Abstract
Abdominal aortic calcification (AAC) and the systemic inflammation response index (SIRI) have been linked to both all-cause and cardiovascular disease (CVD)-related mortality. Whether combining AAC and SIRI improves the predictive ability for adverse outcomes remains poorly unexplored. The present study aims to investigate the joint associations of AAC and SIRI with the risk of all-cause and CVD-related mortality in the general population. This prospective cohort study included participants with AAC and SIRI data from the 2013–2014 National Health and Nutrition Examination Survey (NHANES). Primary outcomes were death from any cause (all-cause mortality) and heart or cerebrovascular diseases (CVD-related mortality). AAC was categorized into three groups based on the AAC score: non-AAC (score = 0), low- moderate AAC (score > 0 and < 5), and severe AAC (score ≥ 5). SIRI ( x 109/L) was stratified by tertiles. Multivariable Cox regression analyses and competing risk models were employed to examine the individual associations of AAC and SIRI with the risk of all-cause and CVD-related mortality. Participants were further divided into four groups according to AAC (presence or absence) and SIRI (≤ or > median) to explore their joint association. A total of 2159 participants with a median age of 55 years were included in this study. 1031 (47.8%) were males and 1128 (52.2%) were females. For race, 317 (14.7%) were mexican american, 226 (10.5%) were other hispanic, 878 (40.7%) were white, 431 (20.0%) were black, and 307 (14.2%) were other race. During a median of 73 months follow-up, 119 deaths were recorded, 41 of which were CVD-related cases. AAC was presented in 553 participants (355 with low-moderate AAC and 198 with severe AAC), and the median SIRI was 1.05 × 109/L. After adjusting for potential confounding factors, AAC and SIRI were significantly associated with the risks of all-cause (AAC: HRsevere AAC vs. non−AAC = 2.903, 95% CI: 1.855 ~ 4.543, p for trend < 0.001; SIRI: HRtertile 3 vs. tertile 1 = 2.077, 95% CI: 1.264 ~ 3.411, p for trend = 0.001) and CVD-related death (AAC: HRsevere AAC vs. non−AAC = 4.579, 95% CI: 2.019 ~ 10.381, p for trend < 0.001; SIRI: HRtertile 3 vs. tertile 1 = 3.215, 95% CI: 1.253 ~ 8.246, p for trend = 0.006). These associations remained statistically significant even after mutual adjustment. Participants with both AAC presence and elevated SIRI had higher risk of adverse outcomes. Severe AAC and elevated SIRI were independently associated with an increased risk of all-cause and CVD-related mortality in the general population. Notably, individuals with both AAC presence and increased SIRI exhibited the greatest mortality risk. The combined assessment of AAC and SIRI may provide novel predictive value, offering a more comprehensive approach to identifying high-risk individuals and refining risk stratification strategies.
Similar content being viewed by others
Introduction
Abdominal aortic calcification (AAC), a common manifestation of vascular calcification, is a complex, multifactorial process characterized by osteogenic differentiation of vascular smooth muscle cells, chronic low-grade inflammation, and dysregulated mineral metabolism, ultimately leading to excessive deposition of hydroxyapatite crystals within the arterial wall1,2,3. Kauppila developed a scoring method to assess AAC severity based on the visual identification of diffuse white stippling or linear calcifications along the aortic walls in lateral spine radiographs3,4. Several studies demonstrated AAC was a promising marker of adverse outcomes5,6,7,8,9,10,11. However, relying solely on this marker may limit its ability to capture the multidimensional aspects of an individual’s health and reduce its predictive accuracy12.
Chronic low-grade inflammation has been implicated in various diseases, including cancer and atherosclerosis13. The Systemic Inflammation Response Index (SIRI), calculated from peripheral neutrophil, monocyte, and lymphocyte counts, serves as a novel marker that effectively reflects the balance between inflammation and immune status14,15. Recently, SIRI has gained attention for its potential predictive value in populations with cancer16, acute ischemic stroke15, and coronary artery disease17, suggesting its promise as a tool for risk stratification.
Since inflammation may promote arterial calcification and directly impact mortality through various mechanisms18,19, investigating the combined effect of AAC and SIRI in predicting adverse outcomes may help identify individuals at higher risk and provide valuable clinical insights. However, it remains unclear whether combining AAC and SIRI provide a complementary information on future mortality predicting. Therefore, this study aims to evaluate the joint associations of AAC and SIRI with the risk of all-cause and cardiovascular-related mortality in the general population.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is an ongoing, cross-sectional, nationally representative study conducted by the National Center for Health Statistics (NCHS) at the U.S. Centers for Disease Control and Prevention (CDC). This survey, employing a complex, stratified, multistage probability sampling design, aims to monitor the health and nutritional status of the U.S. population. The protocol and data collection procedures of NHANES were approved by the NCHS Research Ethics Review Board, and all participants provided informed consent at the time of enrollment.
