Abstract
Purpose To analyze vascular morphologies of layers of the choroid and compare between acute and chronic central serous chorioretinopathy (CSC). Methods A total of 161 (79 acute and 82 chronic) CSC eyes followed up for 6 months were enrolled. Baseline optical coherence tomography (OCT) and OCT angiography (OCTA) data were collected, and choriocapillaris (CC), Sattler’s layer, and Haller’s layer en-face slabs were obtained from OCT and OCTA. Vessel parameter analysis was performed using the OCTA Vascular Analyzer. Results Acute CSC showed higher node numbers, vessel area density, and total vessel length at the CC along with reduced diameter variation, skew, and kurtosis when compared to chronic CSC (all P ≤ 0.032). Acute CSC showed reduced diameter, diameter variation, and skew at Sattler’s layer when compared to chronic CSC (all P ≤ 0.033). Finally, acute CSC showed reduced diameter kurtosis at Haller’s layer when compared to chronic CSC (P = 0.017). There were significant correlations noted among the above-mentioned parameters between each layer, with correlations being more prominent between Sattler’s and Haller’s layers. Conclusion CC change in chronic CSC involved decreased nodes, density, and greater diameter variation, which in turn led to increases in diameter and diameter variations of larger vessels.
Similar content being viewed by others
Introduction
Central serous chorioretinopathy (CSC), a retinal disorder characterized by serous detachment of the neurosensory retina, has various clinical manifestations, courses, and outcomes1. The pathophysiology of CSC involves a dysfunctional retinal pigment epithelium (RPE) and abnormal choroidal vasculature2.
Abnormal choroidal vessel dilation and increased vascular permeability are considered to be major contributors to serous retinal detachment, with their role having been proven by numerous imaging techniques, including enhanced-depth imaging optical coherence tomography and optical coherence tomography angiography (OCTA)2. The key features found by imaging studies include a thickened choroid, dilation of the large choroidal vessels in Haller’s layer, and relative thinning of Sattler’s layer and the choriocapillaris3. With the distinct characteristics observed in the choroidal vasculature across different layers, CSC is a representative of the pachychoroid spectrum diseases.
CSC is often described to be either acute or chronic according to the period of subretinal fluid (SRF) accumulation. Acute CSC is generally self-limiting, resolving spontaneously within 3 months. Chronic CSC, marked by persistent fluid presence for more than 6 months, can involve prolonged SRF accumulation and consequent atrophy of the RPE, which may lead to persistent visual impairment4.
Studies have documented different structural changes between acute and chronic CSC at the choroid level. Findings include differences in choriocapillaris density and flow deficit pattern, Haller vessel morphologies, and patterns on OCT and OCTA5,6,7. Global differences also exist between acute and chronic CSC in the choroidal structure on ultra-widefield indocyanine green angiography, implying variations in choroidal vessel involvement8.
In this study, we analyzed changes in choroidal vessels between acute and chronic CSC using en-face OCT and OCTA imaging, performing a detailed layer-by-layer comparison. The analysis was automated by the OCTA Vascular Analyzer (OCTAVA) (https://github.com/GUntracht/OCTAVA), a previously proven open-source toolbox for vessel analysis9. Correlation of the parameters between layers were also investigated.
Results
In total, 79 acute CSC cases and 82 chronic CSC cases were included in the analysis. Among 161 subjects, 111 were male (68.9%). Sex distribution did not differ between cases of acute and chronic CSC (19 women and 60 men with acute CSC vs. 31 women and 51 men with chronic CSC, P = 0.086). The mean ages for the acute and chronic CSC cohorts were 51.2 ± 11.7 and 55.1 ± 9.2 years, respectively (P = 0.015). The mean SFCT values were 394.01 ± 54.77 and 371.70 ± 60.38 μm at baseline (P < 0.001) and 364.20 ± 52.08 and 361.17 ± 63.50 μm (P = 0.741) at Month 6 for the acute and chronic CSC groups, respectively. SFCT decreased at 6 months from baseline in both groups (both P < 0.001); however, the decrement of SFCT was greater in the acute CSC group (29.81 ± 19.88 μm) compared to the chronic CSC group (10.52 ± 24.52 μm) (P < 0.001).
