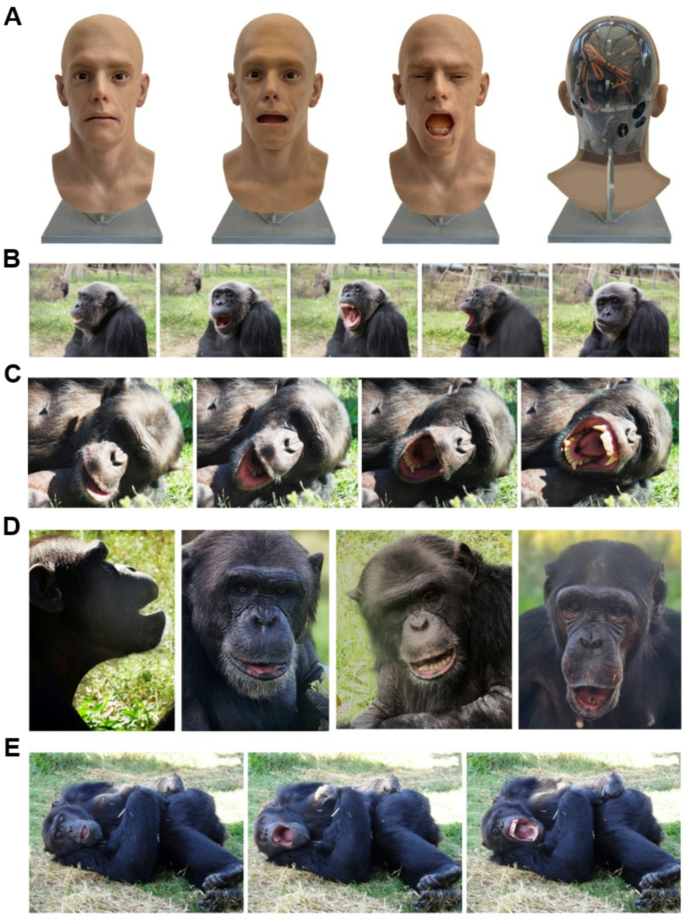
Abstract
This study explores contagious yawning in adult chimpanzees (Pan troglodytes) in the presence of a non-biological humanoid agent, an android. Chimpanzees observed an android portraying specific facial expressions, including yawns and gapes. The results showed that adult chimpanzees exhibited across-agent yawn contagion, with a graded response: the highest contagion occurred when the android displayed a fully wide-open mouth (Yawn condition), a reduced response when the mouth was partially opened (Gape condition), and no contagion when the android’s mouth was closed (Close condition). Additionally, chimpanzees engaged in behaviours associated with drowsiness, such as gathering bedding materials, constructing nests, and lying down, while observing the android yawning. This suggests that yawning by an unfamiliar model may act as a contextual cue for rest, rather than merely triggering a motor resonance response. These findings contribute to the understanding of non-human primates’ susceptibility to contagiously induced behaviours, specifically yawns, even when triggered by an artificial agent. This study highlights the role of social factors in shaping yawn contagion and calls for further research on cross-species and cross-agent interactions.
Similar content being viewed by others
Introduction
Processes essential for social interaction between humans may also play a role in interactions with non-human agents, such as robots or androids. Human-robot interactions tap into mechanisms related to empathy1, perspective-taking2,3, and sensorimotor simulation4,5,6, which are further modulated by the observer’s familiarity with the agent or action7,8,9,10.
Animal research based on observations of non-verbal behaviour analysis has provided insights into the evolutionary origins and mechanisms underlying social interaction11. While most of this research has focussed on interactions between conspecifics, via screen presentations12,13, cross-species research has broadened our understanding of these processes, including contagious yawning14. Here, we expand social animal research by examining interactions beyond biological species to reach across to agents, specifically by investigating the behavioural response of our closest evolutionary relatives (chimpanzees) to a non-conspecific, non-biological agent performing various actions.
Understanding interactions beyond biological agents in a real-word setting can enhance our comprehension of core social mechanisms, by directly examining the contingency of social attributes such as empathy and contagion. Specifically, in this study, we explored contagious yawning in chimpanzees when observing a humanoid android presenting a yawning movement, a control action (gaping), or remaining motionless with its mouth closed.
On contagious yawning, empathy, and imitation
The embodied nature of yawning has been widely used to examine contagious intransitive actions in primates, including humans15 bonobos16 and chimpanzees17, as well as non-primate species, such as dogs18 sheep19 elephants20 and budgerigars21. While several influential theories about the potential multifunctional features of yawning have been postulated in various species22,23,24, the ultimate function of spontaneous yawns remains debated25. Yawning has been associated with physiological26 and social events27, thermoregulatory28, including transitions between rest and arousal29, attention modulation30, and group synchronization31. Furthermore, the contagious aspect of yawning has been intertwined with core elements of social interaction, such as empathy32, (but see33,34) emotion processing35, and imitation36.
The developmental trajectory of contagious yawning (CY) supports its association with empathy-related processes. CY emerges gradually in humans, chimpanzees, and dogs, becoming prominent at around 4 years in humans, 5 years in chimpanzees, and 7 months in dogs37,38,39. This aligns with the maturation of other cognitive and social abilities, such as perspective-taking, attention to and identification of others’ mental states (children40; chimpanzees41; dogs42), and what is sometimes referred to as affective empathy43.
Yawn contagion has also been interpreted as a particular type of emotion contagion44 (but see45). Proponents of this perspective suggest that CY relies on two interconnected processes: non-conscious mimicry and afferent feedback46. Non-conscious mimicry, also known as the “chameleon effect”47,48, refers to the tendency to mirror others’ behaviours involuntarily in social interactions, for example, copying postures, facial expressions, or movements without conscious awareness or intent. Afferent feedback occurs when gestural communication and facial expressions influence emotional experiences, as seen in reciprocal smiling49, or even in whole-body postural effects50,51. Mimicry or imitation52 play a fundamental role in reinforcing social bonds through an emotional feedback loop53. In humans, being mimicked increases affinity, liking, and empathic responses54, including compassion55, and promotes prosocial behaviour not only towards the mimicker but also towards unrelated individuals in the same social context56,57.