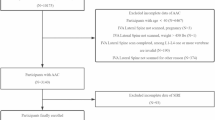
For this study, we utilized publicly available data from the 2013–2014 cycle, which included information on AAC assessment and blood cell examination. All personal identifiable information was excluded, and the methods adhered to relevant regulations and guidelines (https://www.cdc.gov/nchs/nhanes/index.html). The protocol of this study complied with the Declaration of Helsinki, and ethical approval was obtained from Ethics Review Committee of the Xinqiao hospital, Army Medical University, Chongqing, China. The exclusion criteria were: (1) incomplete data on AAC scores or blood cells; (2) history of CVD or cancer; and (3) incomplete baseline characteristics or follow-up information.
AAC severity
AAC severity was assessed as AAC score using the Kauppila semiquantitative scoring system, based on lateral imaging of the thoracolumbar spine obtained through dual-energy X-ray absorptiometry (DXA)4. These assessments were performed by trained NHANES staff at a mobile examination center. Detailed information is available on the NHANES website (https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DXXAAC_H.htm). Briefly, the anterior and posterior aortic walls in DXA were divided into four anatomical segments corresponding to lumbar vertebral levels L1–L4. Calcification was identified in each of these eight vascular segments (four anterior/posterior pairs) based on the presence of either diffuse stippling or linear calcifications along the aortic walls. Each segment was scored as follows: “0” for no calcification, “1” if calcification covered one-third or less of the aortic wall, “2” if more than one-third but less than two-thirds was calcified, and “3” if more than two-thirds was calcified. The AAC score was calculated separately for each segment, yielding a total score ranging from 0 to 24. AAC severity was categroized as : non-AAC (score = 0), low- moderate AAC (score > 0 and < 5), and severe AAC (score ≥ 5)20,21.
SIRI
Blood samples from participants were collected and analyzed using the Beckman Coulter DXH 800 Hematology Analyzer at the NHANES mobile examination center. Detailed information is available on the NHANES website (https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CBC_H.htm). Lymphocyte (x 109/L), neutrophil (x 109/L) and monocyte counts (x 109/L) in peripheral blood were obtained. The SIRI was calculated using the following formula: monocyte count × neutrophil count / lymphocyte count, and presented as x 109 /L22,23.
Mortality follow-up
Participants were followed until death or the cut-off date of December 31, 2019. Mortality information was obtained from the NHANES-linked National Death Index (NDI) public access files. Detailed information on the linking methods is available in the NCHS documentation. Causes of death were classified according to the International Classification of Diseases (ICD-10). The primary outcomes of our study were death from any cause (all-cause mortality), and from heart or cerebrovascular diseases (CVD-related mortality: ICD-10 codes I00–I78 and I80–I99).
Other variables
Socio-demographic data including age (year), sex (male and female), body mass index (BMI, kg/m2), race/ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, or other), education level (less than high school, high school, or above), lifestyle factors, and medical history such as smoking status, drinking status, hypertension, diabetes, hypercholesterolemia, CVD, and arthritis, were downloaded from the NHANES website. Additionally, laboratory parameters, including serum creatinine (Scr, µmol/L), vitamin D (nmol/L), and drug usage, were obtained. Smoking status was categorized into two groups (current/former smokers vs. never smokers). Current/former smokers were defined as participants smoked at least 100 cigarettes in life, thus less than 100 were grouped as never smokers. Drinking status was classified as current/former drinkers vs. never drinkers. Participants had at least 12 drinks of any type of alcoholic beverage were defined as current/former drinkers, while less than 12 drinks were categorized as never drinkers. Arthritis was defined as participants with osteoarthritis or rheumatoid arthritis. The estimated glomerular filtration rate (eGFR) was calculated using the MDRD (Modification of Diet in Renal Disease) study equation: eGFR (ml/min*1.73 m²) = 186.3 × Scr^−1.154 × age^−0.203 × (0.742 for females) × (1.21 for Black individuals)24.
Statistics analyses
Descriptive analyses
Baseline characteristics were presented as mean ± standard deviation or median (25th–75th percentile) for continuous variables, depending on whether the data followed a normal distribution, and as frequency and percentage for categorical variables. The study population was divided into three groups based on AAC severity and SIRI tertiles, respectively. Differences in continuous variables were analyzed using one-way ANOVA or the Kruskal-Wallis H test, while categorical variables were compared using the χ2 test or Fisher’s Exact test.
