Abstract
People with neurological disorders and foot drop may suffer from cognitive-motor interference during walking. Functional Electrical Stimulation (FES) targets foot drop during gait but its effects on cognition remain underexplored. Fifteen individuals (4 males, 11 females; mean age 35.5 ± 12.5 years) with various neurological disorders, who had been using FES for at least three months, were recruited from our outpatient clinic. Subjects were assessed during walking with and without FES (FES CONDITION) and under single- and dual-task walking (TASK). The dual-task consisted of counting backwards from a number near 100 in steps of seven while walking. Cognition rate served as outcome parameter, with high values indicating subjects could efficiently maintain both cognitive task performance and walking speed. A linear mixed model analysis was conducted with FES CONDITION and TASK as fixed and SUBJECTS as random effects. The cognition rate during dual-task walking was significantly better with FES vs. without. FES showed minor effects on dorsiflexion in swing but larger effects on overall gait, as reflected in walking speed, step length and step width. While dual-task led to inferior results in gait, FES counteracted this effect and improved cognition rate. These findings suggest that FES not only addresses gait pattern and stability but also frees cognitive resources for walking. This shift in focus may enhance environmental awareness, social interaction or multitasking and thereby improving overall independence and quality of life, which is particularly relevant for patients with neurological disorders and an increased risk of falls.
Similar content being viewed by others
Introduction
Persons with neurological disorders often show altered walking patterns characterized by higher gait variability1 and an increased incidence of falls compared to age-matched healthy individuals, with step length being a primary determinant2.Conditions such as cerebral palsy, spinal cord injuries, traumatic brain injuries, stroke, multiple sclerosis, and hereditary spastic paraplegia disrupt upper motor neuron pathways, thus leading to altered motor control3.
A common impairment among these conditions is a lesion of dorsiflexor muscles and a resulting foot drop, which decreases toe clearance during swing phase and thereby increases risk of toe dragging and falling4.Ankle-foot orthoses (AFOs) are widely used to prevent foot drop by stabilizing the foot. However, they typically limit push-off power5, restrict ankle movement to some degree and show drawbacks regarding size, weight, appearance and usable footwear6.
Since its introduction in 1961, functional electrical stimulation (FES) has become more and more popular as an alternative treatment for foot drop. With intact lower motor neurons and peripheral nerves, impaired muscles can be reactivated7.In the literature the effects of FES are differentiated into immediate effects of FES (orthotic) and therapeutic effects, which are described as long-term carry-over effects of FES that are present even when the device is not worn6,8,9.
Despite a direct (bio-)mechanical effect of AFOs, FES has the advantage of re-engaging the patient’s own muscle function, thus promoting neuroplasticity when used over a longer period9. This neuroplasticity is assumed to be the leading mechanism to achieve therapeutic effects10 with patients showing greater neural plasticity experience better therapeutic effects11.Additionally, FES could potentially help reintegrate the affected limb into the body schema, enabling patients to regain or enhance body ownership and spatial awareness12,13. Nonetheless structural but also dynamic anatomical deformities might lead to a necessity of additional conventional orthosis such as foot orthosis or an additional stabilisation of the ankle by a stabilising AFO. Combinations of both FES and conventional orthosis might address these issues.
However, the effects of FES on gait function vary in the literature. While some authors have shown that dorsiflexion during swing phase is improved with FES14,15,16,17 the evidence remains limited18. FES has been associated with improvements in walking speed similar to those provided by AFOs9,19,20,21,22,23. Although no improvement has been seen in individuals with cerebral palsy (orthotic effect)17 FES has also been linked to greater subjective experience regarding stability during gait, quality of gait pattern, effort of walking21 and satisfaction23 compared to AFOs. In addition a reduction in gastrocnemius spasticity16, an increase in M. tibialis anterior volume and strength24, and a decrease in toe dragging and falls25 has been shown.
