5 or less was very small, so we could not investigate the associations in underweight participants. Further studies focusing on this BMI category are suggested.
Conclusion
The results of the current study showed that PhA and ECW/TBW, two markers obtained from BIA, which are usually indicative of inflammation in the body, were correlated with hematological markers. These hematological markers exhibit changes during inflammatory conditions. PhA demonstrated a general association with elevated Hgb, Hct, Platelet, WBC, lymphocyte, and PHR levels. Conversely, ECW/TBW displayed an inverse correlation with Hgb, Hct, Platelet, WBC, and Lymphocyte and a direct correlation with granulocyte and PLR, RPR, and GLR ratios. Overall, the results indicated that ECW/TBW, compared to PhA, exhibited a stronger relationship with hematological markers, possibly due to its capacity to signify inflammation in the body and represent the fluid balance, potentially impacting the concentration of these hematological markers. Therefore, the stronger association with all hematological markers seems reasonable.
Moreover, these connections were more evident in people with a normal weight than those with a BMI ≥ 25. As a result, it is proposed that obesity should be recognized as a potential factor influencing the linear correlation between PhA and ECW/TBW with hematological markers. It is worth noting that PhA and ECW/TBW measures were explicitly linked to lymphocytes and granulocytes but not monocytes. Furthermore, this correlation was only observed in individuals with a BMI ≥ 25. Future research should investigate the potential underlying mechanisms for these disparities.
Data availability
Data is available upon request from Dr. Seyyed Reza Sobhani (Corresponding author).
References
Barbosa-Silva, M. C. & Barros, A. J. Bioelectrical impedance analysis in clinical practice: A new perspective on its use beyond body composition equations. Curr. Opin. Clin. Nutr. Metab. Care. 8(3), 311–317 (2005).
Gray, J. & Kennedy, T. Skeletal muscle mass and extracellular water/total body water independently predict phase angle percentile in young men. Curr. Developments Nutr. 4, nzaa05616 (2020).
Martins, P. C., Junior, C. A. S. A., Silva, A. M. & Silva, D. A. S. Phase angle and body composition: A scoping review. Clin. Nutr. ESPEN 56, 237–250 (2023).
Barrea, L. et al. Phase angle as an easy diagnostic tool of Meta-Inflammation for the nutritionist. Nutrients 13(5), 1446 (2021).
Ceolin, J. et al. Phase angle of bioimpedance as a marker of inflammation in cardiovascular diseases: A systematic review. Nutrition 112, 112064 (2023).
Giannakopoulou, S. P., Antonopoulos, A. & Panagiotakos, D. Serum inflammatory markers used in cardiovascular disease risk prediction models: A systematic review. Angiology 33197241239691. (2024).
Khorasanchi, Z. et al. The association of hematological inflammatory markers and psychological function in COVID-19 patients: A cross-sectional study. Physiol. Rep. 11(24), e15889 (2023).
Tudurachi, B. S., Anghel, L., Tudurachi, A., Sascău, R. A. & Stătescu, C. Assessment of inflammatory hematological ratios (NLR, PLR, MLR, LMR and Monocyte/HDL-Cholesterol Ratio) in acute myocardial infarction and particularities in young patients. Int. J. Mol. Sci. 24(18) (2023).
da Silva, B. R. et al. Phase angle and cellular health: Inflammation and oxidative damage. Rev. Endocr. Metab. Disord. 24(3), 543–562 (2023).
Hori, T. et al. Phase angle and extracellular water-to-total body water ratio estimated by bioelectrical impedance analysis are associated with levels of hemoglobin and hematocrit in patients with diabetes. Heliyon 9(4). (2023).
Vannini, F. D., Antunes, A. A., Caramori, J. C., Martin, L. C. & Barretti, P. Associations between nutritional markers and inflammation in Hemodialysis patients. Int. Urol. Nephrol. 41, 1003–1009 (2009).
Lee, G. R. & Kim, E. Y. Usefulness of phase angle on bioelectrical impedance analysis as a surveillance tool for postoperative infection in critically ill patients. Front. Med. 10, 1111727 (2023).
Tohidinezhad, F. et al. The burden and predisposing factors of non-communicable diseases in Mashhad university of medical sciences personnel: A prospective 15-year organizational cohort study protocol and baseline assessment. BMC Public. Health. 20, 1–15 (2020).
Prevention CfDCa. National Health and Nutrition Examination Survey (NHANES): anthropometry procedures manual. 2007 [cited September, 2024]. [cited September, 2024]. Available from: https://stacks.cdc.gov/view/cdc/50334
Varlet-Marie, E. et al. Leg electrical resistance predicts venous blood viscosity and hematocrit. Clin. Hemorheol. Microcirc. 71(4), 397–402 (2019).
Tran, A. K., Sapkota, A., Wen, J., Li, J. & Takei, M. Linear relationship between cytoplasm resistance and hemoglobin in red blood cell hemolysis by electrical impedance spectroscopy & eight-parameter equivalent circuit. Biosens. Bioelectron. 119, 103–109 (2018).
Khatun, S. et al. Glycated hemoglobin (HbA1c) is independently associated with the bioelectrical impedance phase angle in junior sumo wrestlers: A pilot study. Physiological Rep. 12(9), e16045 (2024).
Kim, D. H. & Oh, D-J. Phase angle values, a good indicator of nutritional status, are associated with median value of hemoglobin rather than hemoglobin variability in Hemodialysis patients. Ren. Fail. 43(1), 327–334 (2021).
Hung, S. C. et al. Association of fluid retention with anemia and clinical outcomes among patients with chronic kidney disease. J. Am. Heart Assoc. 4(1), e001480 (2015).