Association of AAC and SIRI with mortality in Cox proportional hazards models
The cumulative risks of all-cause and CVD-related death were displayed using Kaplan-Meier curves, and the log-rank test was performed to assess differences across AAC severity and SIRI tertiles. Cox proportional hazards models were used to evaluate the associations of AAC and SIRI with all-cause and CVD-related mortality. Schoenfeld residuals were tested to ensure the proportional hazards assumption was met for each covariate, any covariate violating this assumption was modeled as an interaction term with follow-up time. We modeled AAC severity and SIRI tertiles as categorical, with non-AAC and tertile 1 of SIRI as the reference group, respectively. Due to skewed distributions and for clinical interpretability, three continuous variables were transformed into categorical variables based on clinically relevant cutoff values (age [<60 and ≥ 60], BMI [<25, 25–30 and ≥ 30], and eGFR [<60 and ≥ 60]). To explore the joint association of AAC and SIRI with adverse outcomes, participants were divided into four groups based on the presence of AAC and SIRI levels relative to the median: (1) non-AAC and SIRI ≤ median, (2) non-AAC and SIRI > median, (3) AAC and SIRI ≤ median, and (4) AAC and SIRI > median.
Three models were fitted to explore the association of AAC and SIRI with mortality. Model 1 was adjusted for age, sex, and race. Model 2, based on Model 1, was further adjusted for BMI, education level, smoking status, and drinking status. Model 3 was further adjusted for hypertension, diabetes, hypercholesterolemia, arthritis, eGFR, antihypertensive drugs, hypoglycemic drugs, lipid-lowering drugs, and vitamin D. Arthritis and vitamin D were selected for confounding adjustment based on accumulating evidence indicating their associations with the increased risk of mortality25,26,27,28,29.
Association of AAC and SIRI with mortality in competing risk models
Fine-Gray regression analyses were used to estimate the cumulative risks of all-cause and CVD-related mortality while accounting for competing risks30,31. Deaths from non-CVD causes were considered as competing risks for CVD-related mortality, and deaths from accidents were treated as competing risks for all-cause mortality. The adjusting factors in these analyses were consistent with those in the Cox models.
Incremental value of adding AAC and SIRI on clinical outcomes predicting
Time-dependent ROC curves for models incorporating AAC, SIRI, separately and jointly were plotted, and the area under the curves (AUCs) were calculated to compare the incremental predictive ability of adding AAC and SIRI to traditional risk factors for mortality outcomes32.
Subgroup analyses
Subgroup analyses were conducted to evaluate the robustness of the associations of AAC and SIRI with mortality, and interactions text with age (<60 and ≥ 60), sex (male and female), smoking status (Current/former and never smokers), hypertension, diabetes, hypercholesterolemia, arthritis and eGFR (<60 and ≥ 60) were conducted to explore if any effect modifiers existing.
Sensitivity analyses
Several sensitivity analyses were conducted to test the robustness of study results: analysis considering for sample weights, clustering and stratification of NHANES study design, and analysis excluding participants with follow-up period less than 2 years.
Results
Baseline characteristics
The present study finally included 2159 individuals (Fig. 1), with a median age of 55.0 years, 47.8% (1031) of whom were male. AAC was present in 553 (25.6%) participants (with 355 (16.4%) classified as low-moderate AAC and 198 (9.2%) as severe AAC), and the median SIRI was 1.05 × 109/L. Baseline characteristics of the study population, stratified by AAC severity and SIRI tertiles (T1, 0.12–0.83 × 109/L; T2, 0.83–1.32 × 109/L; T3, 1.32–10.38 × 109/L), are presented in Table 1 (Differences in baseline characteristics across AAC severity) and Table 2 (Differences in baseline characteristics across SIRI tertiles), respectively. Participants with severe AAC were more likely to be older, smokers, and have hypertension, diabetes, hypercholesterolemia, and arthritis. They also tended to have a lower BMI, reduced eGFR, and elevated vitamin D levels. Significant differences in age, sex, BMI, race, smoking and drinking status, hypertension, diabetes, arthritis, eGFR, and the use of antihypertensive and hypoglycemic drugs were observed across SIRI tertiles.
Associations of AAC and SIRI separately with mortality
The median follow-up period was 73 months (range: 66–79). By the census date of December 31, 2019, 119 all-cause deaths were recorded, of which 41 were CVD-related. As shown in Fig. S1 (death rates across AAC severity or SIRI tertiles), participants with higher AAC severity and higher SIRI tertiles exhibited elevated rates of all-cause and CVD-related death. The cumulative risks of these events, stratified by AAC severity and SIRI tertiles, were displayed using Kaplan-Meier curves. The log-rank test demonstrated that participants with severe AAC and those in the highest SIRI tertile group had significantly higher risks of both all-cause and CVD-related death (Fig. 2, cumulative risks of adverse outcomes across AAC severity or SIRI tertiles; all log-rank tests, p < 0.001).
