Abstract
It remains unclear that trade-off between the benefits of regular physical activity (PA) and the potentially harmful effects of exposure to PM2.5 (particulate matter with aerodynamic diameter < 2.5 μm) in highly polluted regions. Therefore, we aimed to investigate the modification of PM2.5 on the associations of PA volume and intensity with depressive symptoms and glaucoma in individuals with or without depressive symptoms. Data of this study was obtained from the China Health and Retirement Longitudinal Survey (CHARLS) implemented during 2011 to 2020. PA volume and intensity were measured by a standardized questionnaire; a machine learning prediction model was applied to ascertain the PM2.5 concentrations. Cox proportional hazards regression models were employed to explore associations. A total of 20,930 participants were finally included, with 11,214 analyzed for PA and depressive symptom risk and 16,965 analyzed for PA and glaucoma risk. PA volume or intensity was independent protective factor for incident depressive symptoms, while PM2.5 was independent risk factor for depressive symptoms and glaucoma in participants with or without depressive symptoms. Among participants with low PM2.5 exposure (< 35 micrograms per cubic meter [µg/m3]), PA volume or intensity showed an inverse association with the risk of incident depressive symptoms, but insignificant associations between PA volume or intensity and glaucoma risk were observed in either participants with or without depressive symptoms. Among participants with high PM2.5 exposure (≥ 35 µg/m3), higher PA volume or intensity increased the risks of depressive symptoms and glaucoma. Higher PA level was associated with a reduced risk of depressive symptoms only among participants with low PM2.5 exposure, and higher PA did not decrease the risk of glaucoma regardless of PM2.5 level. Our findings recommend regular PA to prevent depressive symptoms in less polluted regions and reinforce the importance of air quality improvement.
Similar content being viewed by others
Introduction
Depression is a prevalent mental health disorder characterized by persistent low mood and diminished interest1. With the acceleration of urbanization and industrialization process, depression has emerged as the third most common non-fatal disease worldwide2; the lifetime prevalence of depression is estimated to be approximately 11% and this rate continues to increase within the general population3,4,5. As the country with the highest prevalence of depression globally, China has seen a reduction in the incidence of depression due to the implementation of intervention measures. However, it is still difficult to detect the reduction of the disease burden of depression nationwide6. Mild depressive symptoms can affect an individual’s work ability, while severe depressive symptoms, including low mood, loss of pleasure, and cognitive impairment, are strongly associated with suicidal behavior. Consequently, both mild and severe depressive symptoms may bring substantial burden to society7,8. Several factors, such as genetic predisposition, behavioral lifestyle, and environmental influences, may contribute to the development of depression9,10. Given the current limitations in sufficiently effective treatments for depression, a deeper understanding of its causes and prevention strategies remains a critical priority for both clinical practice and public health.
Studies have demonstrated that physical activity (PA) is beneficial for mental health11,12. A meta-analysis of prospective studies revealed that individuals with higher levels of PA exhibited a 17% lower risk of developing depression compared to those with lower PA levels13. Air pollution (AP), particularly ambient PM2.5 (particulate matter with aerodynamic diameter < 2.5 μm), elevates the risk of numerous diseases, including depression and glaucoma14,15. PA significantly increases the inhalation of air pollutants, potentially diminishing or even offsetting its health benefits16,17. 80% of countries worldwide have adopted national policies to encourage regular PA, yet over half of the global population resides in regions that fail to meet the World Health Organization’s minimum air quality standards (PM2.5 ≤ 35 µg/m3)18. Consequently, it raises significant public concern whether regular PA should be recommended in highly polluted areas16.
The findings of several studies investigating the potential implications of prolonged exposure to PM2.5 on the preventive benefits of regular PA remain inconclusive19,20,21,22. While some studies suggest that PA may amplify the adverse effects of AP on various health outcomes19,20, others propose that PA may either be independent of or even protective against the harmful impacts of AP21,22. Moreover, most of studies are frequently conducted in areas with low AP levels, thereby limiting their generalization to highly polluted regions, such as mainland China. Additionally, earlier studies focused solely on the volume or type of PA, neglecting the role of PA intensity — a critical gap highlighted by the 2020 European Society of Cardiology guidelines on cardiology and exercise23.
Glaucoma is the leading cause of irreversible vision loss globally24. Its chronic and progressive nature often leads to significant psychological distress among patients25. Studies have showed that a glaucoma diagnosis can contribute to depressive symptoms, and depressive symptoms may, in turn, influence the onset and progression of the disease26. Several studies have examined the potential association between higher levels of PA and a reduced risk of glaucoma; however, the evidence remains conflicting27,28,29. In addition, it is unclear whether the effects of PA on glaucoma differ between individuals with and without depressive symptoms. Recent studies suggest that long-term exposure to ambient AP may influence the associations between PA and various health outcomes, including hypertension, diabetes, stroke, etc16,17. Nevertheless, the impact of prolonged PM2.5 exposure on the association between PA and glaucoma in individuals with depressive symptoms remains poorly understood.