Social motivations, such as the desire to establish bonds, can increase non-conscious mimicry in humans58,59. Similarly, in many animal species, offspring copy their parents, for example, in hunting or self-maintenance behaviours60. These phylogenetically conserved mechanisms support social learning61. Moreover, imitation-based social interactions have been reported across various species. For instance, capuchin monkeys (Sapajus apella) exhibit greater affiliative behaviour towards humans who imitate them62. Consistent with the idea that humans preferentially mimic socially significant individuals63, yawn contagion is more likely to occur between socially bonded individuals in several species, including chimpanzees64, bonobos65, gelada monkeys66, and possibly dogs67,68.
Robots and human-like agents in social interaction
The advancements in artificial intelligence and computer science have enabled the creation of robots (hereafter: “android”) and agents with human-like features (e.g., realistic gestures and speech) to study social interaction69,70. Typically, the term “robot” refers to a physically embodied system, whereas “agent” denotes a software-based system71,72. Neuroimaging studies have investigated how humans process actions performed by androids73, revealing that the same observation network is engaged when observing either a human or an android performing an action74,75,76, though this remains a topic of debate. At a behavioural level, humans tend to apply their common social norms when interacting with androids, adjusting their responses based on the android’s human-like facial features77. For example, androids with infant-like facial features are perceived as more sociable and friendly78, and participants tend to cooperate with a software agent that has a human face to the same extent as they would with another person79. Similarly, when an android mimics human behaviour72 or demonstrates perspective-taking abilities80, people develop more positive attitudes towards it. Generally, humans tend to attribute human-like qualities to non-biological agents that possess anthropomorphic characteristics81,82.
To understand the evolutionary roots of flexible “cross-agent” social interaction mechanisms, we evaluated behavioural responses of chimpanzees to facial movements performed by an unfamiliar, non-biological agent, namely a human-like android. To this end, we developed an android head capable of executing facial motor actions with precise motor and temporal accuracy (see Fig. 1a). Specifically, we tested whether adult chimpanzees would exhibit yawning when observing an android simulating yawns. We predicted that chimpanzees would yawn more frequently in the Yawn condition compared to the Gape (opening of the mouth but not as wide as in a yawn with neutral, non-emotional expression and no particular significance (control movement)) and Close (mouth closed, no movement) conditions (see Fig. 1a). Additionally, we recorded baseline measurements of chimpanzee behaviour on a typical day to compare against experimental conditions. To further explore the chimpanzees’ responses to the android, we analysed additional behaviours, including the duration of lying down (a resting position indicative of low arousal) during and after the android’s movements across the three experimental conditions.
Methods
Participants
Participants were 14 adult chimpanzees (Pan troglodytes spp.) aged 10 to 33 years (\(\bar{X}\) = 22.57, SD = 8.06), including 10 males and 4 females (see Table 1). The chimpanzees had lived at the Fundació Mona (Spain) for between 1 and 12 years (\(\bar{X}\) = 7.36, SD = 4.34), after being confiscated or rescued from the pet and entertainment industry to be permanently housed at the centre. All chimpanzees were socially housed in two stable groups within a naturalistic enclosure designed to promote species-typical behaviours. The groups had access to both indoor and outdoor areas and were provided with daily environmental enrichment. No individuals were housed in isolation. Although some chimpanzees initially exhibited abnormal and anxiety-like behaviours, such as stereotypies or overgrooming, previous studies on their rehabilitation process83 have shown that desirable behaviours and welfare indices increased over time, while undesirable behaviours decreased.
Ethical considerations
All experimental procedures were non-invasive and complied with the ethical guidelines of the Animal Behaviour Society, which establish the standard and safe Guidelines for the Use of Animals in Research. The study was reviewed and approved by the Ethics Board of Fundació Mona and the Psychology Department Research Ethics Committee of City St. George’s University of London (SREC 14–15 01 CA 16 06 2015).
Design and stimuli
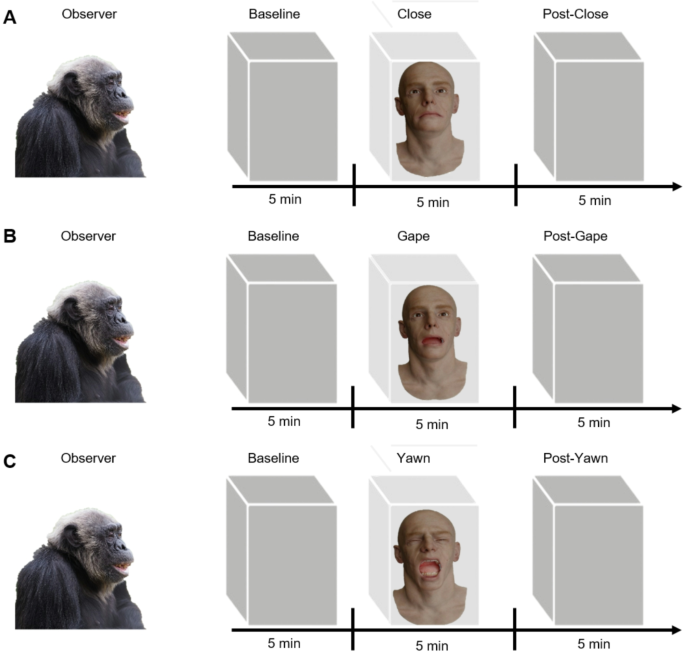
The study used a within-subjects design, with an independent variable of exposure to an android performing one of three facial expressions: Yawning, Gaping, or a neutral expression (closed mouth) (see Fig. 1a). A human-like android was designed with realistic biological features and motion dynamics. The android measured 45 cm in height, 20 cm in width, and weighed 3.8 kg. Thirty three servo motors were integrated to generate controlled facial movements. When powered on, the android’s neutral expression corresponded to the Close condition (mouth closed, no movement). All programmed facial movements lasted 10 s from onset to offset. To ensure precise and consistent movement, the servos were programmed as follows: (1) Close condition (neutral expression), nine servos maintained the neutral face, ensuring that no unintentional expressive movement occurred; (2) Gape condition (Non-yawning mouth opening). Twelve servos (two on each side above the mouth and two on each side on the lower part of the mouth) controlled the mouth movement, opening it to a maximum of 1.5 cm, sustaining the expression for 6 to 8 s, and then closing the mouth. The remaining active servos acted as support for the rest of the face to remain static. The entire cycle lasted 10 s. Eight mini servos around the eyebrow regions were designed to exemplify the corrugator muscle movement, which forms part of the yawning expression. Finally, the (3) Yawn condition required 6 mini servos to create the internal space necessary for the movement command. These “space facilitator servos”, were placed in the back of the cheek area to maintain the facial structure in the same position and therefore prevent the portrayal of more than one expression at the same time. The android’s mouth opened to a maximum of 5.5 cm, mimicking air intake, while the eyes closed and reopened as the mouth closed.