Kaplan-Meier curves for adverse outcomes stratified by AAC severity or SIRI tertile. (A) All-cause death by AAC severity; (B) CVD-related death by AAC severity; (C) All-cause death by SIRI tertile; (D) CVD-related death by SIRI tertile. AAC abdominal aortic calcification, SIRI systemic inflammation response index, CVD cardiovascular disease.
Table S1 (associations of AAC severity with adverse outcomes) and Table S2 (associations of SIRI tertiles with adverse outcomes) summarize the hazard ratios (HRs) for all-cause and CVD-related mortality, according to AAC severity and SIRI tertiles. AAC severity was positively associated with adverse outcomes across all models. After adjusting for age, sex, race, education level, BMI, smoking status, drinking status, hypertension, diabetes, hypercholesterolemia, arthritis, eGFR, antihypertensive medications, hypoglycemic medications, lipid-lowering drugs, and vitamin D, participants with severe AAC had an increased risk of both all-cause (Table S1: HR = 2.903, 95% CI: 1.855–4.543) and CVD-related death (HR = 4.579, 95% CI: 2.019–10.381) compared to those without AAC. Similarly, individuals in the highest SIRI tertile group were at increased risk of all-cause (Table S2: HR = 2.077, 95% CI: 1.264–3.411) and CVD-related death (HR = 3.215, 95% CI: 1.253–8.246).
The associations of AAC (Table S3, associations of AAC severity with adverse outcomes accounting for competing risks) and SIRI (Table S4, associations of AAC severity with adverse outcomes accounting for competing risks) with adverse outcomes were estimated in competing risk model. The magnitude of associations was similar in models without competing risks (All-cause death: HRsevere AAC vs. non−AAC = 2.959, 95% CI: 1.870 ~ 4.683; HRtertile 3 vs. tertile 1 = 2.075, 95% CI: 1.250 ~ 3.445; CVD-related death: HRsevere AAC vs. non−AAC = 4.085, 95% CI: 1.722 ~ 9.693; HRtertile 3 vs. tertile 1 = 3.279, 95% CI: 1.168 ~ 9.208).
Association of combining AAC severity and SIRI with mortality
The association between the combination of AAC severity and SIRI with all-cause and CVD-related mortality was assessed using multivariable Cox models that included both variables. The results demonstrated that AAC severity and SIRI remained independently associated with adverse outcomes, even after mutual adjustment (Fig. S2, associations of AAC and SIRI with adverse outcomes adjusting for each other). These associations persisted when accounting for competing risk factors (Fig. S3, competing risk associations of AAC and SIRI with adverse outcomes adjusting for each other).
To further investigate the joint effect of AAC and SIRI, a new variable was created based on the presence of AAC and the median SIRI value (Non-AAC & ≤ median-SIRI, Non-AAC & > median-SIRI, AAC & ≤ median-SIRI, AAC & > median-SIRI). Kaplan-Meier curves revealed that participants with both AAC and high SIRI exhibited the highest cumulative risks of all-cause (Fig. 3A, cumulative risks of all-cause mortality across combination of AAC and SIRI, log-rank test, P < 0.001) and CVD-related death (Fig. 3B, cumulative risks of CVD-related mortality across combination of AAC and SIRI, log-rank test, P < 0.001) compared to the other three groups. The HRs for adverse outcomes based on AAC-SIRI groups are shown in Table 3 (joint associations of AAC and SIRI with adverse outcomes), indicating that participants with both AAC presence and elevated SIRI faced the highest risk of all-cause and CVD-related mortality. This association remained apparent in competing risk models (Table S5, joint associations of AAC and SIRI with adverse outcomes accounting for competing risks).
Incremental value of adding AAC and SIRI to traditional risk factors on adverse events predicting
Time-dependent ROC curves were generated, and AUCs were calculated to assess the predictive power for adverse events among models with different predictive variables. As illustrated in Fig. 4 (the comparison of AUCs of models including different variables), incorporating AAC enhanced AUCs compared to traditional risk factor models. Specifically, for all-cause mortality, the AUC improved from 0.816 to 0.827 at 4 years, from 0.830 to 0.841 at 5 years, and from 0.812 to 0.819 at 6 years. For CVD-related mortality, the AUC increased from 0.865 to 0.893 at 4 years, from 0.880 to 0.911 at 5 years, and from 0.867 to 0.884 at 6 years. Similarly, the addition of SIRI also enhanced predictive accuracy for adverse outcomes, with improvements for all-cause death (AUC from 0.816 to 0.822 at 4 years, from 0.830 to 0.838 at 5 years, from 0.812 to 0.824 at 6 years) and CVD-related death (AUC from 0.865 to 0.872 at 4 years, from 0.880 to 0.889 at 5 years, from 0.867 to 0.884 at 6 years). The model incorporating both AAC and SIRI demonstrated the most significant improvement in AUCs, indicating the highest predictive power for both all-cause and CVD-related mortality.