To address the aforementioned gaps, we conducted a comprehensive analysis using data from a nationwide study in China. Our objectives were threefold: (1) to evaluate the association between PA, including its volume and intensity, and depressive symptom; (2) to examine whether PA, in terms of volume and intensity, reduces the risk of glaucoma among individuals with depressive symptoms; and (3) to assess whether prolonged PM2.5 exposure modifies the associations between regular PA and the risk of depressive symptoms and glaucoma.
Methods
Study design and participants
The participants in this study were drawn from the China Health and Retirement Longitudinal Survey (CHARLS), a nationally representative longitudinal survey of Chinese individuals aged 45 and above, as well as their partners. A detailed description of this cohort has been previously published30. Briefly, CHARLS utilized probability proportional to size (PPS) sampling to randomly select 150 districts and counties across 28 provincial administrative units. The study initially included 17,708 participants as the baseline in 2011, with follow-ups conducted in 2013, 2015, and 2018 using face-to-face computer-assisted personal interviews (CAPIs). Each follow-up wave incorporated new participants. As of the latest survey, a total of 25,586 individuals have been enrolled in CHARLS. All participants provided written informed consent, which was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015).
Out of the total participants, 4,656 were excluded due to being under 45 years of age or lacking key information, such as age and sex. Finally, 20,930 individuals were included. To explore the association between PA and depressive symptoms, we further excluded participants with only one survey of depressive symptoms or missing PA information at baseline (n = 3,942), as well as those diagnosed with depressive symptoms at baseline (n = 5,774). Consequently, 11,214 eligible individuals were included in this analysis. To examine the association between PA and glaucoma incidence, we excluded participants with only one survey of glaucoma or missing PA information at baseline (n = 3,620), those diagnosed with glaucoma at baseline (n = 25), and those lacking depressive symptom scores at baseline (n = 320). Ultimately, 16,965 participants (5,750 with depressive symptoms and 11,215 without depressive symptoms) were included in the study (Figure S1).
PA evaluation
CHARLS employed a self-reported standardized questionnaire, comparable to the International Physical Activity Questionnaire (IPAQ)31, to evaluate the PA levels of all participants. Three types of PA levels were evaluated: (1) Mild PA, defined as walking for leisure or recreation, walking at work, and traveling between places; (2) Moderate PA, encompassing activities such as brisk walking, Tai Chi, mopping, or light loading; (3) Vigorous PA, including activities like digging, heavy lifting, or quick cycling. The intensity of each activity was quantified in metabolic equivalents (MET), where 1 MET equals 1 kcal/kg of body weight per hour. We assigned MET values of 2, 4, and 8 to mild, moderate, and vigorous activities, respectively16. Participants were asked to report the frequency (0–7 days) and typical duration per day (no activity, 10–29, 30–119, 120–239, and ≥ 240 min) of PA during a typical week. Following previous literature32,33, the median time for each PA duration category was used to calculate PA duration, as specific durations were unavailable. The “≥240 minutes” category was treated as 240 min. The total time spent on each activity (hours per week) was calculated by multiplying the frequency of PA by the duration of each activity. For each participant, the daily PA volume (MET-h/day) was calculated using the following steps: (1) determining the PA volume of each activity by multiplying the total time of each activity by its MET value; and (2) summing the PA volumes of all activities and dividing by 7 days. Participants were then categorized into four groups based on quartiles of daily PA volume: ≤4.10 MET-h/day, >4.10–9.87 MET-h/day, >9.87–25.17 MET-h/day, and >25.17 MET-h/day. The average PA intensity for each participant was calculated by dividing the daily PA volume by the daily total PA time34. Average PA intensity was classified into three categories based on prior literature16: light (<3.0 MET), moderate (3.0-<6.0 MET), and vigorous (≥ 6.0 MET).
Assessment of ambient PM2.5 and temperature
Monthly mean PM2.5 concentrations from 2011 to 2020 were obtained from the Tracking Air Pollution in China database (http://tapdata.org.cn). A two-stage machine learning prediction model was implemented, integrating synthetic minority oversampling and tree-based gap-filling methodologies, to estimate PM2.5 concentrations35. This model demonstrated robust predictability for PM2.534. Since precise addresses of participants were unavailable, county-level PM2.5 concentrations were used as a proxy for individual exposure levels based on participants’ residential addresses. Each individual’s PM2.5 exposure level was calculated as the average concentration between cohort entry and the onset of illness or until the end of the cohort (2020) if participants who did not develop illness during follow-up. For participants without illness who were lost to follow-up or changed addresses during the follow-up period, PM2.5 exposure level was averaged over the period between the time they entered the cohort and the time of their last follow-up before they were lost to follow-up or changed address.