The main goal of FES in foot drop management is to achieve an adequate foot lift during swing phase. While studies demonstrate significant improvements, a recent pilot study of our group, showed faster walking speed over time but revealed no significant improvement in dorsiflexion during swing26,27. Nevertheless, both the literature and our pilot data indicate high patient satisfaction, with reports of enhanced comfort, stability, and safety, making FES a preferred choice among patients21.
An underexplored but potentially important aspect of FES therapy is its impact on real-life multitasking demands. Cognitive-motor interference occurs frequently in everyday situations, which often require the simultaneous performance of cognitive and motor tasks to effectively interact with the environment. Given that attention is a limited resource, splitting it between two tasks can lead to decreased performance in one or both tasks28,29. This is especially relevant for patients with neurological disorders, where higher gait variability1, an increased risk of falling30, and dual-task interferences can significantly impact walking speed and cognitive performance, with weaker cognitive functioning often leading to slower walking speeds31.
Thus, for individuals with neurological conditions and foot drop, a rehabilitation tool addressing both gait and cognitive performance may be superior for everyday interactions. This study aims to evaluate the long-term effects of FES on gait kinematics, spatio-temporal parameters, and cognitive performance during single-task (ST) and dual-task (DT) walking, hypothesizing that FES will enhance both gait and cognitive performance.
Methods
Subjects
Subjects were recruited in the Orthotics and Prosthetics Department of the Orthopedic Hospital of Heidelberg University and provided written informed consent to participate in this study. The study received approval from the Ethics Committee of the Medical Faculty of Heidelberg University (S-503/2022) and all methods were carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki).
First inclusion criterion was to have used one of the FES systems Evomove (Evomotion GmbH, Lueneburg, Germany) or L300Go (Otto Bock HealthCare Deutschland GmbH, Duderstadt, Germany) as continuously for at least the past three months. Both devices use accelerometer and gyroscope data to detect gait phases and are individually adjusted by a certified local orthotist to ensure optimal outcomes for each subject.
Further inclusion criteria were: ability to provide consent for study participation, presence of a neurological disorder with upper motor neuron lesions leading to foot drop, age between 18 and 70 years and the ability to walk unaided for at least 15 min. Exclusion criteria were an equinus foot deformity exceeding 5° of plantarflexion, any previous surgical interventions, or botulinum toxin injections in the leg muscles within the past six months. Table 1 summarises information on all subjects, including demographic information and diagnosis.
Study design
This within-subject observational study employed conventional 3D gait analysis and clinical examination to assess lower extremity joint range of motion, spasticity, strength, and selective motor control. Gait kinematics and spatio-temporal parameters were evaluated when the participants were walking on level ground with standardized footwear (Deichmann SE, Essen, Germany). The main study consisted of a more extensive measurement protocol including the following conditions first without FES, followed by with FES: standard level ground, single-task and dual-task walking followed by walking up and down a 10° inclined ramp. The four walking conditions—without FES vs. with FES and single-task (ST) vs. dual-task (DT) walking—were conducted in a fixed sequence: without FES during single- and dual-task walking, followed by with FES during single- and dual-task walking (Fig. 1). Subjects walked at a self-selected speed towards a cone at the end of the 15-meter walkway and returned to the starting position (total distance = 30 m). Subjects could freely choose whether to turn right or left around the cone.
For the dual-task condition, subjects completed one practice trial followed by two recorded trials. The additional cognitive task required counting backward from a randomised starting number near 100 in steps of seven. It has been shown that the task is difficult enough to alter gait and that the method is reliable for repeated trials32,33. In the familiarisation trial, the starting number was set to 100, while for the two subsequent actually recorded trials, starting numbers were randomised near 100 to minimise learning effects and only numbers were chosen which avoid multiples of seven to prevent calculation advantages. If an error occurred, subjects were instructed to continue counting, with the next correct step of seven being counted as correct. The correct and total numbers of responses were extracted from the recorded video.