AUCs of time dependent ROC curves for adverse outcomes. (A) All-cause death; (B) CVD-related death. Traditional risk factors included age, sex, race, BMI, education level, smoking status, drinking status, hypertension, diabetes, hypercholesterolemia, arthritis, eGFR, antihypertensive drugs, hypoglycemic drugs, lipid lowering drugs and vitamin D. AUCs area under curve, ROC receiver operator characteristic, CVD cardiovascular disease, AAC abdominal aortic calcification, SIRI systemic inflammation response index, BMI body mass index, eGFR estimated glomerular filtration rate.
Subgroup analyses
Analyses according to age, sex, smoking status, hypertension, diabetes, hypercholesterolemia, arthritis and eGFR were performed to evaluate the robustness of results, and interaction test was conducted. The positive associations of AAC and SIRI with adverse outcomes were consistent in different subgroups, however, a potential interaction effect between SIRI and hypercholesterolemia was found (Fig. 5, subgroup analyses and interaction test). The association of SIRI with all-cause mortality was stronger in participants without hypercholesterolemia (HRtertile 3 vs. tertile 1 = 3.229, 95% CI: 1.597 ~ 6.527) compared with those with hypercholesterolemia (HRtertile 3 vs. tertile 1 = 1.218, 95% CI: 0.569 ~ 2.608, p for interaction = 0.044).
Forest plot of HRs (95% CIs) for adverse outcomes from subgroup analyses. Models were adjusted for age, sex, race, BMI, education level, smoking status, drinking status, hypertension, diabetes, hypercholesterolemia, arthritis, eGFR, antihypertensive drugs, hypoglycemic drugs, lipid lowering drugs and vitamin D. *, p value of interaction text for all-cause mortality. &, p value of interaction text for CVD-related mortality. Sky blue represents results of AAC. Red represents results of SIRI. CVD cardiovascular disease, AAC abdominal aortic calcification, SIRI systemic inflammation response index, HR hazard ratios, CI confidence interval, eGFR estimated glomerular filtration rate.
Sensitivity analyses
Consistent with the main analyses, the severity of AAC (Table S6, associations of AAC severity with adverse outcomes accounting for NHANES design) and SIRI (Table S7, associations of SIRI tertiles with adverse outcomes accounting for NHANES design) remained independently associated with death events even when accounting for sample weights, clustering, and stratification. This association was also upheld in models excluding subjects with follow-up periods shorter than 2 years (AAC: Table S8; SIRI: Table S9, associations of AAC severity and SIRI tertiles with adverse outcomes excluding participants with short follow-up periods). Additionally, the relationships between AAC-SIRI and adverse outcomes were robust across all sensitivity analyses (Table S10, joint associations of AAC and SIRI with adverse outcomes accounting for NHANES design; Table S11, joint associations of AAC and SIRI with adverse outcomes excluding participants with short follow-up periods).
Discussion
Our study investigated the individual and combined associations of AAC and SIRI with future mortality in the general population. The key findings are as follows: 1.Both severe AAC and elevated SIRI were independently associated with an increased risk of all-cause and CVD-related mortality. 2.Individuals with both AAC presence and elevated SIRI faced the highest risk of all-cause and CVD-related death, and incorporating these markers enhanced the predictive ability of traditional risk factors. These associations remained robust even after accounting for competing risks.
Several studies had explored the association of AAC with adverse outcomes33,34,35. However, most of these investigations have been limited to CKD patients or older women at elevated cardiovascular risk, leaving the prognostic value of AAC in the general population less well defined. Peter et al., in a study of 2,515 participants from the Framingham Heart Study, identified a significant association between the highest tertile of AAC and an increased risk of CVD-related death (HR = 2.26, 95% CI: 1.66–3.09)5. A subsequent observational study reinforced these findings, demonstrating that a higher AAC score predicted greater risks of both CVD-related and all-cause mortality6. Similarly, another analysis from the Framingham Heart Study affirmed these associations, further establishing AAC as a predictor of adverse outcomes7. Our findings align with these prior studies, showing that greater AAC severity is independently associated with an elevated risk of all-cause and CVD-related mortality, even after adjusting for traditional risk factors. In our cohort, individuals with severe AAC had a 2.903-fold higher risk of all-cause mortality and a 4.579-fold higher risk of CVD-related death compared to those without AAC, with risk increasing in step with AAC severity. These associations remained consistent across various subgroups. Time-dependent ROC curve analysis further demonstrated that incorporating AAC into traditional risk models improved predictive accuracy for mortality. Expanding upon previous research, we accounted for additional cardiovascular risk factors and employed competing risk models, confirming that AAC remains a significant predictor of adverse outcomes. These findings strengthen the evidence supporting AAC as a crucial factor in risk stratification within the general population.