Monthly average temperature data for cities in CHARLS from 2011 to 2020 were extracted from the National Earth System Science Data Center (http://www.geodata.cn). The assessment of individual temperature exposure followed a similar approach to that of PM2.5. Specifically, each participant’s temperature exposure level was calculated as the average from cohort entry (2011) until either the onset of the disease or the end of the cohort (2020) for participants who did not develop the disease during the follow-up period. For participants who did not develop the disease but were lost to follow-up or changed their addresses after cohort entry, temperature exposure was averaged from cohort entry to the last follow-up date.
Outcome measurement
CHRLAS utilized the 10-item Center for Epidemiology Studies Depression (CESD-10) questionnaire36 to assess depressive symptoms. The CESD-10 provides a comprehensive evaluation, with responses scored on a four-point scale ranging from 0 to 3. The total CESD-10 score ranges from 0 to 30, with higher scores indicating more severe depressive symptoms. Previous studies have demonstrated the good reliability of the CESD-1037. In line with established literature36, participants with a CESD-10 score exceeding 10 points were classified as having depressive symptoms.
Covariates
Several potentially confounding covariates, including demographic characteristics, socioeconomic status, and lifestyle behaviors, were included in this study. Specifically, these covariates included age (≤ 55; 55–65; >65 years), sex (male; female), education level (primary and below; junior and senior high school; university and above), marital status (married; unmarried), body mass index (BMI) (underweight; normal; overweight), residence (rural; urban), chronic disease (no; yes), living standard (categorized by the quartile of family assets at the baseline: high; middle; low; very low), disability in activities of daily living (ADL disability) (yes; no), smoking status (current; former; never), drinking status (current; former; never), cooking fuel (biomass; fossil fuel; clean fuel; others), and heating fuel (biomass; fossil fuel; clean fuel; others).
Statistical analysis
The Cox proportional hazards model was employed to estimate hazard ratios (HRs) and 95% confidence intervals (95%CIs) for the associations between PA (volume and intensity), PM2.5 levels, and the risk of developing depressive symptoms or glaucoma. Weighted Schoenfeld residuals were calculated to test the proportional hazards assumption, and no violations were detected (P > 0.05). The following covariates were included in the Cox proportional hazards models: age, sex, education level, marital status, BMI, residence, chronic disease, living standard, ADL disability, smoking status, drinking status, cooking fuel, heating fuel, PM2.5 (for the association with PA volume), PA volume (for the association with PM2.5), or both PM2.5 and PA volume (for the association with PA intensity), and ambient temperature (a regression spline with 3 degrees of freedom was applied to accommodate the nonlinear relationship between temperature and outcome variables).
Stratified analyses were conducted to evaluate the associations between PA (volume or intensity) and the risk of depressive symptoms or glaucoma by PM2.5 level, using a cutoff of 35 µg/m³ based on the China Ambient Air Quality Standard (GB3095-2012). Tests for trend were performed by treating the median of each PA category as a continuous variable in the models. A two-sample z-test was used to assess whether there were significant differences in effect estimates between individuals with and without depressive symptoms38.
Additionally, the joint effects of PM2.5 and PA (volume or intensity) were examined by including both main effects and interaction terms of PM2.5 groups and PA (volume or intensity) categories in the Cox proportional hazards model. The effects of eight joint categories of PM2.5 (low and high) and PA volume (first to fourth quartiles) were calculated by comparing each group to the reference group, which consisted of participants in the first quartile of PA volume and exposed to low PM2.5. Similarly, participants were classified into six groups to estimate the combined effects of PM2.5 (low and high) and PA intensity (light, moderate, and vigorous), with the group of light PA intensity and low PM2.5 as the reference.
To ascertain the strength and reliability of our results, we conducted sensitivity analyses: (1) to address the possibility of reverse causality that could bias the study results, participants who had an outcome at the first visit were excluded; (2) to better control for socioeconomic status, the average number of years of schooling at the county level (based on the population census of China) was incorporated into the Cox models; and (3) to assess whether the main findings varied based on different PM2.5 cutoff points, the median PM2.5 concentration (47.8 µg/m³) was used as an alternative cutoff for reanalysis.
All statistical analyses were performed using R software (version 4.2.2, R Development Core Team 2019, https://www.r-project.org), and statistical significance was declared if P < 0·05.