Comparison of choroidal vascular metrics of each layer between acute and chronic CSC
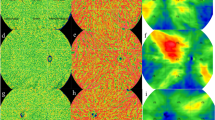
In the choriocapillaris, node number, VAD, VLD, and total length were higher in acute CSC patients compared to chronic CSC patients, whereas diameter variation, skew, and kurtosis were greater in chronic CSC patients compared to acute CSC patients (all P ≤ 0.032) on OCTA imaging. Node number was higher in acute CSC patients and mean diameter and diameter variation were greater in chronic CSC patients (all P ≤ 0.016) on OCT imaging. The comparison of choriocapillaris metrics is summarized in Table 1. Figure 1 shows higher densities of a more regular-diameter choriocapillaris in a patient acute CSC compared to a patient with chronic CSC, and vessel visualization was better with OCTA compared to OCT.
Comparison of the choriocapillaris vasculature between acute and chronic CSC on OCTA, OCT, and processed images. (Top) Raw en-face slab obtained at the choriocapillaris level. (Middle) Binarized image heat-mapped according to vessel diameter. (Bottom) Skeletonized images. (A column) OCTA imaging of an acute CSC case. (B column) OCTA imaging of a chronic CSC case. (C column) OCT imaging of the acute CSC case shown in (A). (D column) OCT imaging of the chronic CSC case shown in (B). Higher densities of a more regular-diameter choriocapillaris are observable in the acute CSC case compared to the chronic CSC case on OCTA and OCT imaging, and vessel visualization is better with OCTA compared to OCT.
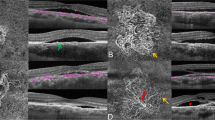
Considering Sattler’s layer, the mean and median diameters and diameter variation were greater in chronic CSC patients compared to acute CSC patients (all P ≤ 0.033) on OCTA imaging. Node number, VLD, and total length were greater in acute CSC patients and mean length, mean and median diameters, and diameter variation were higher in chronic CSC patients (all P ≤ 0.023) on OCT imaging. The comparison of choriocapillaris metrics is summarized in Table 2. Figure 2 shows a greater diameter with higher diameter variations of choroidal vessels in chronic CSC compared to acute CSC. Vessel visualization was better with OCTA compared to OCT.
Comparison of the Sattler’s layer vasculature between acute and chronic CSC on OCTA, OCT, and processed images. (Top) Raw en-face slab obtained from Sattler’s layer. (Middle) Binarized image heat-mapped according to vessel diameter. (Bottom) Skeletonized images. (A column) OCTA imaging of an acute CSC case. (B column) OCTA imaging of a chronic CSC case. (C column) OCT imaging of the acute CSC case shown in (A). (D column) OCT imaging of the chronic CSC case shown in (B). Greater diameter and diameter variation of choroidal vessels are observable in the chronic CSC case compared to the acute CSC case on OCTA and OCT imaging. Vessel visualization is better with OCTA compared to OCT.
For Haller’s layer, diameter skew was higher on OCTA imaging and diameter kurtosis was higher on OCT imaging in chronic CSC patients compared to acute CSC patients (P = 0.012 and P = 0.017, respectively). The comparison of choriocapillaris metrics is summarized in Table 3. Figure 3 shows greater diameter variations of choroidal vessels in chronic CSC compared to acute CSC. Vessel visualization was better with OCT compared to OCTA.