For 3D-motion capture, a Vicon System (Oxford, UK) with consisting of twelve cameras (T40s, 120 Hz) was utilised. Reflective, skin mounted markers were placed on each subject according to the Plug-In-Gait (PiG) protocol34,35,36 with an additional marker on the hallux to calculate toe clearance. Ground reaction forces were measured by two force plates (1080 Hz, AMTI, Inc., Watertown, MA, USA) embedded inconspicuously in the walkway. Joint kinematics and kinetics were calculated using the conventional inverse dynamics approach with PiG software (Vicon, Oxford, UK) and normalized to body weight, resulting in joint moments expressed in Nm/kg and powers in W/kg.
Outcome measures
Gait parameters
Hip, knee, and ankle kinematics were calculated and the focus was set onto those variables influencing toe clearance including ankle dorsiflexion, and knee flexion of the affected leg during swing phase, and hip abduction of the contralateral leg during stance. General gait function was evaluated with spatio-temporal parameters, including walking speed, step length, and step width.
Cognition rate
The cognition rate was calculated by dividing the number of correct answers by the total number of answers, then dividing by the walking time37. With high cognition rate indicating that subjects can efficiently maintain both cognitive task performance and walking speed, while a low cognition rate suggests either less correct answers relative to total answers, slower walking speed, or both.
Statistical analyses
Linear mixed model analysis was conducted to evaluate the effects of FES CONDITION (without FES vs. with FES), TASK (ST vs. DT), and their interaction (FES CONDITION *TASK) on gait parameters. In the mixed model, FES CONDITION and TASK were treated as fixed effects, while SUBJECTS were considered as a random effect to account for inter-individual variability. P-values, β-coefficients, and 95% confidence intervals (CIs) were calculated for each dependent variable to determine the main effects of FES CONDITION and TASK as well as their interaction. For better clarity, the results tables for the linear mixed model (LMM) are divided into two: one table for means (SDs) and p-values, and another for β-coefficients and 95% CIs.
Additionally, isolated comparisons of the effects of FES CONDITION and TASK were performed using Bonferroni-corrected paired t-tests. For spatio-temporal parameters, isolated comparisons for FES CONDITION included ST: without FES vs. with FES, and DT: without FES vs. with FES. For TASK, comparisons included without FES: ST vs. DT and with FES: ST vs. DT. The cognition rate during DT walking was also compared between without FES and with FES.
The significance level was set at p < 0.05, and statistical analyses were performed using SPSS (version 27; SPSS, Chicago, IL, USA). Descriptive statistics included mean values with standard deviations and mean differences.
Results
Demographics
As part of a larger study, fifteen subjects with neurological impairments at first motor neuron level—such as cerebral palsy, incomplete spinal cord injury, multiple sclerosis, traumatic brain injury, and hereditary spastic paraplegia—who exhibited foot drop and had used functional electrical stimulation for at least three months were included in this analysis. Table 1 presents their demographical data.
Spatio-temporal parameters
The linear mixed model revealed significant main effects for both FES CONDITION (β = 0.06, p = 0.012, 95% CI = 0.01 to 0.11) and TASK (β = -0.09, p = 0.001, 95% CI = 0.04 to 0.14) on walking speed, but no significant interaction between them (Tables 2 and 3).
With FES, walking speed increased by 0.06 m/s during single-task walking and by 0.07 m/s during dual-task walking. Dual-task walking led to a reduction in walking speed by 0.09 m/s, regardless of without or with FES (Table 4).
The effects on step length followed a similar pattern. Both FES CONDITION (β = 0.03, p = 0.002, 95% CI = 0.01 to 0.05) and TASK (β = -0.03, p = 0.003, 95% CI = 0.01 to 0.04) showed significant main effects, but no interaction (Tables 2 and 3). With FES, step length increased by approximately 0.03 m during both ST and DT. During DT, step length decreased by 0.03 m without FES as well as with FES (Table 3).