Results
Baseline characteristics
A total of 20,930 individuals were included in this analysis, with an average age of 58.6 years (standard deviation, SD = 9.8). The mean survival time of participants from baseline to the onset of depressive symptoms, participants with depressive symptoms from baseline to onset of glaucoma, and participants without depressive symptoms from baseline to the onset of glaucoma were 6.0 years (SD = 2.7), 6.2 years (SD = 1.4), and 6.1 years (SD = 1.5), respectively. More than half of participants were female (10,571, 50.5%) and resided in rural regions (11,673, 55.8%). At baseline, the prevalence of depressive symptoms and glaucoma was 38.7% (n = 8,107) and 3.7% (n = 765), respectively (Table 1). During the follow-up period, 4,405 individuals (43.5%) newly developed depressive symptoms, while 446 participants (2.9%) were diagnosed with new cases of glaucoma. The median PM2.5 concentration was 47.8 mg/m3, ranging from 35.6 mg/m3 to 61.7 mg/m3 (Table 2).
Associations of PA or PM2.5 with depressive symptoms and Glaucoma
Table 3 presents the independent associations of PA and PM2.5 exposure with incident depressive symptoms and glaucoma. Higher levels of PM2.5 were associated with an increased risk of developing depressive symptoms and glaucoma. With the first quartile of PA volume as reference, the adjusted HRs (95%CIs) for the second, third, and fourth quartiles of PA volume were 0.96 (0.88–1.04), 0.88 (0.80–0.96), and 0.83 (0.79–0.87), respectively. Inverse associations were observed between PA intensity and depressive symptoms. No statistically significant associations were found between PA (volume or intensity) and glaucoma, regardless of whether participants had depressive symptoms.
Associations of PA with depressive symptoms and Glaucoma stratified by PM2.5 level
Table 4 displays the associations between PA volume and the risk of depressive symptoms and glaucoma, stratified by PM2.5 level. A significant interaction was observed between PM2.5 and PA volume in relation to the occurrence of depressive symptoms (Pinteraction < 0.001). For individuals exposed to low PM2.5, the HRs were as follows: 1.00 (reference) for the first quartile of PA volume, 0.97 (0.89–1.07) for the second quartile, 0.87 (0.79–0.97) for the third quartile, and 0.82 (0.77–0.87) for the fourth quartile (Ptrend = 0.012). For those exposed to high PM2.5, the HRs (95% CI) were 1.00 (reference), 0.91 (0.75–1.11), 0.94 (0.75–1.16), and 0.85 (0.70–1.03) (Ptrend = 0.141). We observed a significant interaction between the PA volume and exposure to PM2.5 in relation to glaucoma development (Pinteraction < 0.001). However, no significant association between PA volume and glaucoma was found at either high or low PM2.5 concentrations, regardless of whether participants had depressive symptoms.
Table 5 presents the associations of PA intensity with depressive symptoms and glaucoma, stratified by PM2.5 levels. A significant interaction was observed between PA intensity and PM2.5 exposure (Pinteraction < 0.001). Specifically, participants who engaged in vigorous PA had a 25% lower hazard of depressive symptoms compared to those engaged in light PA, but this association was only evident among individuals exposed to low PM2.5 levels. However, no statistically significant association was found between PA intensity and depressive symptoms among those exposed to high PM2.5 levels. A significant interaction was also observed between PA intensity and PM2.5 exposure in relation to incident glaucoma (Pinteraction < 0.001). Furthermore, no significant association was found between PA intensity and the occurrence of glaucoma, regardless of whether individuals were exposed to high or low PM2.5 levels, whether in participants with or without depressive symptoms.
Joint effects of PA and PM2.5 on depressive symptoms and Glaucoma
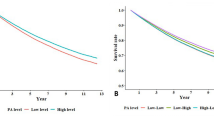
The joint effects of PA (both volume and intensity) and PM2.5 exposure are shown in Fig. 1. Individuals exposed to high PM2.5 levels consistently exhibited a higher risk of developing depressive symptoms and glaucoma compared to those exposed to low PM2.5 levels, regardless of their PA volume or intensity. Additionally, participants with low PM2.5 exposure and high levels of PA (either in volume or intensity) had the lowest risk of depressive symptoms. However, no significant changes in glaucoma risk were observed at low PM2.5 exposure combined with varying levels of PA (regardless of volume or intensity), irrespective of whether participants had depressive symptoms at baseline.