Comparison of the Haller’s layer vasculature between acute and chronic CSC on OCTA, OCT, and processed images. (Top) Raw en-face slab obtained from Haller’s layer. (Middle) Binarized image heat-mapped according to vessel diameter. (Bottom) Skeletonized images. (A column) OCTA imaging of an acute CSC case. (B column) OCTA imaging of a chronic CSC case. (C column) OCT imaging of the acute CSC case shown in (A). (D column) OCT imaging of the chronic CSC case shown in (B). Greater diameter variation of choroidal vessels is observable in the chronic CSC case compared to the acute CSC case on OCTA and OCT imaging. Vessel visualization is better with OCT compared to OCTA.
Correlation of choroidal vascular metrics between layers
Node number, VAD, VLD, and total length of the choriocapillaris negatively correlated with both the diameter and diameter variation skew of Sattler’s layer (both P ≤ 0.002) on OCTA imaging. Separately, diameter variation, skew, and kurtosis of the choriocapillaris positively correlated with both the diameter and diameter variation of Sattler’s layer (all P ≤ 0.041). Correlations between the vascular metrics of the choriocapillaris and Sattler’s layer are summarized in Table 4.
Node number and VAD of the choriocapillaris on OCTA imaging negatively correlated with node number, VLD, and total length as well as the median diameter of Haller’s layer on OCT imaging (all P ≤ 0.049). Meanwhile, diameter skew and kurtosis of the choriocapillaris positively correlated with those of Haller’s layer (P ≤ 0.004). Correlations between vascular metrics of choriocapillaris and Haller’s layer are summarized in Table 5.
The mean diameter and diameter variation of Sattler’s layer on OCTA imaging positively correlated with diameter skew and kurtosis of Haller’s layer on OCT imaging (all P ≤ 0.014). Correlations between vascular metrics of the choriocapillaris and Haller’s layer are summarized in Table 6.
Discussion
This study analyzed choroidal vessel structure differences between acute and chronic CSC, highlighting changes in the choriocapillaris, Sattler’s layer, and Haller’s layer. Correlations between the metrics of choroidal vessels of each layer was also evaluated. Results of the study identified quantifiable vascular differences that could potentially inform the pathophysiology of the disease and future diagnostic and therapeutic approaches.
There is a consensus that SFCT is increased in CSC whether it is acute or chronic in nature10,11,12. It is explained by choroidal hyperpermeability and increased luminal area of the choroidal vessels due to vortex vein congestion in CSC, which in turn causes serous retinal detachment13. However, discrepancies exist in the choroidal thickness between acute and chronic CSC. In this study, SFCT was higher in acute CSC compared to chronic CSC. Several previous papers have offered similar results to those of this study14, while others did not find significant differences in thickness between acute and chronic CSC5,8,15,16. In studies that recorded no statistically significant differences, choroidal thickness tended to be greater in acute CSC15. A volumetric analysis, rather than a single-point measurement of choroidal thickness, is required to resolve this discrepancy.
At the choriocapillaris level, acute CSC was associated with a greater number of nodes and increased VAD and VLD values, which are indicative of more intact choroidal blood flow and vessel density. This aligns with findings of a study on the early stages of pachychoroid17. The area of flow impairment in the choriocapillaris was found to be increased in eyes with pachychoroid—even more so when epitheliopathy was present. This finding suggests a decrease in vessel area and density in the more chronic or severe stages of pachychoroid disease. CSC, along with pachychoroid epitheliopathy, is a common manifestation of pachychoroid spectrum disease. Greater diameter variation, skew, and kurtosis at the choriocapillaris were observed in chronic CSC, which may present as a compensatory response to the decreased choriocapillaris flow and compromised vascular integrity in the chronic state.
According to our data, patients with chronic CSC presented with greater vessel variation, skew, and kurtosis within Sattler’s and Haller’s layers. This observation suggests that chronic CSC is characterized by abnormal choroidal vessel constriction or dilatation and irregular vessel morphology, not only at the choriocapillaris level but also in medium- and large-sized choroidal vessels. This trend is potentially due to long-term alterations in vascular stability in addition to decreased blood flow.