All motion parameters (e.g., time, speed, trajectory, velocity, and muscle simulation motion pattern) were programmed and automatically adjusted using C/C++, Python, Java, and MATLAB. The movements were designed to replicate human facial biological motion, maintaining smooth, human-like transitions while ensuring that each action adhered to the 10-second duration limit.
Although the android’s silicone facial layers closely resembled human skin, some inherent textural differences remained. Nevertheless, a transparent rear panel revealed its internal mechanical components (See Fig. 1a), making its artificial nature explicit, despite its otherwise realistic human-like appearance when viewed frontally.
During the Close condition, the android remained expressionless with its mouth closed and lips sealed for the entire 5-minute presentation phase. In the Gape condition, the model performed non-yawning mouth openings at regular intervals. In the Yawn condition, the model displayed full yawns as described above. During all conditions, the android was positioned within the chimpanzee’s “full visual field” (approximately 0–45°) or “peripheral visual field” (45–110°) relative to the sagittal plane of the participant’s eyes. The actions were repeated for 5 min, occurring a minimum of 15 times and a maximum of 20 times per condition. The experimenter, hidden behind a screen, remotely controlled the android’s actions via a button-operated remote panel (Fig. 2).
Trial structure of three exposure conditions and an initial baseline measurement. All sessions started with a five-minute baseline phase followed by a five-minute exposure condition (A) Close, (B) Gape, (C) Yawn and a five-minute post-stimulus observation phase (Post-Close, Post-Gape, Post-Yawn). Photos RMJM.
Procedure and data collection
Behavioural data was collected during an initial, 5-minute baseline period, during which the box containing the android was covered with a black cloth. This phase served as reference for each participant’s typical daily behaviour (Fig. 2). Each chimpanzee completed four 15-minute sessions, structured into three consecutive 5-minute phases: baseline, stimulus exposure (Yawn, Gape, or Close condition), and post-stimulus (Post-Yawn, Post-Gape, Post-Close), during which the android’s box was covered again. One keeper and one experimenter were present throughout all the sessions but remained behind a panel, to prevent any influence on the chimpanzees’ behaviour. The android was only visible within a 45° angle. As the chimpanzees could move freely within their enclosure, they might not have always had a direct view of the android. To account for this, the experimenter recorded in real time the duration (in seconds) that each chimpanzee spent looking at the android in each condition. The behaviour was scored by the experimenter with the use of a monitor behind the panel; the particular behaviours were corroborated from the additional cameras and later coded from video by the naïve coders.
Each condition aimed to expose participants to between 15 and 20 instances of the android’s facial expressions. Furthermore, if a yawn or gape occurred outside the participant’s visual field, the expression was repeated while the chimpanzee was facing the android, ensuring visibility, without exceeding 20 presentations per condition. Individual sessions were separated by a minimum of 5 min and a maximum of 15 days (for 50% of the sessions, there were more than 12 h between sessions). To minimise interference with the centre’s daily routines, sessions conducted on the same day were separated by an average of 30 min. All conditions (except for the baseline phase) were counterbalanced across participants using a Latin Square design. The chimpanzees were tested individually between 09:30 and 18:00, in a familiar enclosure. All sessions were recorded with two Canon Legria HF G25, one at each side of the android, a Sony HDR-305 CX740VE above the android, and a Panasonic Lumix DMC-FZ200 directly below the android, all facing towards the enclosure to capture the chimpanzee positioned directly in front of the android. Additionally, two GoPro HERO + LCD cameras recorded supplementary angles: one inside the android’s transparent protective cube to document its facial movements and another on a tripod to capture areas outside the android’s 45º field of view. Behaviours such as Yawn, Gape and Close were previously defined in the literature, see Campbell, Provine, Madsen13,15,39. The chimpanzees’ behaviours were scored from video recordings using a focal continuous timed-event sampling77, focusing on the duration and frequencies of behaviours displayed.
Analysis
Two coders naïve to the purpose of the study, independently analysed all the videos and rated the number of chimpanzee yawns and gapes in the 5-minute baseline, stimulus (Yawn, Gape and Close) and post-stimulus phases (Post-Yawn, Post-Gape, Post-Close). Coder inter-rater reliability frequency of chimpanzee yawns was extremely high (agreement = 99%, Cohen’s Kappa = 0.99, 100% of sessions scored). Observers also coded lying-down behaviour and duration of looking at the stimuli. Lying down was defined as the whole body in a horizontal position, either on the ground or in a suspended hammock, with at least one shoulder in contact with the floor or the hammock. Inter-rater reliability for duration of lying down was perfect (agreement 100%, 100% of sessions scored).