Joint effects of PM2.5 and PA volume or PA intensity on incident depressive symptoms or glaucoma. (A) Joint effects of PM2.5 and PA volume on incident depressive symptoms; (B) Joint effects of PM2.5 and PA intensity on incident depressive symptoms; (C) Joint effects of PM2.5 and PA volume on incident glaucoma in participants with depressive symptoms; (D) Joint effects of PM2.5 and PA intensity on incident glaucoma in participants with depressive symptoms; (E) Joint effects of PM2.5 and PA volume on incident glaucoma in participants without depressive symptoms; (F) Joint effects of PM2.5 and PA intensity on incident glaucoma in participants without depressive symptoms. Cox proportional hazard model, and adjusted for age, sex, education level, marital status, residence, body mass index, disability in activities of daily living, chronic disease, smoking status, drinking status, living standard, heating fuel, cooking fuel, temperature, (further adjusted for PA volume when analyzing the joint effects of PM2.5 and PA intensity). PA, physical activity; PM2.5, fine particulate matter.
Sensitivity analyses
Sensitivity analyses yielded consistent findings when: (1) individuals who developed depressive symptoms or glaucoma during the initial follow-up were excluded (Table S1); (2) county-level average years of education were incorporated into the models (Table S2); and (3) participants were categorized into low and high PM2.5 groups based on the median PM2.5 concentration (47.8 µg/m³, Table S3).
Discussion
This study is the first prospective cohort study to examine the joint effects of long-term PM2.5 exposure and PA (volume and intensity) on the incidence of depressive symptoms and glaucoma among individuals with depressive symptoms residing in highly polluted regions. Among participants with low PM2.5 exposure, both PA volume and intensity showed an inverse association with the risk of incident depressive symptoms. However, no significant associations were observed between PA and glaucoma risk, regardless of whether participants had depressive symptoms. In contrast, among those exposed to high PM2.5 levels, the associations between PA and the risk of depressive symptoms and glaucoma were either insignificant or even positive. Furthermore, the associations of PA with the risk of depressive symptoms or glaucoma were significantly modified by ambient PM2.5 levels.
Our study, alongside several others13,14 (despite inconsistent evidence from other studies39,40), has identified independent effects of AP and PA on depressive symptoms. Specifically, significant associations were observed between PM2.5/PA and incident depressive symptoms. Furthermore, some observational epidemiological studies suggest that the health benefits of PA may be diminished by long-term exposure to AP41,42. Our findings indicate that PA is effective in improving depressive symptoms only at low PM2.5 levels (< 35 µg/m³), while the joint effect of PM2.5 and PA demonstrated a harmful impact on the development of depressive symptoms at high PM2.5 exposure levels. A nationwide cohort study16 in China involving 54,797 participants, focusing on AP and blood pressure, also found that individuals can benefit from higher PA levels when PM2.5 concentrations are below 60 µg/m³. This recommended threshold aligns with studies conducted in Taiwan21 and Henan Province43. In these studies, when PM2.5 levels exceed this threshold, individuals consistently face a higher risk of hypertension and metabolic diseases compared to those exposed to subthreshold PM2.5 levels16,43. Under such conditions, high PM2.5 exposure may counteract or even reverse the positive health effects of PA, regardless of PA volume or intensity. A key contribution of our study is its emphasis on the need to control AP levels to lower thresholds (e.g., below the WHO’s Interim Target 1 of 35 mg/m3)18 to maximize the mental health benefits of adequate PA.
This study did not observe an independent impact of PA on glaucoma in individuals with or without depressive symptoms, which aligns with a previous investigation in the UK Biobank44. Although the relationship between AP and glaucoma has gained attention in recent years45, the biological mechanisms linking long-term PM2.5 exposure to glaucoma remain poorly understood. Our findings suggest that the impact of AP on glaucoma may be independent of human behaviors, such as PA. However, it is important to note that the joint effects of PA and AP may significantly influence the development of glaucoma in participants exposed to high PM2.5 levels, regardless of their depressive status. Thus, even individuals with high PA levels may not be protected from the risk of glaucoma associated with AP, particularly those living in areas with high PM2.5 exposure. Limited evidence suggests that the association between PM2.5 and glaucoma may not be related to changes in intraocular pressure but rather to potential neurotoxic and/or vascular effects46. While PA may not be an effective intervention for improving glaucoma in individuals with depressive symptoms, other lifestyle factors, such as dietary behavior and its interaction with AP, warrant further investigation in future studies47.
The health effects of PM2.5 and PA on depressive symptoms may share common mechanisms, including acute neuroendocrine and inflammatory responses13,48. PA can increase the inhalation of air pollutants by elevating the ventilation rate, leading to a higher proportion of pollutants being deposited in the airways and a shift from nasal to oral breathing49. Furthermore, as PA intensity increases, so does the volume of AP inhaled. Previous studies have shown that high-intensity PA results in approximately twice the inhalation of air pollutants compared to moderate PA50,51. This highlights the importance of guiding PA practices in areas with high AP. For example, individuals should check local air quality before engaging in physical activities. When AP levels are high, outdoor activities should be avoided and replaced with indoor alternatives. If outdoor activities are unavoidable, protective measures such as wearing specialized masks (air filters) should be implemented. Additionally, creating cleaner spaces (e.g., green belts) and encouraging exercise in cleaner areas could be effective measures. Considering indoor PA with air filtration systems could be a beneficial choice for mitigating AP exposure.