Step width significantly decreased with FES (β = -0.91, p = 0.019, 95% CI = -1.66 to -0.15), but TASK did not show a significant effect (β = 0.39, p = 0.308, 95% CI = -1.14 to 0.37) (Tables 2 and 3). With FES, step width decreased by 0.91 cm during ST and by 1.12 cm during DT (Table 3). The non-significant comparison of ST vs. DT revealed an increase in step width by 0.60 cm without FES and 0.39 cm with FES.
Kinematics
In terms of kinematics, small but significant effects were observed in maximum toe clearance and maximum knee flexion during swing phase.
Maximum toe clearance during swing increased with FES (β = 12.11, p = 0.002, 95% CI = 4.57 to 19.65), but no significant effect of TASK was found. In the ST condition, maximum toe clearance increased from 127.4 mm (± 36.4) without FES to 141.1 mm (± 39.5) with FES. In the DT condition, it increased from 123.7 mm (± 37.4) to 133.7 mm (± 39.8) (Tables 2 and 3).
For maximum knee flexion during swing, the TASK effect was small (β = -1.61, p = 0.024, 95% CI = 0.22 to 3.00), demonstrating a decrease in knee flexion during DT, regardless of without FES or with. Without FES, maximum knee flexion decreased from 55.4° (± 9.3) during ST to 54.3° (± 9.7) during DT. Similarly, with FES, maximum knee flexion decreased from 56.5° (± 9.8) during ST to 54.9° (± 10.6) during DT (Table 4).
All other analysed sagittal kinematic variables, including dorsiflexion at heel-strike, maximum dorsiflexion during swing phase, and minimum toe clearance at mid-swing, variables potentially influencing overall toe clearance and foot lift, showed no significant main effect or interaction effects (Tables 2 and 3).
Cognition rate
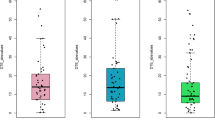
The cognition rate was only measured during dual-task walking, allowing exclusively for the analysis of FES CONDITION effects. With FES, the cognition rate was significantly higher, with a mean difference of 0.25 (p = 0.005, 95% CI = 0.09 to 0.40), compared to without FES (Table 4).
Excluded variables
Due to the lack of normally distributed data and clear statistical effects, further analysis was not performed for max. hip flexion during swing phase and coefficients of variation (CoV) for all represented variables. CoV was calculated by dividing the standard deviation by the mean and multiplying by 100. The only noteworthy result was a tendency toward higher step length CoV during dual-task walking.
Discussion
Regarding the literature, FES has predominantly been applied as an isolated foot drop orthosis with the main focus on analysing immediate and therapeutic effects6,9, with only a few studies addressing complex gait tasks, such as stepping accuracy38 or obstacle avoidance39,40. However, we suggest that FES may contribute to broader effects of FES on gait function, stability and even the interplay between cognitive function and motor control effort.
In the present study, we investigated the effects of FES on single- and dual-task walking in patients with neurological conditions such as cerebral palsy, incomplete spinal cord injury, multiple sclerosis, traumatic brain injury, and hereditary spastic paraplegia with foot drop. Using a repeated-measures design, we analysed the main effects of FES CONDITION and TASK as well as the FES CONDITION*TASK interaction on participants’ gait and cognitive performance outcomes.
The results in Tables 2 and 3, and 4 show minor effects of FES on foot lift kinematics but greater effects on overall gait function, as reflected in spatio-temporal parameters, regardless of cognitive load. While dual-task walking led to worsened gait function, FES counteracted this decline and improved cognitive performance.
The TASK condition led to a worsening of most spatio-temporal parameters, except for step width, whereas FES improved these parameters, resulting in faster walking speed, longer step length, and narrower step width. Except for patients with cerebral palsy14,17 an increase in walking speed aligns with existing literature9,19,20,23,41 which goes together with higher step length. To our knowledge, there is currently no literature on effects of FES on step width. Our findings show that FES leads to a significant reduction in step width, suggesting a more stable and secure gait. One study on ankle-foot orthosis (AFO) demonstrated similar results in walking speed42, with faster walking under AFO use, irrespective of the TASK condition and without AFO*TASK interaction.