Inspection of raw data and Shapiro-Wilks tests of normality showed that all variables, except for Time spent Looking (TL) were significantly different from normality (p < 0.05). Therefore, non-parametric tests were used for analyses. Friedman test was used to analyse if the number of observed behaviours (yawns, gapes) varied across the different conditions. Wilcoxon tests were used for paired-wise comparisons. Values are reported as frequency of yawns and gapes per session, their mean and SEM per experimental condition, and the total number of yawns and gapes across all conditions. A secondary analysis was performed on the combined experimental and post experimental phases (10-min period, i.e., Yawn + Post-Yawn). All tests were two-tailed unless specified, and significance levels set at 0.05. Data were analysed using SPSS Statistics 25 for Windows (IBM Inc.).
Results
Yawn behaviour
Eight of the 14 (57.1% (N = 14)) chimpanzees displayed contagious yawning when observing the yawns of an android (see Fig. 1 (B) and (C)). Overall, the Yawn condition elicited 22 yawns (\(\bar{X}\) = 1.57, SD = 2.38) and the Post-Yawn condition elicited 23 yawns (\(\bar{X}\) = 1.64, SD = 2.24). Combining both Yawn conditions, the chimpanzees exhibited 45 yawns (\(\bar{X}\) = 3.21, SD = 3.62). They displayed no yawns in the Gape condition and 13 yawns in the Post-Gape condition (\(\bar{X}\) = 0.93, SD = 1.44). The Gape condition elicited 16 gapes (\(\bar{X}\) = 1.14, SD = 1.79) and the Post-Gape condition elicited 7 gapes (\(\bar{X}\) = 0.5, SD = 1.09). Combining both Gape conditions 23 gapes were displayed (\(\bar{X}\) = 1.64, SD = 2.27) (See Fig. 1(D)). No gapes occurred in the Yawn or in the Post-Yawn conditions, and no yawns or gapes were observed in the Close condition. Four yawns (\(\bar{X}\) = 0.29, SD = 0.83) and one gape (\(\bar{X}\) = 0.07, SD = 0.27) were observed in the Post-Close condition. During the Baseline, we observed 5 yawns (\(\bar{X}\) = 0.36, SD = 0.93) and zero gapes (Fig. 3a and b).
Total number of behaviours displayed. (A) Frequency of yawns and gapes in all the experimental conditions (Close and Post-Close, Gape and Post-Gape, Yawn and Post-Yawn). (B) Frequency of yawns (left) and gapes (right) during the exposure and post exposure period (5 min each) (C) Time spent lying down (left) and looking at the android (right) during the exposure and post exposure period.
Overall, the number of chimpanzee yawns differed significantly across the three exposure conditions (Friedman’s X2(2) = 16.00, p = 0.001). Follow-up Wilcoxon tests showed significant differences between the number of yawns in the Yawn and the Gape condition (Z=-2.55, p = 0.011), and the Yawn and the Close condition (Z= -2.56, p = 0.011).
When merging the exposure and post-exposure conditions, the number of yawns was also significant across the three exposure conditions (Friedman’s X2(2) = 10.46, p = 0.005). Follow-up Wilcoxon tests showed significant differences between all Yawn conditions (Yawn + Post Yawn) vs. all Closed (Closed + Post Closed) (Z=-2.503, p = 0.012) and all Yawn (Yawn + Post Yawn) vs. all Gape (Gape + Post Gape) (Z=-2.047, p = 0.041). The frequency of yawning in the Baseline and Yawn conditions was not influenced by the time of day of testing (09:00–12:00; 12:00–15:00, 15:00–18:00, Friedman test, Baseline: X2(2) = 1.72, p = 0.434; Yawn: X2(2) = 0.56, p = 0.890). Finally, using a Latin square to control for the order of condition presentation, a Kruskal-Wallis test showed no order effects (H = 0.97, p = 0.809). This means the number of yawns in the Yawn condition was not influenced by the order of presentation, whether the Yawn condition was presented as the first, second or third condition after the baseline.
Lying-down behaviour
To explore chimpanzees’ behaviours indicative of relaxation or drowsiness, we compared the duration of Lying-down (LD) behaviour (reported in sec per condition) across the three stimulus conditions (see Table 2). The same percentage of chimpanzees who exhibited yawn contagion exhibited lying-down behaviour (57.1%) (See Fig. 1(e)). We found a significant increase in lying-down behaviour in the Yawn condition compared to the Close condition (Wilcoxon test, Z = -2.023, p = 0.043). No other significant differences were found. The mean time spent lying down in Yawn + Post-Yawn condition (\(\bar{X}\) = 108.84 s, SD = 133.91) was longer than in the Gape + Post-Gape condition (\(\bar{X}\) = 88.26 s, SD = 152.43) and in the Close + Post-Close condition (\(\bar{X}\) = 62.77 s, SD = 160.79) (Fig. 3(c)). Differences between time spent lying down in the Close + Post-Close and Gape + Post-Gape conditions were not significant (Z=-1.46, p = 0.144).
More time was spent lying down in the Yawn condition (LD = 639.9 s, \(\bar{X}\) = 45.71, SD = 105.95) and Post-Yawn condition (LD = 883.8 s, \(\bar{X}\) = 63.13, SD = 99.51) (Total LD = 1,523.7 s, \(\bar{X}\) = 108.84, SD = 133.91), than in the Gape condition (LD = 590.5 s, \(\bar{X}\) = 42.18, SD = 81.7) and Post-Gape condition (LD = 645.1 s, \(\bar{X}\) = 46.08, SD = 87.89) (Total LD = 1,235.6 s, \(\bar{X}\) = 88.26, SD = 152.43) and in the Close condition (LD = 340.4 s, \(\bar{X}\) = 24.31, SD = 62.29) and Post-Close condition (LD = 538.4 s, \(\bar{X}\) = 38.46, SD = 98.50) (Total LD = 878.8 s, \(\bar{X}\) = 62.77, SD = 160.79). There was no lying down behaviour during the Baseline (LD = 0 s) .