The correlation analysis revealed that node number, VAD, VLD, and total length of the choriocapillaris negatively correlate with diameter metrics of Sattler’s and Haller’s layers, suggesting that, as choroidal vessel diameter increases in deeper layers, both the density and length of the choriocapillaris decrease. This inverse relationship may suggest a compensatory response of medium and large choroidal vessels to the choriocapillaris flow decrease. Alternatively, it could reflect a redistribution of blood flow within the choroid or an underlying vascular remodeling process, though the direction of this relationship remains uncertain. Furthermore, positive correlations between vessel diameter skew and kurtosis in Sattler’s and Haller’s layers with those of the choriocapillaris may reflect an interconnected process of vascular remodeling that progressively affects choroidal layers in CSC eyes. The direction of this process remains to be clarified. The automated analysis of these metrics has demonstrated reliability in vascular structure assessment, as supported by prior validating similar methodologies, including those applied to the choriocapillaris9,18,19.
This study has some limitations, including its retrospective design and the potential for selection bias inherent in a single-center cohort study. The study groups were not age-matched, which may introduce confounding effects, as choroidal thickness and choriocapillaris flow density can vary significantly with age20. For the segmentation of layers, we adopted the automated segmentation provided by the machine’s review software. Although this method has been commonly used in previous studies, variations in vessel analysis due to the segmentation method may still exist. Also, the potential underestimation or misrepresentation of blood flow in deeper choroidal layers in OCTA images may exist due inherent nature of the imaging technique21. the potential underestimation or misrepresentation of blood flow in deeper choroidal layers due to significant beam attenuation and projection artifacts inherent in current OCTA imaging devices. Additionally, in this study, CSC cases were simply classified into acute and chronic types; however, the actual classification of CSC may be more complex22. A more refined classification approach may facilitate a better understanding of the underlying mechanisms and could improve the accuracy of comparing clinical characteristics across subtypes. Future studies with larger, prospective cohorts and multimodal imaging could further elucidate the differences in choroidal changes between acute and chronic CSC and confirm the diagnostic and prognostic utility of the findings of this study.
In conclusion, this study found significant vascular and structural differences between acute and chronic CSC, revealing that patients with acute CSC present healthier vessel morphology at the choriocapillaris, while those with chronic CSC display greater choroidal vessel diameter irregularities at all three layers of the choroid. These insights may guide personalized management strategies, with choroidal imaging serving as a promising modality to improve disease monitoring in CSC patients.
Methods
Subjects and imaging
A total of 161 eyes from 161 patients diagnosed with CSC by fundoscopic exam, OCT, OCTA, and fluorescein angiography and followed-up for more than 6 months were enrolled in this study at Bucheon St. Mary’s Hospital, The Catholic University of Korea. The cohort included 161 CSC cases. In cases of bilateral CSC, one eye was randomly selected for the analysis. Subjects were classified as having chronic CSC if there was persistent fluid present for 6 months after the onset of symptoms; otherwise, they were classified as having acute CSC. This study was approved by the Bucheon St. Mary’s Hospital Institutional Review Board (IRB), with adherence to ethical guidelines as per the Declaration of Helsinki. The need for informed consent was waived based on the retrospective chart review nature of the study by the Bucheon St. Mary’s Hospital IRB.
All subjects underwent OCT and OCTA imaging acquired using the Cirrus H6000 system (Zeiss, Oberkochen, Germany). Volumetric OCT and OCTA images were acquired at the 6- × 6-mm posterior pole of the eye. Cases with signal strength of 8 or higher were included. OCT and OCTA en-face slabs of the choriocapillaris were obtained using the Cirrus Review Software (ver. 11.5.2; Zeiss). En-face slabs of Sattler’s and Haller’s layers were obtained by adjusting the z-axis plane to 60–100 μm and 150–200 μm below the RPEfit line, respectively.