We found that FES generally enhanced toe clearance, though none of the analysed kinematic variables showed significant improvements. However, many studies report a significant increase in maximum dorsiflexion during swing14,43 and higher minimum toe clearance14,41. The lack of significant improvements in our study regarding dorsiflexion during swing could be attributed to factors such as cumulative effects across joints from both contralateral and ipsilateral sides, an underpowered sample size, or specific FES device limitations. Nevertheless, consistent with the tendencies observed in our pilot study27, we found no significant effect of FES on foot drop. The only significant kinematic variable identified was maximum toe clearance in swing, which is more indicative of a stable gait initiation for the next heel-strike rather than enhanced toe clearance during swing phase.
During dual-task walking with FES, subjects demonstrated a significant improvement in cognition rate compared to walking without FES. Indicating that subjects using FES could more effectively balance cognitive load while maintaining walking, which is crucial for environmental interactions. In existing FES literature on more complex gait tasks, only stepping accuracy and obstacle avoidance tasks have been studied38,39,40 and not specifically on additional cognitive load itself. Although both AFO and FES effectively improved walking speed, FES allowed patients after stroke to avoid obstacles significantly better than AFO39,40. Additionally, during a target accuracy task, medio-lateral accuracy improved significantly with FES38, aligning with our findings. With FES during dual-task walking, cognition rate improved significantly, alongside a decrease in step width.
Drake, et al.42utilised a similar study design with AFOs and dual-task walking in patients after stroke, although with a different cognitive task involving feedback on an auditory memory task. Participants using AFO increased their walking speed regardless of TASK condition, yet no significant cognitive improvement was observed. Even though, the cognitive task was different, the significant results of our study may suggest that FES offers superior cognitive-motor interference during dual-task walking compared to AFO. Future research is needed to examine cognitive-motor interference further especially in different dual-task conditions as well as with different treatment methods.
The underlying neurophysiological mechanisms by which FES enhances cognition rate and gait function remain unclear. The primary efferent task of FES is to supports or take over the foot lift execution and thereby indirectly reduce the motor control effort.
However, the altered afferent feedback may be of higher clinical relevance. By modifying proprioceptive input, FES can reduce cognitive and motor control effort by shifting motor control from motor cortex (M1) to sensory cortex (S1), making tasks like foot lifting less demanding. Enhanced sensory feedback allows M1 to rely more on S1 for movement execution which reduces the need for detailed motor planning44.
Furthermore, the afferent rhythmic feedback from FES may modulate the central pattern generator45,46 and thereby reduces the need for conscious control of walking and freeing up resources for additional (cognitive) tasks. Additionally, the afferent feedback may induce supraspinal neuroplasticity, resulting in increased voluntary motor control10, muscle volume and strength24 potentially reducing motor effort.
Most likely, these mechanisms interact, with afferent feedback prompting neuroplastic changes at spinal level for an automatic, unconscious gait and at supraspinal level for improved voluntary control and a reduced need for detailed motor planning, while the efferent stimulation ensures sufficient foot lift. Ultimately, even minor improvements from efferent stimulation in foot lift may be sufficient for adequate toe clearance and the freedom for the aforementioned beneficial afferent effects to evolve.
Overall, we account the accumulation of the effects in walking speed, step width and cognition rate as main clinical relevance for the subjects. For each parameter by itself, the effects of FES are relatively modest but for walking speed still in the clinically meaningful range of 0.05 to 0.082 m/s47,48 with an improvement in 0.07 m/s with FES during dual-task walking.
