Abstract
This study aimed to identify the recovery predictors for patients with pyogenic liver abscess (PLA) and diabetes who are undergoing antibiotic therapy, and to develop an effective nomogram for predicting the antibiotic treatment duration (ORT). This retrospective study included consecutive PLA patients with diabetes who received antibiotic treatment, with ORT defined as the time from the initiation of antibiotic therapy to its cessation. Univariate and multivariate analyses were performed to identify the main predictors of ORT. Kaplan-Meier survival curves and a nomogram were subsequently constructed to predict ORT, and the accuracy of the nomogram was assessed using Harrell’s C-statistic and calibration curves. A total of 139 PLA patients with diabetes were included, with a median ORT of 17 days (interquartile range: 13–22 days). The study found that fever (P < 0.01), pre-treatment septic shock (P < 0.01), abscess diameter greater than 5 cm (P < 0.01), and elevated white blood cell count (P = 0.04) were independent risk factors for prolonged ORT, suggesting that patients with these factors had a significantly longer ORT compared to those without them. Prognostic analysis showed that patients exhibiting more predictive factors (e.g., high fever, shock, larger abscess, elevated white blood cell count) had a significantly extended ORT. Based on these factors, we developed a nomogram to predict ORT, with a Harrell’s C-statistic of 0.75, indicating good predictive accuracy. The calibration curve for predicting ORT demonstrated good consistency between the expected and actual results. This nomogram provides clinicians with a simple and practical tool to assess patient prognosis and guide the appropriate cessation of antibiotic treatment.
Similar content being viewed by others
Introduction
Pyogenic liver abscess (PLA) is a common clinical hepatic infectious disease, and its occurrence is usually closely related to factors such as bacterial infections, biliary diseases, liver damage, and impaired immune function1,2. Despite significant advancements in antibiotic therapy and minimally invasive treatments in modern medicine, the treatment of liver abscesses still presents certain challenges3. Globally, the incidence of liver abscesses has been increasing annually, especially among diabetic patients, who experience higher morbidity and mortality rates4,5. According to existing epidemiological studies, diabetes is one of the independent risk factors for liver abscesses, and the weakened immune system in diabetic patients makes them face greater challenges in infection control6.
In clinical practice, the treatment of PLA primarily relies on antibiotic therapy, but the treatment outcomes are often influenced by factors such as the patient’s physical condition, comorbidities, and the type of pathogenic microorganisms7. There is currently no effective tool that can universally predict the duration of antibiotic treatment required for patients. Existing studies mainly focus on the clinical features, treatment strategies, and microbiological characteristics of PLA7,8. However, systematic studies on the factors influencing recovery in diabetic patients are relatively limited. Research has shown that the effectiveness of antibiotic therapy is closely related to various factors, including the size of the primary infection site, levels of various inflammatory markers, and the severity of comorbidities9. For diabetic patients with PLA, studies have found that their recovery time is significantly longer than that of non-diabetic patients, which is closely related to the impaired immune response and chronic hyperglycemia associated with diabetes10. However, there is no specific study on the independent factors affecting the hospital stay of these patients.
Therefore, this study aims to identify the recovery predictors for diabetic patients with PLA undergoing antibiotic treatment and to develop an effective nomogram for predicting the ORT through univariate and multivariate analysis. Through retrospective analysis, this study will explore how factors such as chills and fever, pre-treatment shock, and abscess diameter affect the recovery time of patients, providing scientific evidence for clinical decision-making. Furthermore, by developing the nomogram tool, this study aims to help clinicians more accurately predict recovery time and provide a basis for the rational adjustment of antibiotic use, thereby improving treatment outcomes and reducing unnecessary treatment burdens.
Materials and methods
Patient selection
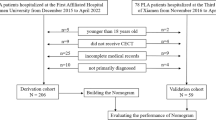
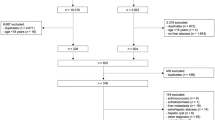
This study conducted a retrospective analysis of 139 patients with PLA treated at the Eighth People’s Hospital of Qingdao from January 2015 to December 2022. This study adopted a comprehensive diagnostic criterion, requiring at least two of the following core criteria for confirmation: (1) ultrasound or CT showing typical features of a pyogenic liver abscess; (2) isolation of pathogenic bacteria from pus or blood cultures; (3) confirmation through pathology from aspiration or surgical specimens. Supportive criteria included: (1) common clinical manifestations such as chills, fever, and limb weakness, with tenderness in the liver area being the main physical sign; (2) improvement in symptoms after antibiotic treatment. All patients were screened to exclude other causes, such as hepatocellular carcinoma and amebic liver abscess.