SIRI, a marker of systemic inflammation, was initially identified as a predictor of poor prognosis in cancer patients16,36,37. More recently, studies have begun to explore its association with CVD38,39. However, evidence on the relationship between SIRI and adverse outcomes in the general population remains limited. A cohort study in a Chinese population found that individuals in the highest SIRI quartile had an increased risk of all-cause mortality compared to those in the lowest quartile (HR = 1.393, 95% CI: 1.296–1.498)40. Similarly, Xia et al., using NHANES data, reported that participants with SIRI > 1.43 had a higher risk of both all-cause (HR = 1.39, 95% CI: 1.26–1.52) and CVD-related mortality (HR = 1.39, 95% CI: 1.14–1.68) compared to those with SIRI < 0.6841. Our study reinforces these findings, demonstrating a 2.077-fold and 3.215-fold increased risk of all-cause and CVD-related mortality, respectively, among individuals in the highest SIRI tertile. Subgroup analyses revealed similar associations, though a significant interaction was observed between SIRI and hypercholesterolemia. Notably, the risk of all-cause mortality associated with high SIRI was more pronounced in participants without hypercholesterolemia (HR = 3.229, 95% CI: 1.597–6.527) compared to those with hypercholesterolemia (HR = 1.218, 95% CI: 0.569–2.608, p for interaction = 0.044). However, given the observational nature of our study and the absence of prespecified subgroups, these interaction findings should be interpreted with caution. Further research is needed to confirm and elucidate the underlying mechanisms. Expanding upon previous studies, we applied competing risk models to further investigate the association between SIRI and adverse outcomes, yielding consistent results. These findings reinforce the prognostic value of SIRI in risk stratification within the general population.
Recognizing the limitations of using AAC alone to identify individuals at risk of adverse outcomes—and given the established link between inflammation and vascular calcification—our study explored the combined impact of AAC and SIRI on clinical prognosis. To our knowledge, this is the first study to classify participants based on both metrics. Our findings demonstrate that AAC and SIRI independently predict all-cause and CVD-related mortality, with their associations remaining significant even after mutual adjustment. Notably, individuals with both AAC and elevated SIRI faced the highest risk. By integrating AAC and SIRI, this study provides a novel and more comprehensive approach to risk stratification.
The key strength of this study is that it is the first to assess the combined prognostic value of AAC and SIRI—both easily obtainable from routine physical exams—and their potential for risk stratification. Additional strengths include its prospective design based on a general population, a representative U.S. sample, and the use of competing risk models. However, several limitations should be noted. First, as NHANES includes only American adults, the findings may not be generalizable to other populations. Second, residual confounding from unmeasured factors cannot be entirely excluded. Lastly, the relatively small sample size and event incidence warrant further validation in future studies.
Conclusions
AAC and SIRI were positively associated with the risk of all-cause and CVD-related mortality in the general population. Participants with AAC presence and increased SIRI were at high risk of future all-cause and CVD-related death. Our study provides complementary information for identifying individuals at higher risk, and potentially improving risk stratification strategies.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Chao, C. T. et al. Natural and non-natural antioxidative compounds: potential candidates for treatment of vascular calcification. Cell. Death Discovery. 5, 145. https://doi.org/10.1038/s41420-019-0225-z (2019).
Pan, X. et al. Mammalian sirtuins and their relevance in vascular calcification. Front. Pharmacol. 13, 907835. https://doi.org/10.3389/fphar.2022.907835 (2022).
Lewis, J. R. et al. Abdominal aortic calcification identified on lateral spine images from bone densitometers are a marker of generalized atherosclerosis in elderly women. Arterioscler. Thromb. Vasc. Biol. 36 (1), 166–173. https://doi.org/10.1161/atvbaha.115.306383 (2016).
Kauppila, L. I. et al. New indices to classify location, severity and progression of calcific lesions in the abdominal Aorta: a 25-year follow-up study. Atherosclerosis 132 (2), 245–250. https://doi.org/10.1016/s0021-9150(97)00106-8 (1997).