Androne, A-S. et al. Hemodilution is common in patients with advanced heart failure. Circulation 107(2), 226–229 (2003).
Dunn, C. D. Effect of food or water restriction on erythropoiesis in mice: Relevance to anemia of space flight. Am. J. Physiol. 238(5), R301–R305 (1980).
Giglio, J. M., Alippi, R. M., Barceló, A. C. & Bozzini, C. E. Mechanism of the decreased erythropoiesis in the water deprived rat. Br. J. Haematol. 42(1), 93–99 (1979).
Devarasu, N. & Sudha, G. F. Dual-frequency bioelectrical phase angle to estimate the platelet count for the prognosis of dengue fever in Indian children. Biomedical Engineering/Biomedizinische Technik. 65(4), 417–428 (2020).
da Silva¹, B. R. et al. Phase angle and cellular health: Inflammation and oxidative damage. Reviews Endocr. Metabolic Disorders. 24(3), 543–562 (2023).
Lukaski, H. C. & Talluri, A. Phase angle as an index of physiological status: Validating bioelectrical assessments of hydration and cell mass in health and disease. Rev. Endocr. Metab. Disord. 24(3), 371–379 (2023).
Kelesidis, T. et al. The role of platelet-activating factor in chronic inflammation, immune activation, and comorbidities associated with HIV infection. AIDS Rev. 17(4), 191 (2015).
Detopoulou, P., Fragopoulou, E., Nomikos, T. & Antonopoulou, S. Associations of phase angle with platelet-activating factor metabolism and related dietary factors in healthy volunteers. Front. Nutr. 10, 1237086 (2023).
Burgers, J. A., Bruynzeel, P. L., Mengelers, H. J., Kreukniet, J. & Akkerman, J. W. Occupancy of platelet receptors for platelet-activating factor in asthmatic patients during an allergen-induced bronchoconstrictive reaction. J. Lipid Mediat. 7(2), 135–149 (1993).
Foy, B. H., Sundt, T. M., Carlson, J. C. T., Aguirre, A. D. & Higgins, J. M. Human acute inflammatory recovery is defined by co-regulatory dynamics of white blood cell and platelet populations. Nat. Commun. 13(1), 4705 (2022).
Saravi, B. et al. Capillary leak and endothelial permeability in critically ill patients: A current overview. Intensive Care Med. Experimental. 11(1), 96 (2023).
Sakai, Y. & Kobayashi, M. Lymphocyte ‘homing’and chronic inflammation. Pathol. Int. 65(7), 344–354 (2015).
Ueda, Y., Kondo, M. & Kelsoe, G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 201(11), 1771–1780 (2005).
Cancello, R. et al. Phase angle (PhA) in overweight and obesity: Evidence of applicability from diagnosis to weight changes in obesity treatment. Rev. Endocr. Metab. Disord. 24(3), 451–464 (2023).
Jeong, H. R., Lee, H. S., Shim, Y. S. & Hwang, J. S. Positive associations between body mass index and hematological parameters, including RBCs, WBCs, and platelet counts, in Korean children and adolescents. Children 9(1), 109 (2022).
Jamshidi, L. & Seif, A. Association between obesity, white blood cell and platelet count. Zahedan J. Res. Med. Sci. 19(2). (2017).
Li, N. et al. Correlation of white blood cell, neutrophils, and hemoglobin with metabolic syndrome and its components. diabetes, metabolic syndrome and obesity. 1347-55. (2023).
Ando, M. et al. Body water balance in Hemodialysis patients reflects nutritional, circulatory, and body fluid status. J. Biorheol. 32(2), 46–55 (2018).
Puspasari, A., Maharani, C., Mus, R., Setyawati, I. & Hastuti, P. Higher circulating white blood cell and lymphocyte counts in obese metabolic syndrome patients: A preliminary population-based study in Yogyakarta, Indonesia. Malaysian J. Med. Health Sci. 16 (2020).
Kosovski, I-B. et al. Characterizing human peripheral blood lymphocyte phenotypes and their correlations with body composition in Normal-Weight, overweight, and obese healthy young adults. Medicina 60(9), 1523 (2024).
Kubiak, A., Ziółkowska, E. & Korycka-Wołowiec, A. The diagnostic pitfalls and challenges associated with basic hematological tests. Acta Haematol. Pol. 53(2), 104–111 (2022).
Böhm, A. & Heitmann, B. The use of bioelectrical impedance analysis for body composition in epidemiological studies. Eur. J. Clin. Nutr. 67(1), S79–S85 (2013).
Demirci, B. G. & Karakan, M. Ş. ADMA, neutrophil to lymphocyte, platelet to lymphocyte ratios and phase angle: Effects on inflammation and nutrition in hemodialysis patients. Eur. Res. J., 1–6.
Acknowledgements
We thank Mashhad University of Medical Sciences for providing the data.
Funding
Mashhad university of medical science (grant number: 4031222).
Author information
Authors and Affiliations
Contributions
F.K. and M.M. wrote the main manuscript text. F.K. also contributed to conceptualization and statistical analysis. S.R.S. provided scientific consultation and is a corresponding author. Z.K. contributed to statistical analysis and is a corresponding author. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Mashhad university of medical sciences ethical committee (grant number: 4031222). Written informed consent was obtained from all participants prior to their participation in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kamrani, F., Mohammadzadeh, M., Sobhani, S.R. et al. Phase angle and water cell distribution as inflammation indicators linked to hematological markers across BMI categories. Sci Rep 15, 16147 (2025). https://doi.org/10.1038/s41598-025-98430-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98430-0