The following patients were excluded from the study: (1) pregnant or breastfeeding women; (2) patients currently participating in or having participated in clinical trials within the past six months; (3) patients diagnosed with fever caused by non-infectious diseases, such as autoimmune diseases (e.g., rheumatoid arthritis), neoplastic diseases, or post-surgical absorbent fever; (4) patients with non-abscess liver space-occupying lesions, such as liver cysts, malignant liver tumors, or benign tumors; (5) patients without diabetes; (6) patients with unclear bacterial culture results.
As this was a retrospective study, no formal sample size calculation or power analysis was performed. We included all eligible consecutive patients diagnosed and treated at our institution during the study period (n = 139) to maximize the representativeness and generalizability of our findings.
Data collection
Data were collected from the hospital’s case management system, including the patients’ basic information (age, gender), clinical manifestations (chills and high fever, abdominal pain, septic shock), comorbidities (biliary stones, hypertension, pneumonia), and whether they underwent biliary puncture drainage. The laboratory indicators collected included white blood cell count (WBC), hemoglobin (HB), procalcitonin (PCT), C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and D-dimer (D-D). Additionally, the types of bacteria were recorded, as well as the time from the initiation of antibiotic treatment to its cessation. Liver abscess size was measured as the maximum diameter observed on pre-treatment CT imaging. ORT (antibiotic treatment duration) was defined as the time from the initiation to the cessation of antibiotic therapy. Upon diagnosis of pyogenic liver abscess, all patients received empirical antibiotic therapy with third-generation cephalosporins, which was subsequently adjusted based on individual antibiotic susceptibility results. In addition, ultrasound-guided percutaneous drainage procedures were performed by the same senior physician for all cases. All patients recovered and were discharged.
Statistical analysis and nomograph construction
The statistical analysis in this study was performed using R software (version 4.3.2; http://www.r-project.org/). Categorical variables were described using frequencies and percentages, while continuous variables were expressed as mean ± standard deviation (x̅±s) or median (P_25, P_75). Univariate regression analysis was performed to identify variables associated with the ORT, followed by multivariate Cox regression analysis to determine independent predictors. The stepwise method was used for variable selection in Cox regression analysis to identify independent risk factors related to ORT. Additionally, variance inflation factor (VIF) analysis was conducted to assess multicollinearity among the included variables. All VIF values were found to be below 2 (age: 1.134; fever: 1.272; shock: 1.051; drainage: 1.352; abscess size: 1.762; bacterial species: 1.509; white blood cell count: 1.267; AST: 1.353), indicating no significant multicollinearity. Based on the independent prognostic factors identified in the multivariate regression analysis, Kaplan-Meier survival curves were plotted and a nomogram was constructed to predict the ORT. To ensure the stability and accuracy of the model, internal validation was performed, and the model’s discriminative ability and predictive accuracy were assessed using the concordance index (C-index) and calibration curves. A p-value of less than 0.05 was considered statistically significant.
Results
Basic information of patients
From January 2015 to December 2022, this study selected 139 patients with bacterial PLA and diabetes who were treated at the Eighth People’s Hospital of Qingdao. All patients had positive bacterial cultures and were switched to sensitive antibiotics based on the drug sensitivity results after initial empirical treatment. As shown in Table 1, there were 85 male patients (61.2%) and 54 female patients (38.2%), with a significantly higher proportion of male patients. The average age of the patients was 61.4 ± 16.2 years. Among all the patients, 62 (44.6%) experienced chills and high fever, and 25 (18.0%) had septic shock before treatment. Regarding other comorbidities, 25 patients (18.0%) had a history of biliary stones, 63 patients (45.3%) had hypertension, and 36 patients (25.9%) had pneumonia. After bacterial culture, 109 cases (78.4%) were infected with Klebsiella pneumoniae, which was the dominant pathogen. Other pathogens included Escherichia coli (17 cases), Staphylococcus aureus (7 cases), Pseudomonas aeruginosa (3 cases), and Streptococcus viridans (3 cases), which were grouped together as other bacterial infections (30 cases, 21.6%). Among the abscess locations, 97 patients (69.8%) had right lobe abscesses, 24 patients (17.3%) had left lobe abscesses, and 18 patients (12.9%) had multiple abscesses within the liver. A total of 115 patients (82.7%) underwent ultrasound-guided biliary puncture drainage, all performed by a senior ultrasound physician at the hospital. A typical CT scan of the process of percutaneous puncture drainage treatment for pyogenic liver abscess is shown in Fig. 1. After appropriate treatment, the median ORT of the patients was 17 days (range: 13–22 days).