Analysis of choroidal vessels
Subfoveal choroidal thickness (SFCT) was measured by drawing a perpendicular distance between Bruch’s membrane and the choroid–scleral border at the fovea in the Cirrus Review Software. Analysis of choroidal vessels in the choriocapillaris, Sattler’s layer, and Haller’s layer slabs on both OCT and OCTA images were performed using the OCTAVA toolbox ran on MATLAB 2024a (The MathWorks, Inc., Natick, MA, USA)9. OCTAVA can calculate quantitative metrics of vasculatures, including node numbers; vessel area density (VAD); total vessel length; vessel length density (VLD); vessel length (mean and median); variation, skew, and kurtosis of vessel length; vessel diameter (mean and median); and variation, skew, and kurtosis of vessel diameter. Brief descriptions for each metrics are as follows: (1) nodes were defined as a connection point where endpoints of multiple vessels meet; (2) VAD indicates the perfused blood vessel area in a binarized OCTA image divided by total image area; (3) VLD indicates the total length of perfused vessels measured along the vessel centerline divided by the total image area on a skeletonized OCTA image; (4) the average and distribution of vessel length indicate the length of each identified vessel segment; and (5) the average and distribution of vessel diameters indicate diameter measurements acquired using a local thickness algorithm in ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA) applied to the binarized OCTA image. Kurtosis was used to evaluate the presence of extreme values and structural variability in vascular features.
Statistical analysis
Statistical analysis was performed using MATLAB 2024a. A chi-squared test was used to compare categorical variables between groups. An independent t test was conducted to compare continuous variables between groups when normal distribution was confirmed. In cases where normality was not achieved, the Mann–Whitney U test was applied. For the analysis of correlations between parameters, Pearson’s correlation coefficient was used. Continuous variables are described as mean ± standard deviation values. P < 0.05 was considered statistically significant.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Feenstra, H. M. A. et al. Central serous chorioretinopathy: an evidence-based treatment guideline. Prog Retin Eye Res. 101, 101236. https://doi.org/10.1016/j.preteyeres.2024.101236 (2024).
Fung, A. T., Yang, Y. & Kam, A. W. Central serous chorioretinopathy: A review. Clin. Exp. Ophthalmol. 51, 243–270. https://doi.org/10.1111/ceo.14201 (2023).
Pujari, A. et al. Optical coherence tomography angiography in central serous chorioretinopathy: the current clinical role and future perspectives. Surv. Ophthalmol. 67, 68–82. https://doi.org/10.1016/j.survophthal.2021.05.003 (2022).
Gilbert, C. M., Owens, S. L., Smith, P. D. & Fine, S. L. Long-term follow-up of central serous chorioretinopathy. Br. J. Ophthalmol. 68, 815–820. https://doi.org/10.1136/bjo.68.11.815 (1984).
Lee, W. J., Lee, J. W., Park, S. H. & Lee, B. R. En face choroidal vascular feature imaging in acute and chronic central serous chorioretinopathy using swept source optical coherence tomography. Br. J. Ophthalmol. 101, 580–586. https://doi.org/10.1136/bjophthalmol-2016-308428 (2017).
Savastano, M. C. et al. Retina 38, 1211–1215, doi:https://doi.org/10.1097/iae.0000000000001678 (2018).
Lu, H., Wang, Z., Xin, Z. & Yang, J. To evaluate the microcirculation of retinochoroid capillary between acute and chronic central serous chorioretinopathy with OCTA. Med. (Baltim). 100, e27069. https://doi.org/10.1097/md.0000000000027069 (2021).
Lee, P. K., Ra, H., Han, S. Y., Baek, J. & QUANTITATIVE ANALYSIS OF CHOROIDAL MORPHOLOGY USING MULTIMODAL IMAGING IN ACUTE AND PERSISTENT CENTRAL SEROUS CHORIORETINOPATHY. Retina 43, 832–840, doi:https://doi.org/10.1097/iae.0000000000003715 (2023).
Untracht, G. R. et al. An open-source toolbox for quantitative analysis of optical coherence tomography angiography images. PLoS One. 16, e0261052. https://doi.org/10.1371/journal.pone.0261052 (2021).