Our study has several notable strengths. Unlike previous research that primarily focused on the modifying effects of AP17,43, this study explores the combined effects of PA and AP. Our findings suggest that individuals living in areas with high levels of PM2.5 pollution do not benefit from engaging in intense outdoor PA. In contrast to earlier studies that emphasized the impact of AP on physical functioning, our research underscores the sensitivity of mental health to PM2.5 exposure, highlighting the need to maintain lower levels of air pollutants. Second, this study is based on a nationally representative sample with a long follow-up period, utilizing well-validated spatiotemporal models for PM2.5 exposure and incorporating extensive covariate data. However, our findings should be interpreted in the context of several limitations. First, the AP data were derived from residential addresses, overlooking other locations where individuals spend significant time, such as workplaces. Second, depressive symptoms were assessed using questionnaires, which may introduce some misclassification. Third, the effects of ecological factors, green environments, and other evaluation indicators were not examined due to a lack of available data.
Conclusions
This study is among the few to provide evidence from highly polluted areas, suggesting that high levels PM2.5 exposure may counteract or even reverse the benefits of PA in preventing depressive symptoms. Our findings also revealed that PA in areas with high AP levels may increase the risk of glaucoma. Therefore, interventions aimed at reducing AP or improving air quality should be prioritized to maximize the protective effects of PA on mental health.
Data availability
The CHARLS datasets for this study are publicly available and can be found here: http://charls.pku.edu.cn/.
References
Zhang, X. et al. Association between indoor air pollution and depression: a systematic review and meta-analysis of cohort studies. BMJ Open. 14, e075105. https://doi.org/10.1136/bmjopen-2023-075105 (2024).
GBD 2017 DALYs and & Collaborators, H. A. L. E. Global, regional, and National disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet (London England). 392, 1859–1922. https://doi.org/10.1016/s0140-6736(18)32335-3 (2018).
Lim, G. Y. et al. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 8, 2861. https://doi.org/10.1038/s41598-018-21243-x (2018).
Moreno-Agostino, D. et al. Global trends in the prevalence and incidence of depression:a systematic review and meta-analysis. J. Affect. Disord. 281, 235–243. https://doi.org/10.1016/j.jad.2020.12.035 (2021).
Zhao, Y. J. et al. Prevalence of major depressive disorder among adults in China: A systematic review and Meta-Analysis. Front. Psychiatry. 12, 659470. https://doi.org/10.3389/fpsyt.2021.659470 (2021).
Wang, R. et al. cMIND Diet, Indoor Air Pollution, and Depression: A Cohort Study Based on the CLHLS from 2011 to 2018. Nutrients 15, (2023). https://doi.org/10.3390/nu15051203
Cai, H. et al. Prevalence of suicidality in major depressive disorder: A systematic review and Meta-Analysis of comparative studies. Front. Psychiatry. 12, 690130. https://doi.org/10.3389/fpsyt.2021.690130 (2021).
Zhdanava, M. et al. The prevalence and National burden of Treatment-Resistant depression and major depressive disorder in the united States. J. Clin. Psychiatry. 82 https://doi.org/10.4088/JCP.20m13699 (2021).
Fan, S. J. et al. Ambient air pollution and depression: A systematic review with meta-analysis up to 2019. Sci. Total Environ. 701, 134721. https://doi.org/10.1016/j.scitotenv.2019.134721 (2020).
Nisar, A. et al. Prevalence of perinatal depression and its determinants in Mainland China: A systematic review and meta-analysis. J. Affect. Disord. 277, 1022–1037. https://doi.org/10.1016/j.jad.2020.07.046 (2020).
Kim, S. Y. et al. Physical activity and the prevention of depression: A cohort study. Gen. Hosp. Psychiatry. 60, 90–97. https://doi.org/10.1016/j.genhosppsych.2019.07.010 (2019).
Zhang, S., Xiang, K., Li, S. & Pan, H. F. Physical activity and depression in older adults: the knowns and unknowns. Psychiatry Res. 297, 113738. https://doi.org/10.1016/j.psychres.2021.113738 (2021).
Pearce, M. et al. Association between physical activity and risk of depression: A systematic review and Meta-analysis. JAMA Psychiatry. 79, 550–559 (2022).
Borroni, E., Pesatori, A. C., Bollati, V., Buoli, M. & Carugno, M. Air pollution exposure and depression: A comprehensive updated systematic review and meta-analysis. Environ. Pollut. 292, 118245. https://doi.org/10.1016/j.envpol.2021.118245 (2022).