Selection of risk factors
We performed univariate and multivariate analyses, with the results shown in Table 2; Fig. 2. In the univariate analysis, we found that age (P = 0.026, HR = 0.987), high fever (P = 0.002, HR = 0.570), septic shock (P < 0.01, HR = 0.340), abscess puncture drainage (P = 0.011, HR = 0.556), abscess size (P < 0.01, HR = 0.867), bacterial species (P = 0.040, HR = 1.542), white blood cell count (P = 0.024, HR = 0.963), and AST levels (P < 0.01, HR = 0.995) were significantly correlated with the duration of antibiotic treatment (ORT). Further multivariate Cox regression analysis, including the significant variables from univariate analysis, showed that age (P = 0.024, HR = 0.673), high fever (P < 0.01, HR = 0.500), septic shock (P < 0.01, HR = 0.34), and abscess size (P < 0.01, HR = 0.51) were associated with the hospital stay. Kaplan-Meier survival curves were subsequently plotted (see Fig. 3), but no significant differences were observed between the older and younger groups.
Kaplan-Meier survival analysis curves of partial risk factors. (A): Treatment time curve for patients with or without chills and high fever; (B): Treatment time curve for patients with or without septic shock; (C): Treatment time curve for patients with abscesses smaller than 5 cm and those with abscesses 5 cm or larger; (D): Treatment time curve for patients with normal white blood cell count and those with elevated white blood cell count.
Nomogram creation
Finally, a nomogram was constructed to predict the duration of antibiotic treatment for PLA patients with diabetes based on the three independent predictive factors: high fever, septic shock, abscess size, and WBC count (see Fig. 4). After internal validation, the model’s Harrell’s C-statistic was 0.75, and the calibration curve shown in Fig. 5 demonstrated good consistency between the predicted treatment time and the observed days.
To illustrate the use of the nomogram, consider a diabetic PLA patient diagnosed by bacterial culture and imaging, with an abscess maximum diameter of 8 cm (50 points), septic shock prior to treatment (68 points), and a white blood cell count of 12 × 10⁹/L (18 points). The patient’s total score would be 136 points, corresponding to an estimated antibiotic treatment duration of approximately 25 days. This means that the patient may require 25 days of continuous antibiotic treatment for recovery.
Clinical outcomes and ORT
All patients started empirical antibiotic therapy after a clear diagnosis, and the antibiotics were switched to sensitive ones based on drug sensitivity results. The drug sensitivity test showed that 95% of Klebsiella pneumoniae strains were sensitive to third-generation cephalosporins or carbapenems. All ultrasound-guided percutaneous biliary drainage procedures were performed by the same senior ultrasound physician at the hospital. For multi-loculated abscesses, the larger abscess cavity was chosen for puncture, and all punctures were successful on the first attempt. All patients were eventually discharged after recovery, with no mortality. The median ORT for the patients was 17 days (range: 13–22 days).
Discussion
Pyogenic liver abscess (PLA) is a disease caused by bacterial invasion of the liver through various mechanisms, leading to hepatic suppurative inflammation. In the early reports on PLA, scholars generally considered biliary tract diseases as the primary cause of PLA and believed that Escherichia coli was the most common pathogen11,12,13. However, with improvements in healthcare conditions, the spectrum of pathogens causing PLA has undergone significant changes. Over the past 20 years, Klebsiella pneumoniae has gradually become the predominant pathogen of PLA14,15,16. Additionally, we observed that most patients with liver abscesses typically have a short hospital stay and a good prognosis after active drainage and antibiotic therapy17,18. However, diabetic patients have been shown to have a worse prognosis, as multiple studies have confirmed19. The incidence of diabetes continues to rise globally, with China having the largest number of diabetic patients, accounting for about one-quarter of the global diabetic population20.