Time looking at the android
To explore the chimpanzees’ attention to the android, we compared the duration of time in looking (TL) behaviour within the 45 degree angle (Table 2). No differences were statistically significant, suggesting all three conditions were of equal interest to the chimpanzees (Fig. 3(c)). A significant positive correlation was found between total Time Looking at the stimuli and number of yawns in the Yawn + Post-Yawn conditions (rs=0.564, p = 0.035), but not between total TL and the other conditions, TL Gape + Post-Gape vs. Yawn + Post-Yawn, r = 0.225, p = 0.440, possibly driven by the fact that there were no yawns displayed in those conditions, and TL Close + Post-Close vs. Yawn + Post-Yawn, 0r = 0.491, 0p = 0.071.
The analysis of average time spent looking (TL) at the stimuli showed that chimpanzees spent more time looking during the Yawn (TL = 1,367 s), and Post-Yawn conditions (TL = 318.5 s), i.e. total Time Looking during Yawn + Post-Yawn (TL = 1,685.5 s) than the Time Looking during the Gape (TL = 1,141 s) and Post-Gape conditions (TL = 675.5 s), i.e., total Gape + Post-Gape (TL = 1,816 s), or the Close (TL = 910 s) and Post-Close conditions (TL = 352 s), i.e. total Close + Post-Close (TL = 1,262 s).
Discussion
To our knowledge, this is the first study to explore contagious yawning in response to an inanimate agent, an android, presented in real time. More than half of the adult chimpanzees exhibited yawn contagion across agents. Specifically, their yawning frequency increased significantly in response to the android’s yawning condition compared to the Gape, Close and Baseline conditions. Interestingly, no yawning was observed in the Gape or Close conditions, and only one chimpanzee yawned during the Baseline phase, suggesting that spontaneous yawning was rare under these circumstances. In addition, chimpanzees spent more time lying down in the Yawn condition than in the Close condition, potentially indicating a soporific effect of observing yawning, as some individuals also gathered bedding materials before lying down.
Our findings align with previous research of intra-species contagious yawning such as in humans84, chimpanzees85 bonobos86,87 orangutans88 and inter-species effect, such as yawning transmission from humans to chimpanzees38 adult dogs14,60 or puppies31, However, in the present study the stimulus was an unfamiliar non-biological agent with human-like features. The chimpanzees’ response suggest that yawning does not necessarily require social familiarity or a conspecific model, but can be triggered by an unfamiliar agent exhibiting biologically relevant cues.
The mechanisms underlying this response remain unclear89. One possibility is that chimpanzees deliberately engaged with the unfamiliar android, intending to interact, imitate, or bond with it90,91. Alternatively, the observed yawning may have triggered an automatic perception-action coupling mechanism, leading to an embodied response without explicit intent92. This interpretation is consistent with theories suggesting that yawning contagion results from basic motor resonance rather than complex social cognition.
Interestingly, the increased yawning frequency in the Yawn condition coincided with greater lying down behaviour, despite the android never performing actions other than yawning, gaping, or closing its mouth. This pattern suggests that yawning may have signalled rest-related associations for the chimpanzees. The chimpanzees’ behaviour possibly resulted from inferences or associations evoked by the observed yawn, or their own yawn, contagiously induced or not. The chimpanzees’ immediate response to seeing the android’s yawning could reflect an uncontrollable urge to react or re-enact. Here, re-enacting would consist of immediately yawning as well, while reacting would possibly involve perceiving the yawn as carrying further information, i.e. the android’s yawns being perceived as a signal that it was the place and time to lie down and rest. Furthermore, the chimpanzees did not display this behaviour in the Gape or Close conditions, giving further support to the inferential processes resulting from only the yawn stimuli. Overall, this is the first study to demonstrate that one of our genetically closest primates displays reproduction of an observed action, and adds associated behaviours93 such as lying down, even when the observed action is performed by a non-biological agent.
These findings warrant further studies of interactions between androids, humans and other species in general, and in particular, action perception, understanding and interpretation. For example, are other movements or actions performed by robotic agents contagious to humans or non-human animals? Conceivably, in humans61 the phylogenetically old phenomenon of spontaneous yawning, particularly its contagious aspect, may have been part of a pre-language form of communication. Contagiously induced or not, in other animals it may have had a functional role in social interactions involving comparable information-processing mechanisms, and maintained through evolution81,82.
Regardless of the potential interpretations, our findings indicate that chimpanzees exhibit yawn contagion, triggered by a non-biological inanimate agent, a humanoid android, that looks as if it is yawning. Yawning, despite its elusive primary functions, may still have an evolutionarily old, non-verbal communicative role, and its contagious aspect may help us find out more about how humans and animals developed adaptive functions, ways of communication, synchronisation and social interaction83,84,94.
Data availability
Data coded is available in Figshare (City St George’s University of London Repository) in the following link: https://figshare.com/s/8379f297b21049c91772.
References
Leite, I. et al. The influence of empathy in human-robot relations. Int. J. Hum. Comput. Stud. 71 (3), 250–260 (2013).
Berlin, M., Gray, J., Thomaz, A. L. & Breazeal, C. Perspective taking: An organizing principle for learning in human-robot interaction. In AAAI 2, pp. 1444–1450 (2006).
Lemaignan, S., Warnier, M., Akin Sisbot, E., Clodic, A. & Alami, R. Artificial cognition for social human-robot interaction: an implementation. Artif. Intell. 247, 45–69 (2017).
Wood, A., Rychlowska, M., Korb, S. & Niedenthal, P. Fashioning the face: sensorimotor simulation contributes to facial expression recognition. Trends Cogn. Sci. 20 (3), 227–240 (2016).
Schillaci, G. Sensorimotor learning and simulation of experience as a basis for the development of cognition in robotics. (2014).
Schillaci, G., Verena, V., Hafner, B. L. & Grosjean, M. Is that me? Sensorimotor learning and self-other distinction in robotics. 8th ACM/IEEE International Conference on Human-Robot Interaction (HRI), pp. 223–224. (IEEE, 2013).