Wilson, P. W. et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 103 (11), 1529–1534. https://doi.org/10.1161/01.cir.103.11.1529 (2001).
Criqui, M. H. et al. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 34 (7), 1574–1579 .https://doi.org/10.1161/atvbaha.114.303268 (2014).
Hoffmann, U., Massaro, J. M. & D’Agostino, R. B. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham heart study. J. Am. Heart Assoc. 5 (2). https://doi.org/10.1161/jaha.115.003144 (2016).
Hendriks, E. J. E. et al. Annularity of Aorto-Iliac arterial calcification and risk of All-Cause and cardiovascular mortality. JACC Cardiovasc. Imaging. 11 (11), 1718–1719. https://doi.org/10.1016/j.jcmg.2018.01.029 (2018).
Kälsch, H. et al. Association of progressive thoracic aortic calcification with future cardiovascular events and all-cause mortality: ability to improve risk prediction? Results of the heinz Nixdorf recall (HNR) study. Eur. Heart J. Cardiovasc. Imaging. 20 (6), 709–717. https://doi.org/10.1093/ehjci/jey173 (2019).
Gebre, A. K. et al. Abdominal aortic calcification is associated with a higher risk of injurious fall-related hospitalizations in older Australian women. Atherosclerosis 328, 153–159. https://doi.org/10.1016/j.atherosclerosis.2021.05.003 (2021).
Bartstra, J. W., Mali, W., Spiering, W. & Jong, P. A. Abdominal aortic calcification: from ancient friend to modern foe. Eur. J. Prev. Cardiol. 28 (12), 1386–1391. https://doi.org/10.1177/2047487320919895 (2021).
O’Connor, S. D., Graffy, P. M. & Zea, R. Does nonenhanced CT-based quantification of abdominal aortic calcification outperform the Framingham risk score in predicting cardiovascular events in asymptomatic adults?? Radiology 290 (1), 108–115. https://doi.org/10.1148/radiol.2018180562 (2019).
Candore, G., Caruso, C., Jirillo, E. & Magrone, T. V. Low grade inflammation as a common pathogenetic denominator in age-related diseases: novel drug targets for anti-ageing strategies and successful ageing achievement. Curr. Pharm. Design. 16 (6), 584–596. https://doi.org/10.2174/138161210790883868 (2010).
Cao, Y. et al. Levels of systemic inflammation response index are correlated with tumor-associated bacteria in colorectal cancer. Cell Death Dis. 14 (1), 69. https://doi.org/10.1038/s41419-023-05602-9 (2023).
Wang, R. H. et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front. Immunol. 14, 1115031. (2023).
Qi, Q. et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 122 (14), 2158–2167. https://doi.org/10.1002/cncr.30057 (2016).
Ma, M. et al. Impacts of systemic inflammation response index on the prognosis of patients with ischemic heart failure after percutaneous coronary intervention. Front. Immunol. 15, 1324890. (2024).
Kong, P. et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal. Transduct. Target. Therapy. 7 (1), 131. https://doi.org/10.1038/s41392-022-00955-7 (2022).
Fernández-Ruiz, I. Immune system and cardiovascular disease. Nat. Rev. Cardiol. 13 (9). https://doi.org/10.1038/nrcardio.2016.127 (2016).
Lu, Y. Y. Clinical relevance of serum selenium levels and abdominal aortic calcification. Biol. Trace Elem. Res. 199 (8), 2803–2810. https://doi.org/10.1007/s12011-020-02405-3 (2021).
Graumam, R. Q., Pinheiro, M. M., Szejnfeld, V. L., Nery, L. & E.andCastro, C. H. M. High rate of abdominal aortic calcification in COPD patients and its relationship with musculoskeletal fragility. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl . Osteoporosis Found. U. S. A. 34 (1), 69–79. https://doi.org/10.1007/s00198-022-06513-9 (2023).
Dong, J. et al. Pretreatment systemic inflammation response index is predictive of pathological complete response in patients with breast cancer receiving neoadjuvant chemotherapy. BMC cancer. 21 (1), 700. https://doi.org/10.1186/s12885-021-08458-4 (2021).
Liu, H., Chen, X., Wang, Z. & Liu, Y. L. High systemic inflammation response index level is associated with an increased risk of lower extremity deep venous thrombosis: a large retrospective study. Ann. Med. 55 (2), 2249018. https://doi.org/10.1080/07853890.2023.2249018 (2023).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann. Intern. Med. 130 (6), 461–470. https://doi.org/10.7326/0003-4819-130-6-199903160-00002 (1999).