Grant, A., Leung, G. & Freeman, E. E. Ambient air pollution and Age-Related eye disease: A systematic review and Meta-Analysis. Invest. Ophthalmol. Vis. Sci. 63, 17 (2022).
Liu, Q. et al. Long-term exposure to fine particulate matter modifies the association between physical activity and hypertension incidence. J. Sport Health Sci. 11, 708–715. https://doi.org/10.1016/j.jshs.2022.01.004 (2022).
Li, Y. et al. Associations of long-term exposure to air pollution and physical activity with the risk of systemic inflammation-induced Multimorbidity in Chinese adults: results from the China multi-ethnic cohort study (CMEC). BMC Public. Health. 23, 2556. https://doi.org/10.1186/s12889-023-17518-2 (2023).
World Health Organization. WHO global air quality guidelines: Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide, (2021). https://www.who.int/publications/i/item/97892400342282021
Yu, Y. et al. Ozone exposure, outdoor physical activity, and incident type 2 diabetes in the SALSA cohort of older Mexican Americans. Environ. Health Perspect. 129, 97004. https://doi.org/10.1289/ehp8620 (2021).
Furlong, M. A., Alexander, G. E., Klimentidis, Y. C. & Raichlen, D. A. Association of air pollution and physical activity with brain volumes. Neurology 98, e416–e426. https://doi.org/10.1212/wnl.0000000000013031 (2022).
Guo, C. et al. Independent and opposing associations of habitual exercise and chronic PM(2.5) exposures on hypertension incidence. Circulation 142, 645–656. https://doi.org/10.1161/circulationaha.120.045915 (2020).
Kim, S. R., Choi, S., Keum, N. & Park, S. M. Combined effects of physical activity and air pollution on cardiovascular disease: A Population-Based study. J. Am. Heart Association. 9, e013611. https://doi.org/10.1161/jaha.119.013611 (2020).
Pelliccia, A. et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 42, 17–96. https://doi.org/10.1093/eurheartj/ehaa605 (2021).
Berchuck, S., Jammal, A., Mukherjee, S., Somers, T. & Medeiros, F. A. Impact of anxiety and depression on progression to glaucoma among glaucoma suspects. Br. J. Ophthalmol. 105, 1244–1249 (2021).
Kong, X., Yan, M., Sun, X. & Xiao, Z. Anxiety and depression are more prevalent in primary angle closure Glaucoma than in primary Open-Angle Glaucoma. J. Glaucoma. 24, e57–63. https://doi.org/10.1097/ijg.0000000000000025 (2015).
Shin, D. Y., Jung, K. I., Park, H. Y. L. & Park, C. K. The effect of anxiety and depression on progression of glaucoma. Sci. Rep. 11, 1769 (2021).
Meier, N. F., Lee, D. C., Sui, X. & Blair, S. N. Physical activity, cardiorespiratory fitness, and incident Glaucoma. Med. Sci. Sports Exerc. 50, 2253–2258 (2018).
Lin, S. C., Wang, S. Y., Pasquale, L. R., Singh, K. & Lin, S. C. The relation between exercise and glaucoma in a South Korean population-based sample. PLoS One. 12, e0171441 (2017).
Madjedi, K. M. et al. The association of physical activity with Glaucoma and related traits in the UK biobank. Ophthalmology 130, 1024–1036 (2023).
Zhao, Y., Hu, Y., Smith, J. P., Strauss, J. & Yang, G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int. J. Epidemiol. 43, 61–68. https://doi.org/10.1093/ije/dys203 (2014).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports. Exerc. 35, 1381–1395. https://doi.org/10.1249/01.mss.0000078924.61453.fb (2003).
Jiang, H. et al. Does physical activity attenuate the association between ambient PM(2.5) and physical function? Sci. Total Environ. 874, 162501. https://doi.org/10.1016/j.scitotenv.2023.162501 (2023).
Han, S. et al. Systemic inflammation accelerates the adverse effects of air pollution on metabolic syndrome: findings from the China health and retirement longitudinal study (CHARLS). Environ. Res. 215, 114340. https://doi.org/10.1016/j.envres.2022.114340 (2022).
Tanasescu, M. et al. Exercise type and intensity in relation to coronary heart disease in men. Jama 288, 1994–2000. https://doi.org/10.1001/jama.288.16.1994 (2002).
Geng, G. et al. Tracking air pollution in China: near Real-Time PM(2.5) retrievals from multisource data fusion. Environ. Sci. Technol. 55, 12106–12115. https://doi.org/10.1021/acs.est.1c01863 (2021).
Andresen, E. M., Malmgren, J. A., Carter, W. B. & Patrick, D. L. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for epidemiologic studies depression Scale). Am. J. Prev. Med. 10, 77–84 (1994).