The primary focus of this study was to evaluate the impact of FES on gait and cognition. Unfortunately, due to feasibility constraints, a placebo-controlled sham condition could not be integrated in the clinical setting so potential placebo effects cannot be ruled out. Furthermore, since inclusion criteria focused on upper motor neuron damage, the cohort is rather heterogeneous regarding neurological disorders. The response to FES may therefore vary. However, the sample size is too small for stratification by aetiology.
While the stimulation settings are specifically adjusted to meet each subject’s needs to ensure individual benefits, this approach introduces variability with respect to pulse width, frequency, intensity, and timing of the FES. However, it was not the purpose of this work to analyse optimum settings for FES but to reflect best clinical practice ensuring effectiveness of FES and maximizing benefits for each individual.
For people with neurological disorders our findings suggest that FES not only improves spatio-temporal parameters and better controlled foot positioning but also enhances cognitive performance during dual-task walking. This is particularly relevant for individuals at increased risk of falling, who may benefit from a reduced need of attention to walking itself. The consistent effects of FES across both single- and dual-task walking highlight its robustness in improving gait function and stability, regardless of cognitive load. The improved cognition rate with FES during dual-task walking may reflect a reduced need to focus almost exclusively on walking, allowing for more interaction with the environment while maintaining steady gait kinematics. These results support FES as a promising rehabilitation tool to enhance gait function, stability, and cognitive performance in neurological patients with foot drop.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Moon, Y., Sung, J., An, R., Hernandez, M. E. & Sosnoff, J. J. Gait variability in people with neurological disorders: A systematic review and meta-analysis. Hum. Mov. Sci. 47, 197–208. https://doi.org/10.1016/j.humov.2016.03.010 (2016).
Ehrhardt, A. et al. Fall-related functional impairments in patients with neurological gait disorder. Sci. Rep. 10, 21120. https://doi.org/10.1038/s41598-020-77973-4 (2020).
Sheean, G. & McGuire, J. R. Spastic Hypertonia and Movement Disorders: Pathophysiology, Clinical Presentation, and Quantification. PM&R 1, 827–833. https://doi.org/10.1016/j.pmrj.2009.08.002 (2009).
Stewart, J. D. Foot drop: where, why and what to do? Pract. Neurol. 8, 158. https://doi.org/10.1136/jnnp.2008.149393 (2008).
Altschuck, N. et al. Efficacy of prefabricated carbon-composite ankle foot orthoses for children with unilateral spastic cerebral palsy exhibiting a drop foot pattern. J. Pediatr. Rehabil. Med. 12, 171–180. https://doi.org/10.3233/PRM-170524 (2019).
Stein, R. B. et al. Long-Term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabilit. Neural Repair. 24, 152–167. https://doi.org/10.1177/1545968309347681 (2010).
Liberson, W. T., Holmquest, H. J., Scot, D. & Dow, M. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch. Phys. Med. Rehabil. 42, 101–105 (1961).
Taylor, P. N. et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch. Phys. Med. Rehabil. 80, 1577–1583. https://doi.org/10.1016/S0003-9993(99)90333-7 (1999).
Everaert, D. G. et al. Effect of a Foot-Drop stimulator and Ankle–Foot orthosis on walking performance after stroke. Neurorehabilit. Neural Repair. 27, 579–591. https://doi.org/10.1177/1545968313481278 (2013).
Everaert, D. G., Thompson, A. K., Ling, S., Stein, R. B. & C. & Does functional electrical stimulation for foot drop strengthen corticospinal connections?? Neurorehabilit. Neural Repair. 24, 168–177. https://doi.org/10.1177/1545968309349939 (2010).
Merkel, C. et al. Active prosthesis dependent functional cortical reorganization following stroke. Sci. Rep. 7. https://doi.org/10.1038/s41598-017-09325-8 (2017).
Berlucchi, G. & Aglioti, S. M. The body in the brain revisited. Exp. Brain Res. 200, 25–35. https://doi.org/10.1007/s00221-009-1970-7 (2010).