Cai, W. & Tang, X. P. Prevalence of metabolic syndrome in patients with rheumatoid arthritis: an updated systematic review and Meta-Analysis. Front. Med. 9, 855141. (2022).
Schattner, A. The cardiovascular burden of rheumatoid Arthritis - Implications for treatment. Am. J. Med. 136 (12), 1143–1146. https://doi.org/10.1016/j.amjmed.2023.09.004 (2023).
Veronese, N. et al. Osteoarthritis and mortality: A prospective cohort study and systematic review with meta-analysis. Semin. Arthritis Rheum. 46 (2), 160–167. https://doi.org/10.1016/j.semarthrit.2016.04.002 (2016).
Wang, J. et al. Vitamin D status and risk of All-Cause and Cause-Specific mortality in osteoarthritis patients: results from NHANES III and NHANES 2001–2018. Nutrients 14, 21. https://doi.org/10.3390/nu14214629 (2022).
Fan, Y. et al. Vitamin D status and All-Cause mortality in patients with type 2 diabetes in China. Front. Endocrinol. 13, 794947. https://doi.org/10.3389/fendo.2022.794947 (2022).
Lau, B. & Cole, S. R. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 170 (2), 244–256. https://doi.org/10.1093/aje/kwp107 (2009).
Austin, P. C. & Lee, D. S. Introduction to the analysis of survival data in the presence of competing risks. Circulation 133 (6), 601–609. https://doi.org/10.1161/circulationaha.115.017719 (2016).
Kamarudin, A. N. & Cox, T. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med. Res. Methodol. 17 (1), 53. https://doi.org/10.1186/s12874-017-0332-6 (2017).
Rodondi, N. et al. Association between aortic calcification and total and cardiovascular mortality in older women. J. Intern. Med. 261 (3), 238–244. https://doi.org/10.1111/j.1365-2796.2007.01769.x (2007).
Lewis, J. R., Wong, G., Taverniti, A. & Vucak-Dzumhur, M. Association between aortic calcification, cardiovascular events, and mortality in kidney and Pancreas-Kidney transplant recipients. Am. J. Nephrol. 50 (3), 177–186. https://doi.org/10.1159/000502328 (2019).
Suh, S. H. et al. Abdominal aortic calcification and cardiovascular outcomes in chronic kidney disease: findings from KNOW-CKD study. J. Clin. Med. 11 (5). https://doi.org/10.3390/jcm11051157 (2022).
Hua, X. et al. The preoperative systemic inflammation response index (SIRI) independently predicts survival in postmenopausal women with breast cancer. Curr. Probl. Cancer. 44 (4), 100560. (2020).
Chao, B., Ju, X., Zhang, L. & Xu, X. A novel prognostic marker systemic inflammation response index (SIRI) for operable cervical Cancer patients. Front. Oncol. 10, 766. (2020).
Li, J. et al. Prognostic significance of admission systemic inflammation response index in patients with spontaneous intracerebral hemorrhage: A propensity score matching analysis. Front. Neurol. 12, 718032. (2021).
Lin, K. B. et al. Systemic immune inflammation index and system inflammation response index are potential biomarkers of atrial fibrillation among the patients presenting with ischemic stroke. Eur. J. Med. Res. 27 (1), 106. https://doi.org/10.1186/s40001-022-00733-9 (2022).
Jin, Z. et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and All-Cause mortality: A Ten-Year Follow-Up study in 85,154 individuals. J. Inflamm. Res. 14, 131–140. https://doi.org/10.2147/jir.S283835 (2021).
Xia, Y. et al. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J. Clin. Med. 12 (3). https://doi.org/10.3390/jcm12031128 (2023).
Acknowledgements
Dr. Ma is the sole first author of this work. This research is financially supported by Natural Science Foundation of China (No.82400812) and the Natural Science Foundation of Shigatse City (No. RKZ2024ZR-002).
Author information
Authors and Affiliations
Contributions
Study concept and design: Denglu Zhou. Data collection, analysis and interpretation: Tianyi Ma. Drafting manuscript: Shupei Tang. Critical review and final approval of manuscript content: Tianyi Ma, Shupei Tang, Denglu Zhou.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Statements of ethical approvals
All participants signed informed consent at enrollment. The protocol of this study complied with the Declaration of Helsinki, and ethical approval was obtained from Ethics Review Committee of the Xinqiao hospital, Army Medical University, Chongqing, China.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, T., Tang, S. & Zhou, D. A prospective cohort study on the joint associations of abdominal aortic calcification and systemic inflammation response index with mortality risk. Sci Rep 15, 13421 (2025). https://doi.org/10.1038/s41598-025-98485-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98485-z