The elevated blood glucose levels in diabetic patients cause multifaceted effects on their physiological state. Hyperglycemia inhibits the chemotaxis and phagocytic function of white blood cells, lowers immunity, and promotes vascular sclerosis and reduced blood flow, which further restricts the mobilization of white blood cells. These pathophysiological changes lead to more severe clinical symptoms and worse prognosis in diabetic patients with bacterial liver abscess21,22. Currently, there is no dedicated prognosis analysis for PLA patients with diabetes. Our study fills this gap by developing a prognostic nomogram.
Our research indicates that fever, pre-treatment septic shock, abscess size larger than 5 cm, and elevated white blood cell count are independent risk factors for prolonged ORT. These factors are closely related to the recovery time of patients, and our model’s C-index of 0.75 demonstrates higher accuracy compared to previous studies23. Moreover, previous research often focused on extreme indicators that lead to very poor prognosis, such as the studies by Xu et al., which overemphasized the adverse impact of gas formation and shock, while some patients did not show abnormalities in these two indicators, making it difficult to effectively identify the duration of hospitalization for these patients23.
Studies have shown that high fever is an important indicator of bacterial infection activity, and several studies have confirmed that high fever significantly affects the prognosis of patients24,25. Our study supports this view, indicating that fever significantly affects the duration of antibiotic treatment, although it contradicts the conclusions of Yu et al.26. Pre-treatment septic shock is another significant prognostic factor, which is consistent with several previous studies. Septic shock is an important factor affecting the prognosis of patients with septic liver abscess and is usually associated with a longer treatment time27,28. We found that patients with septic shock before treatment had significantly prolonged ORT, which may be related to the increased risk of systemic inflammatory response and multi-organ failure29. Therefore, early identification and active intervention for these patients are crucial. For patients who have not developed shock, white blood cell count remains an effective marker of inflammation, and in our nomogram, we included white blood cell count as a risk factor.
Additionally, abscess size was also identified as a key factor affecting ORT in this study, which is consistent with the research by Chang et al.30. Patients with an abscess diameter larger than 5 cm had a significantly longer treatment time than those with smaller abscesses. These patients often required puncture drainage treatment, which may explain why patients who underwent this more aggressive treatment had worse outcomes compared to those without puncture drainage.
In conclusion, this study highlights the critical role of factors such as fever, pre-treatment septic shock, abscess size, and white blood cell count in predicting the duration of antibiotic treatment for diabetic patients with liver abscesses. We have developed an effective predictive tool to estimate the ORT. The model’s Harrell C-statistic of 0.75 demonstrates good predictive accuracy, and the calibration curve shows good consistency between the predicted and actual ORT, further validating the model’s reliability. This tool not only provides a simple and practical assessment for clinicians but also helps guide the appropriate cessation of antibiotic treatment, thereby reducing unnecessary treatment burdens.
However, this study has several limitations. In order to ensure data accuracy and to specifically develop a predictive model for PLA patients with diabetes, the total number of cases included was limited to 139. Additionally, we were unable to perform external validation of the nomogram, which may restrict the generalizability of our findings to broader and more diverse populations. Future large-scale, multi-center or population-based studies are needed to validate the applicability of these results across different clinical settings. Moreover, due to data limitations, we were unable to evaluate the potential impact of nutritional status indicators, such as serum albumin levels, on the duration of antibiotic therapy. Future studies should incorporate these variables to further enhance the model’s predictive accuracy. Overall, prospective, multi-center studies with external validation are essential to strengthen our findings and extend their clinical applicability.
Data availability
The datasets and analytical data utilized in this experiment are not currently publicly available, but they may be made accessible to corresponding authors upon request.
References
Lardière-Deguelte, S. et al. Hepatic abscess: diagnosis and management. J. Visc. Surg. 152, 231–243. https://doi.org/10.1016/j.jviscsurg.2015.01.013 (2015).