Boey, K. W. Cross-validation of a short form of the CES-D in Chinese elderly. Int. J. Geriatr. Psychiatry. 14, 608–617. (1999).
Gentleman, S. J. F. On Judging the Significance of Differences by Examining the Overlap Between Confidence Intervals. The American Statistician (2001).
Pun, V. C., Manjourides, J. & Suh, H. Association of ambient air pollution with depressive and anxiety symptoms in older adults: results from the NSHAP study. Environ. Health Perspect. 125, 342–348. https://doi.org/10.1289/EHP494 (2017).
Kioumourtzoglou, M. A. et al. The association between air pollution and onset of depression among Middle-Aged and older women. Am. J. Epidemiol. 185, 801–809. https://doi.org/10.1093/aje/kww163 (2017).
Si, Q. & Cardinal, B. J. The health impact of air pollution and outdoor physical activity on children and adolescents in Mainland China. J. Pediatr. 180, 251–255. https://doi.org/10.1016/j.jpeds.2016.10.016 (2017).
Li, F., Lü, L. Y., Liang, J. & Harmer, L. Ambient air pollution in China poses a multifaceted health threat to outdoor physical activity. J. Epidemiol. Community Health. 69, 201–204 (2015).
Hou, J. et al. Long-term exposure to ambient air pollution attenuated the association of physical activity with metabolic syndrome in rural Chinese adults: A cross-sectional study. Environ. Int. 136, 105459. https://doi.org/10.1016/j.envint.2020.105459 (2020).
Madjedi, K. M. et al. The association of physical activity with Glaucoma and related traits in the UK biobank. Ophthalmology 130, 1024–1036. https://doi.org/10.1016/j.ophtha.2023.06.009 (2023).
Yang, X. et al. The association between long-term exposure to ambient fine particulate matter and glaucoma: A nation-wide epidemiological study among Chinese adults. Int. J. Hyg. Environ Health. 238, 113858. https://doi.org/10.1016/j.ijheh.2021.113858 (2021).
Chua, S. Y. L. et al. The relationship between ambient atmospheric fine particulate matter (PM2.5) and Glaucoma in a large community cohort. Invest. Ophthalmol. Vis. Sci. 60, 4915–4923. https://doi.org/10.1167/iovs.19-28346 (2019).
Perez, C. I., Singh, K. & Lin, S. Relationship of lifestyle, exercise, and nutrition with glaucoma. Curr. Opin. Ophthalmol. 30, 82–88 (2019).
Ng, F., Berk, M., Dean, O. & Bush, A. I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 11, 851–876. https://doi.org/10.1017/S1461145707008401 (2008).
Giles, L. V. & Koehle, M. S. The health effects of exercising in air pollution. Sports Med. 44, 223–249 (2014).
Daigle, C. C. et al. Ultrafine particle deposition in humans during rest and exercise. Ultrafine Part. Deposition Hum. Dur. Rest. Exerc. Inhal Toxicol. 15, 539–552 (2003).
Oravisjarvi, K. et al. Effects of physical activity on the deposition of traffic-related particles into the human lungs in Silico. Sci. Total Environ. 409, 4511–4518. https://doi.org/10.1016/j.scitotenv.2011.07.020 (2011).
Acknowledgements
We are grateful to the data support from “National Earth System Science Data Center, National Science & Technology Infrastructure of China. (http://www.geodata.cn)”.
Funding
This work was supported by Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities’ Association (grant numbers 202101AO070272, 202101BA070001-220), Health Commission of Sichuan Province Medical Science and Technology Program (grant numbers 24WSXT004), Research Programme of Panzhihua Science and Technology Bureau (grant number 2022ZD-S-26, 2023ZD-C-1, 2024ZD-S-29), Research Programme of Panzhihua Central Hospital (grant number 202406), and Sichuan Province key clinical specialty construction Project, China.
Author information
Authors and Affiliations
Contributions
Xianzhi Li: Data analysis, Validation, Writing-Original original draft preparation and Editing. Yajie Li and Bin Yu: Study design, and Writing-Original original draft preparation. Haorong Meng, Shunjin Liu, and Yunyun Tian: Data cleaning and Data collection. Xiangyi Xing, Meiying Shen, and Li Yin: Study design, Validation, Supervision, and Writing-Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from all participants in CHARLS, which was approved by the Biomedical Ethics Review Committee of Perking University (IRB00001052-11015).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Li, Y., Yu, B. et al. PM2.5 exposure modifies the association of physical activity with depressive symptoms and glaucoma in middle aged and elderly Chinese. Sci Rep 15, 14048 (2025). https://doi.org/10.1038/s41598-025-98711-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98711-8