Gardner, T., Goulden, N. & Cross, E. S. Dynamic modulation of the action observation network by movement familiarity. J. Neurosci. 35 (4), 1561–1572 (2015).
Schütz-Bosbach, S. & Prinz, W. Perceptual resonance: action-induced modulation of perception. Trends Cogn. Sci. 11, 8: 349–355 (2007).
Amoruso, L. & Cosimo, U. Familiarity modulates motor activation while other species’ actions are observed: a magnetic stimulation study. Eur. J. Neurosci. 43, 6: 765–772 (2016).
Cross, E. S. et al. Prinz W robotic movement preferentially engages the action observation network. Hum. Brain. Mapp. 33, 9: 2238–2254 (2012).
Argyle, M. Social Interaction: Process and Products. (Routledge, 2017).
Anderson, J. R., Myowa-Yamakoshi, M. & Matsuzawa, T. Contagious yawning in chimpanzees. Proc. R. Soc. Lond. B Biol. Sci. 271 (suppl_6), S468–S470 (2004).
Campbell, M. W., Carter, J. D., Proctor, D., Eisenberg, M. L. & de Waal, F. B. Computer animations stimulate contagious yawning in chimpanzees. Proc. Royal Soc. B: Biol. Sci. 276 (1676), 4255–4259 (2009).
Joly-Mascheroni, R. M., Senju, A. & Shepherd, A. J. Dogs catch human yawns. Biol. Lett. 4 (5), 446–448 (2008).
Provine, R. R., Hamernik, H. B. & Yawning Effects of stimulus interest. Bull. Psychon. Soc. 24 (6), 437–438 (2013).
Norscia, I. et al. Yawn contagion in bonobos: another group, another story. Am. J. Primatol. 84 (3), e23366 (2022).
Campbell, M. W. & Cathleen, R. Cox. Observational data reveal evidence and parameters of contagious yawning in the behavioral repertoire of captive-reared chimpanzees (Pan troglodytes). Sci. Rep. 9 (1), 13271 (2019).
Ake, K. & Kutsukake, N. Contagious yawning in African painted dogs. Anim. Cogn. 26 (4), 1191–1198 (2023).
Yonezawa, T., Sato, K., Uchida, M., Matsuki, N. & Yamazaki, A. Presence of contagious yawning in sheep. Anim. Sci. J. 88 (1), 195–200 (2017).
Rossman, Z. T., Padfield, C., Young, D., Hart, B. L. & Lynette, A. Hart. Contagious yawning in African elephants (Loxodonta africana): responses to other elephants and familiar humans. Front. Veterinary Sci. 7, 493433 (2020).
Gallup, A. C., Swartwood, L., Militello, J. & Sackett, S. Experimental evidence of contagious yawning in budgerigars (Melopsittacus undulatus). Anim. Cogn. 18 (5), 1051–1058 (2015).
Kotake, K. T., Yamaguchi, S. T., Mukai, Y., Zhou, Z. & Norimoto, H. Yawning and its Temperature-Dependent modulation in Leopard geckos. Zoolog. Sci. 42(1) (2024).
Shoup-Knox, M. L., Gallup, A. C., Gallup, G. G. Jr & McNay, E. C. Yawning and stretching predict brain temperature changes in rats: support for the thermoregulatory hypothesis. Front. Evolut. Neurosci. 2, 108 (2010).
Guggisberg, A. G., Mathis, J., Schnider, A. & Hess, C. W. Why do we yawn? Neurosci. Biobehavioral Rev. 30 (6), 855–863 (2010).
Gallup, A. C. Why do we yawn? Primitive versus derived features. Neurosci. Biobehavioral Reviews. 35 (3), 765–769 (2011).
Moyaho, A. A., Urbina, F., Monjaraz Guzmán, E. & Walusinski, O. Yawning: a cue and a signal. Heliyon 3, 11: e00437 (2017).
Deputte, B. L. Ethological study of yawning in primates. I: quantitative analysis and study of causation in two species of old-world monkeys (Cercocebus albigena and Macaca fascicularis). Ethology 98 (3–4), 221–245 (1994).
Gallup, A. C., Omar, T. & Eldakar The thermoregulatory theory of yawning: what we know from over 5 years of research. Front. NeuroSci. 6, 188 (2013).
Guggisberg, A. G., Mathis, J., Herrmann, U. S. & Hess, C. W. The functional relationship between yawning and vigilance. Behav. Brain. Res. 179 (1), 159–166 (2007).
Walusinski, O. How yawning switches, the default-mode network to the attentional network by activating the cerebrospinal fluid flow. Clin. Anat. 27, 201–209. https://doi.org/10.1002/ca.22280 (2013).
Gallup, A. C. The causes and consequences of yawning in animal groups. Anim. Behav. 187, 209–219 (2022).
Gottfried, J., Lacinová, L. & Širůček, J. Contagious yawning and empathy. E-Psychology 9, 4 (2015).
Massen, J. J. & Gallup, A. C. Why contagious yawning does not (yet) equate to empathy. Neurosci. Biobehavioral Reviews. 80, 573–585 (2017).
O’Hara, S. J. & Reeve, A. V. A test of the yawning contagion and emotional connectedness hypothesis in dogs, Canis familiaris. Anim. Behav. 81, 1: 335–340 (2011).
Provine, R. Contagious yawning and laughing: everyday imitation-and mirror-like behavior. Behav. Brain Sci. 28, 2: 142 (2005).
Over, H. & Carpenter, M. The social side of imitation. Child. Dev. Perspect. 7 (1), 6–11 (2013).
Millen, A. & Anderson, J. R. Neither infants nor toddlers catch yawns from their mothers. Biol. Lett. 7 (3), 440–442 (2010).
Madsen, E. A., Persson, T., Sayehli, S., Lenninger, S. & Sonnesson, G. Chimpanzees show a developmental increase in susceptibility to contagious yawning: A test of the effect of ontogeny and emotional closeness. PLoS One 8(10) (2013).