Khim, G., Em, S., Mo, S. & Townell, N. Liver abscess: diagnostic and management issues found in the low resource setting. Br. Med. Bull. 132, 45–52. https://doi.org/10.1093/bmb/ldz032 (2019).
Chen, C. H., Wu, S. S., Chang, H. C. & Chang, Y. J. Initial presentations and final outcomes of primary pyogenic liver abscess: a cross-sectional study. BMC Gastroenterol. 14, 7. https://doi.org/10.1186/1471-230x-14-133 (2014).
Wang, J. L., Hsu, C. R., Wu, C. Y. & Lin, H. H. Diabetes and obesity and risk of pyogenic liver abscess. Sci. Rep. 13, 9. https://doi.org/10.1038/s41598-023-34889-z (2023).
Song, H. W., Wang, X. B., Lian, Y. B. & Wan, T. E. Analysis of the clinical characteristics of 202 patients with liver abscess associated with diabetes mellitus and biliary tract disease. J. Int. Med. Res. 48, 13. https://doi.org/10.1177/0300060520949404 (2020).
Ko, M. C. et al. A cohort study of age and sex specific risk of pyogenic liver abscess incidence in patients with type 2 diabetes mellitus. Med. (Baltim). 98, 7. https://doi.org/10.1097/md.0000000000015366 (2019).
Gundling, F., Secknus, R., Abele-Horn, M. & Mössner, J. Pyogenic liver abscess -: diagnosis and treatment. Dtsch. Med. Wochenschr. 129, 1685–1688. https://doi.org/10.1055/s-2004-829015 (2004).
Doherty, K., Papamargaritis, I., Muir, J., Corrigan, M. & Sieberhagen, C. Presentation, management, and outcomes of pyogenic liver abscess in a single UK centre: a comparison between centres and an evaluation of liver abscess management. Gut 71, A66–A68. https://doi.org/10.1136/gutjnl-2022-BASL.98 (2022).
Lam, J. C. & Stokes, W. Management of pyogenic liver abscesses contemporary strategies and challenges. J. Clin. Gastroenterol. 57, 774–781. https://doi.org/10.1097/mcg.0000000000001871 (2023).
Wang, T. Y. et al. Pyogenic liver abscess risk in patients with newly diagnosed type 2 diabetes mellitus: A nationwide, Population-Based cohort study. Front. Med. 8, 9. https://doi.org/10.3389/fmed.2021.675345 (2021).
McDonald, M. I. Pyogenic liver-abscess - diagnosis, bacteriology and treatment. Eur. J. Clin. Microbiol. Infect. Dis. 3, 506–509. https://doi.org/10.1007/bf02013608 (1984).
Serraino, C. et al. Characteristics and management of pyogenic liver abscess: A European experience. Med. (Baltim). 97, 6. https://doi.org/10.1097/md.0000000000010628 (2018).
Bruns, T. & Stallmach, A. Bacterial hepatobiliary infections pathogen spectrum, antimicrobial resistance and current treatment concepts. Internist 63, 349–366. https://doi.org/10.1007/s00108-022-01277-0 (2022).
Luo, M. et al. Distribution of common pathogens in patients with pyogenic liver abscess in China: a meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 35, 1557–1565. https://doi.org/10.1007/s10096-016-2712-y (2016).
Sundaramoorthy, N. S., Shankaran, P., Gopalan, V. & Nagarajan, S. New tools to mitigate drug resistance in Enterobacteriaceae - Escherichia coli and Klebsiella pneumoniae. Crit. Rev. Microbiol. 49, 435–454. https://doi.org/10.1080/1040841x.2022.2080525 (2023).
Liu, Y., Wang, J. Y. & Jiang, W. An Increasing Prominent Disease of Klebsiella pneumoniae Liver Abscess: Etiology, Diagnosis, and Treatment. Gastroenterol. Res. Pract. 12 (2013). (2013). https://doi.org/10.1155/2013/258514
Roediger, R. & Lisker-Melman, M. Pyogenic and amebic infections of the liver. Gastroenterol. Clin. North. Am. 49, 361–. https://doi.org/10.1016/j.gtc.2020.01.013 (2020).
Dumic, I. et al. Clinical characteristics, diagnosis, treatment, and outcome of patients with liver abscess due to Aspergillus spp: a systematic review of published cases. BMC Infect. Dis. 24, 9. https://doi.org/10.1186/s12879-024-09226-y (2024).