Gawęcki, M., Grzybowski, A. & Święch, A. Biometric risk factors for central serous chorioretinopathy. Ophthalmol. Ther. 12, 1327–1338. https://doi.org/10.1007/s40123-023-00687-z (2023).
Brandl, C., Helbig, H. & Gamulescu, M. A. Choroidal thickness measurements during central serous chorioretinopathy treatment. Int. Ophthalmol. 34, 7–13. https://doi.org/10.1007/s10792-013-9774-y (2014).
Singh, S. R. et al. Central serous chorioretinopathy imaging biomarkers. Br. J. Ophthalmol. 106, 553–558. https://doi.org/10.1136/bjophthalmol-2020-317422 (2022).
Spaide, R. F. et al. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog Retin Eye Res. 86, 100973. https://doi.org/10.1016/j.preteyeres.2021.100973 (2022).
Ambiya, V. et al. Subfoveal choroidal thickness as a predictor of central serous chorioretinopathy. Eye 30, 1623–1629. https://doi.org/10.1038/eye.2016.209 (2016).
Hanumunthadu, D. et al. Evaluation of choroidal layer thickness in central serous chorioretinopathy. J. Ophthalmic Vis. Res. 14, 164–170. https://doi.org/10.4103/jovr.jovr_152_17 (2019).
Jee, D., Yoon, J. H., Ra, H., Kwon, J. W. & Baek, J. Predicting persistent central serous chorioretinopathy using multiple optical coherence tomographic images by deep learning. Sci. Rep. 12, 9335. https://doi.org/10.1038/s41598-022-13473-x (2022).
Baek, J., Kook, L. & Lee, W. K. Choriocapillaris flow impairments in association with pachyvessel in early stages of pachychoroid. Sci. Rep. 9, 5565. https://doi.org/10.1038/s41598-019-42052-w (2019).
Untracht, G. R. et al. Towards standardising retinal OCT angiography image analysis with open-source toolbox OCTAVA. Sci. Rep. 14, 5979. https://doi.org/10.1038/s41598-024-53501-6 (2024).
Marsh-Armstrong, B., Migacz, J., Jonnal, R. & Werner, J. S. Automated quantification of choriocapillaris anatomical features in ultrahigh-speed optical coherence tomography angiograms. Biomed. Opt. Express. 10, 5337–5350. https://doi.org/10.1364/boe.10.005337 (2019).
Lin, C. Y., Huang, Y. L., Hsia, W. P., Wang, Y. & Chang, C. J. Correlation of choroidal thickness with age in healthy subjects: automatic detection and segmentation using a deep learning model. Int. Ophthalmol. 42, 3061–3070. https://doi.org/10.1007/s10792-022-02292-8 (2022).
Wang, J. C. et al. Visualization of choriocapillaris and choroidal vasculature in healthy eyes with En face Swept-Source optical coherence tomography versus angiography. Transl Vis. Sci. Technol. 7, 25. https://doi.org/10.1167/tvst.7.6.25 (2018).
Singh, S. R. et al. Discrepancy in current central serous chorioretinopathy classification. Br. J. Ophthalmol. 103, 737–742. https://doi.org/10.1136/bjophthalmol-2018-312435 (2019).
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (no. NRF001663041G0003101).
Author information
Authors and Affiliations
Contributions
S.H.L., H.R., and J.B. provided and collected data. S.H.L, Y.J., and J.B. analyzed data. S.C and J.B. prepared figures. J.B. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Institutional Review Board of Bucheon St. Mary’s Hospital, which waived the need for written informed consent because of the study’s retrospective design (HC19RESI0086).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jung, Y., Ra, H., Choi, S. et al. Choroidal vessel changes in acute and chronic central serous chorioretinopathy assessed by en-face Layer-by-Layer comparison and correlation. Sci Rep 15, 14894 (2025). https://doi.org/10.1038/s41598-025-98576-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98576-x