Madsen, E. A. & Persson, T. Contagious yawning in domestic dog puppies (Canis lupus familiaris): the effect of ontogeny and emotional closeness on low-level imitation in dogs. Anim. Cogn. 6, 233–240 (2013).
Gzesh, S. M. & Surber, C. F. Visual perspective-taking skills in children. Child. Dev. 56 (5), 1204–1213 (1985).
Povinelli, D. J., Rulf, A. B. & Bierschwale, D. T. Absence of knowledge attribution and self-recognition in young chimpanzees (Pan troglodytes). J. Comp. Psychol. 108 (1), 74–80 (1994).
Maginnity, M. E. & Grace, R. C. Visual perspective taking by dogs (Canis familiaris) in a Guesser-Knower task: evidence for a canine theory of Mind?? Anim. Cogn. 17, 6 (2014).
Losoya, S. H. & Eisenberg, N. Affective empathy. In Interpersonal Sensitivity (35–58). (Psychology Press, 2001).
Palagi, E., Celeghin, A., Tamietto, M., Winkielman, P. & Norscia, I. The neuroethology of spontaneous mimicry and emotional contagion in human and non-human animals. Neurosci. Biobehavioral Reviews. 111, 149–165 (2020).
Gallup, A. C. On the link between emotional contagion and contagious yawning. Neurosci. Biobehavioral Reviews. 121, 18–19 (2021).
Campbell, M. W. & de Waal, F. B. M. Ingroup-Outgroup bias in contagious yawning by chimpanzees supports link to empathy. PLoS One 6(4). (2011).
Chartrand, T. L. & Bargh, J. A. The chameleon effect: the perception-behavior link and social interaction. J. Pers. Soc. Psych. 76, 893–910 (1999).
Yoon, J. & Tennie, C. Contagious yawning: A reflection of empathy, mimicry, or contagion? Anim. Behav. 79, e1–e3 (2010).
Soussignan, R. Duchenne smile, emotional experience, and autonomic reactivity: a test of the facial feedback hypothesis. Emotion 2 (1), 52–74 (2002).
Briñol, P., Petty, R. E. & Wagner, B. C. Body postures effects on self-evaluation: A self-validation approach. Eur. J. Social Psychol. 39, 1053–1064 (2009).
Adelmann, P. K. & Zajonc, R. B. Facial efference and the experience of emotion. Ann. Rev. Psychol. 40, 249–280 (1989).
Zentall, T. R., Sutton, J. E. & Sherburne, L. M. True imitative learning in pigeons. Psychol. Sci. 7, 6: 343–346 (1996).
Carpenter, M., Uebel, J. & Tomasello, M. Being mimicked increases prosocial behavior in 18-month-old infants. Child. Dev. 84 (5), 1511–1518 (2013).
Madsen, E. A., Persson, T., Sayehli, S., Lenninger, S. & Sonesson, G. Chimpanzees show a developmental increase in susceptibility to contagious yawning: a test of the effect of ontogeny and emotional closeness on yawn contagion. PloS One. 8, 10: e76266 (2013).
Mafessoni, F. & Lachmann, M. The complexity of Understanding others as the evolutionary origin of empathy and emotional contagion. Sci. Rep. 9 (1), 5794 (2019).
Lakin, J. L. & Chartrand, T. L. Using non-conscious behavioral mimicry to create affiliation and rapport. Psych Sci. 14 (4), 334–339 (2003).
Van Baaren, R. B., Holland, R. W., Kawakami, K. & Van Knippenberg, A. Mimicry and prosocial behavior. Psychol. Sci. 15 (1), 71–74 (2004).
Chartrand, T. L., Maddux, W. W. & Lankin, J. L. Beyond the perception-behavior link: The ubiquitous utility and motivational moderators of non-conscious mimicry. In Uleman, J, Bargh, J.A., Hassin, R. (eds.) The new unconscious. OUP, N. Y. 334–361 (2005).
Cheng, C. M. & Chartrand, T. L. Self-monitoring without awareness: using mimicry as a non-conscious affiliation strategy. J. Personal. Soc. Psychol. 85, 6 (2003).
McElreath, R. & Strimling, P. When natural selection favors imitation of parents. Curr. Anthropol. 49 (2), 307–316 (2008).
Russon, A. E. Pantomime and imitation in great Apes: implications for reconstructing the evolution of Language. Interact. Stud. 19 (1–2), 200–215 (2018).
Paukner, A., Suomi, S. J., Visalberghi, E. & Ferrari, P. F. Capuchin monkeys display affiliation toward humans who imitate them. Science 325 (5942), 880–883 (2009).
Norscia, I. & Palagi, E. Yawn contagion and empathy in Homo sapiens. PLoS One 6(2) (2011).
Campbell, M. W. & de Waal, F. B. M. Chimpanzees empathise with group mates and humans, but not with baboons or unfamiliar chimpanzees. Proc. Roy Soc. B 11, 291 (2014).
Demuru, E., Deschner, T. & Palagi, E. In bonobos yawn contagion is higher among kin and friends. PloS One 7(11) (2012).
Palagi, E., Leone, A., Mancini, G. & Ferrari, P. F. Contagious yawning in gelada baboons as a possible expression of empathy. Proc. Nat. Acad. Sci. 106 (46), 19262–19267. https://doi.org/10.1073/pnas.0910891106 (2009).
Silva, K., Bessa, J. & de Sousa, L. Auditory contagious yawning in domestic dogs (Canis familiaris): first evidence for social modulation. Anim. Cogn. 15 (4), 721–724. https://doi.org/10.1007/s10071-012-0473-2 (2012).
Romero, T., Konno, A. & Hasegawa, T. Familiarity bias and physiological responses in contagious yawning by dogs support link to empathy. PloS One 8(8) (2013).
Chaminade, T. (ed Hodgins, J.) Artificial agents in social cognitive sciences. Interact. Stud. Social Behav. Communication Biol. Artif. Syst. 7 3 347–353 (2006).