Wang, Y. A., Wang, H. R., Liu, Z. Y. & Chang, Z. H. The Incidence of Septic Pulmonary Embolism in Patients with Klebsiella pneumoniae Liver Abscess: A Systematic Review and Meta-analysis. Gastroenterol. Res. Pract. 8 (2022). (2022). https://doi.org/10.1155/2022/3777122
Hwang, B. F., Sarasmita, M. A. & Li, M. C. Global, regional, and National burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021 (402, Pg 203, 2023). Lancet 402, 1132–1132. https://doi.org/10.1016/s0140-6736(23)02044-5 (2023).
Sharma, V. K. & Singh, T. G. Chronic stress and diabetes mellitus: interwoven pathologies. Curr. Diabetes Reviews. 16, 546–556. https://doi.org/10.2174/1573399815666191111152248 (2020).
Liu, H. Y., Wang, X. R., Gao, H., Yang, C. & Xie, C. G. Physiological and pathological characteristics of vascular endothelial injury in diabetes and the regulatory mechanism of autophagy. Front. Endocrinol. 14, 10. https://doi.org/10.3389/fendo.2023.1191426 (2023).
Xu, S., Shi, B. Q., Chao, L. M., Tan, Y. S. & Zhang, X. J. Prognostic nomogram for the combination therapy of percutaneous catheter drainage and antibiotics in pyogenic liver abscess patients. Abdom. Radiol. 45, 393–402. https://doi.org/10.1007/s00261-019-02359-8 (2020).
Rosenfeld-Yehoshua, N. et al. vol 177, pg 337,. Hyperpyrexia and high fever as a predictor for serious bacterial infection (SBI) in children-a systematic review Eur. J. Pediatr. 179, 353–353 (2020). (2018). https://doi.org/10.1007/s00431-019-03525-2
Schortgen, F. Fever in sepsis. Minerva Anestesiol. 78, 1254–1264 (2012).
Yu, J. et al. Clinical comparison of febrile and afebrile patients with pyogenic liver abscess: A two-centre retrospective study. Saudi J. Gastroenterol. 27, 370–. https://doi.org/10.4103/sjg.sjg_17_21 (2021).
Li, J. et al. Development and validation of a nomogram for predicting sepsis in patients with pyogenic liver abscess. Sci. Rep. 13, 9. https://doi.org/10.1038/s41598-023-37907-2 (2023).
Chou, F. F., Sheenchen, S. M., Chen, Y. S. & Lee, T. Y. The comparison of clinical course and results of treatment between gas-forming and non-gas-forming pyogenic liver-abscess. Arch. Surg. 130, 401–405 (1995).
Ebert, R. V. & Abernathy, R. S. Septic shock. Federation Proc. 20, 179– (1961).
Lee, C. H. et al. Maximal diameter of liver abscess independently predicts prolonged hospitalization and poor prognosis in patients with pyogenic liver abscess. BMC Infect. Dis. 21, 10. https://doi.org/10.1186/s12879-021-05873-7 (2021).
Funding
This study was supported by the Science and Technology Development Project of Qingdao Eighth People’s Hospital Affiliated to Shandong Second Medical University (2023FYM084).
Author information
Authors and Affiliations
Contributions
G JJ was responsible for statistical analysis, participated in data interpretation, and wrote the article. Y HX provided detailed information on pyogenic cultures and assisted in data collection. W H was responsible for designing the research plan, overseeing the entire research process, and providing guidance for the article writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was approved by the Medical Ethics Committee of the Affiliated Eighth People’s Hospital of Shandong Second Medical University, with approval number (QBYLL-KY-2024-039). The study adheres to the principles outlined in the Declaration of Helsinki.
Informed consent
Due to the retrospective nature of this study, the Medical Ethics Committee of the Affiliated Eighth People’s Hospital of Shandong Second Medical University waived the requirement for obtaining informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gai, J., Yan, H. & Wang, H. Development and validation of a nomogram for predicting antibiotic treatment duration in patients with liver abscess complicated by diabetes. Sci Rep 15, 13897 (2025). https://doi.org/10.1038/s41598-025-98643-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98643-3