Nass, C. & Lee, K. M. Does computer-synthesised speech manifest personality? Experimental tests of recognition, similarity-attraction and consistency-attraction. J. Experimental Psychology: Appl. 7 (3), 171–181 (2001).
Franklin, S. & Graesser, A. Is it an agent, or just a program? A taxonomy for autonomous agents. In: (eds Müller, J. P., Wooldridge, M. J. & Jennings, N. R.) Intelligent Agents III Agent Theories, Architectures, and Languages. ATAL 1996. Lecture Notes in Computer Science (Lecture Notes in Artificial Intelligence), vol 1193. (Springer, Berlin, Heidelberg). (1996).
Parise, S., Kiesler, S., Sproull, L. & Waters, K. Cooperating with life-like interface agents. Comput. Hum. Behav. 15 (2), 123–142 (1999).
Saygin, A. P. & Stadler, W. The role of appearance and motion in action prediction. Psychol. Res. 76 (4), 388–394 (2012).
Powers, A. et al. Common ground in dialogue with a gendered humanoid robot. Proceedings of RO-MAN (2005).
Urgen, B. A., Plank, M., Ishiguro, H., Poizner, H. & Saygin, A. P. EEG theta and mu oscillations during perception of human and robot actions. Front. Neurorobotics. 7, 19 (2013).
KilnerJM, Paulignan, Y. & Blakemore, S. J. An interference effect of observed biological movement on action. Curr. Biol. 13 (6), 522–525 (2003).
Gazzola, V., Rizzolati, G., Wicker, B. & Keysers, C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage 35 (4), 1674–1684 (2007).
Yee, N., Bailenson, J. N. & Rickertsen, K. A meta-analysis of the impact of the inclusion and realism of human-like faces on user experiences in interfaces. In: Proc. SIGCHI conference on Human factors in computing systems. 1–10. (2007).
Powers, A. & Kiesler, S. The advisor robot: tracing people’s mental model from a robot’s physical attributes. In: Proc. 1st ACM SIGCHI/SIGART conference on Human-robot interaction. 218–225 (2006).
Bailenson, J. N. Digital chameleons: automatic assimilation of nonverbal gestures in immersive virtual environments. Psychol. Sci. 16 (10), 814–819 (2005).
Torrey, C., Powers, A., Marge, M., Fussell, S. R. & Kiesler, S. Effects of adaptive robot dialogue on information exchange and social relations. In: Proc. 1st ACM SIGCHI/SIGART conference on Human-robot interaction. 126–133 (2006).
Kiesler, S., Powers, A., Fussell, S. R. & Torrey, C. Anthropomorphic interactions with a robot and robot-like agent. Soc. Cogn. 26 (2), 169–181 (2008).
Llorente, M., Riba, D., Ballesta, S., Feliu, O. & Rostán, C. Rehabilitation and socialization of chimpanzees (Pan troglodytes) used for entertainment and as pets: an 8-Year study at Fundació Mona. Int. J. Primatol. 36, 605–624 (2015).
Gallup, A. C. & Jorg, J. M. M. There is no difference in contagious yawning between men and women. Royal Soc. Open. Sci. 3 (9), 160174 (2016).
Vick, S. & Paukner, A. Variation and context of yawns in captive chimpanzees (Pan troglodytes). Am. J. Primatol. 72 (3), 262–269 (2010).
Tan, J., Ariely, D. & Brian Hare. Bonobos respond prosocially toward members of other groups. Sci. Rep. 7(1), 14733 (2017).
Palagi, E., Norscia, I. & Demuru, E. Yawn contagion in humans and bonobos: emotional affinity matters more than species. PeerJ 2, e519 (2014).
Van Berlo, Evy, A. P., Díaz-Loyo, O. E. & Juárez-Mora, M. E. Kret, and Jorg JM massen. Experimental evidence for yawn contagion in orangutans (Pongo pygmaeus). Sci. Rep. 10(1), 22251 (2020).
Adriaense, J. E. C., Koski, S. E., Huber, L. & Lamm, C. Challenges in the comparative study of empathy and related phenomena in animals. Neurosci. Biobehavioral Reviews. 112, 62–82. https://doi.org/10.1016/j.neubiorev.2020.01.021 (2020).
Sadeghi, S., Stephanie, N. L., Schmidt, D., Mier & Hass, J. Effective connectivity of the human mirror neuron system during social cognition. Soc. Cognit. Affect. Neurosci. 17, 8: 732–743 (2022).
Altmann, S. A. The structure of primate social communication. In: (ed Altmann, S. A.) Social Communication among primates. Chicago: University of Chicago Press; 325–362 (1967).
Norscia, I. & Palagi, E. Yawn contagion and empathy in Homo sapiens. PLoS ONE 6(12) (2011).
Guggisberg, A. G., Mathis, J., Schnider, A. & Hess, C. W. Why do we yawn? The importance of evidence for specific yawn-induced effects. Neurosci. Biobehavioral Reviews. 35 (5), 1302–1304 (2011).
Duranton, C. & Gaunet, F. Behavioural synchronization from an ethological perspective: overview of its adaptive value. Adapt. Behav. 24 (3), 181–191 (2016).
Acknowledgements
The authors would like to thank Fundació Mona Primate Sanctuary and the care givers for their invaluable help with this research. RJM is grateful to the City University of London Scholarship, and to Mark Coulier for his help with the development of the android. ML is a Serra Húnter Fellow (Generalitat de Catalunya).
Author information
Authors and Affiliations
Contributions
RJM and BCM conceptualized and designed the study, supervised all aspects of the research, and were responsible for data analysis and interpretation. RJM, CV, AG, and DC conducted the experiments and collected the data. RJM and BCM wrote the manuscript, with contributions from BF and ML. BF and ML provided critical revisions and conceptual insights. ML also contributed to data interpretation and manuscript editing. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Joly-Mascheroni, R., Forster, B., Llorente, M. et al. Chimpanzees yawn when observing an android yawn. Sci Rep 15, 18002 (2025). https://doi.org/10.1038/s41598-025-98639-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98639-z