Gandolla, M. et al. The neural correlates of Long-Term carryover following functional electrical stimulation for stroke. Neural Plast. 2016 (4192718). https://doi.org/10.1155/2016/4192718 (2016).
Böhm, H., Döderlein, L. & Dussa, C. U. Functional electrical stimulation for foot drop in the upper motor neuron syndrome: does it affect 3D foot kinematics during the stance phase of walking? Fuß Sprunggelenk. 18, 115–124. https://doi.org/10.1016/j.fuspru.2020.03.006 (2020).
Mooney, J. A. & Rose, J. A. Scoping review of neuromuscular electrical stimulation to improve gait in cerebral palsy: the Arc of progress and future strategies. Front. Neurol. 10, 887. https://doi.org/10.3389/fneur.2019.00887 (2019).
Pool, D. et al. The orthotic and therapeutic effects following daily community applied functional electrical stimulation in children with unilateral spastic cerebral palsy: a randomised controlled trial. BMC Pediatr. 15, 154. https://doi.org/10.1186/s12887-015-0472-y (2015).
Moll, I. et al. Functional electrical stimulation of the ankle dorsiflexors during walking in spastic cerebral palsy: a systematic review. Dev. Med. Child. Neurol. 59, 1230–1236. https://doi.org/10.1111/dmcn.13501 (2017).
Johnston, T. E., Keller, S., Denzer-Weiler, C. & Brown, L. A. Clinical practice guideline for the use of Ankle-Foot orthoses and functional electrical stimulation Post-Stroke. J. Neurol. Phys. Ther. 45, 112–196. https://doi.org/10.1097/npt.0000000000000347 (2021).
Busk, H., Stausholm, M. B., Lykke, L. & Wienecke, T. Electrical stimulation in lower limb during exercise to improve gait speed and functional motor ability 6 months poststroke. A review with Meta-Analysis. J. Stroke Cerebrovasc. Dis. 29, 104565. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104565 (2020).
Prenton, S., Hollands, K., Kenney, L. & Onmanee, P. Functional electrical stimulation and ankle foot orthoses provide equivalent therapeutic effects on foot drop: A meta-analysis providing direction for future research. J. Rehabil. Med. 50, 129–139. https://doi.org/10.2340/16501977-2289 (2018).
van Swigchem, R., Vloothuis, J., Boer, J. J., Weerdesteyn, V. & Geurts, A. Is transcutaneous peroneal stimulation benificial to patients with chronic stroke using an ankle-foot orthosis? A within-subject study of patients’ satisfaction, walking speed and physical activity level. J. Rehabilitation Medicine: Official J. UEMS Eur. Board. Phys. Rehabilitation Med. 42, 117–121. https://doi.org/10.2340/16501977-0489 (2010).
Kottink, A. I. R. et al. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: A systematic review. Artif. Organs. 28, 577–586. https://doi.org/10.1111/j.1525-1594.2004.07310.x (2004).
Kluding, P. M. et al. Foot drop stimulation versus ankle foot orthosis after stroke: 30-week outcomes. Stroke 44, 1660–1669. https://doi.org/10.1161/strokeaha.111.000334 (2013).
Pool, D. et al. Neuromuscular electrical stimulation-assisted gait increases muscle strength and volume in children with unilateral spastic cerebral palsy. Dev. Med. Child. Neurol. 58, 492–501. https://doi.org/10.1111/dmcn.12955 (2016).
Pool, D., Blackmore, A. M., Bear, N. & Valentine, J. Effects of short-term daily community walk aide use on children with unilateral spastic cerebral palsy. Pediatr. Phys. Ther. 26, 308–317. https://doi.org/10.1097/pep.0000000000000057 (2014).
Bleichner, N., Alimusaj, M., Nees, F., Flor, H. & Wolf, S. I. Orthotic effects of functional electrical stimulation (FES) on gait and dual-task ability in adult patients with upper motor neuron disease. Gait Posture. 106, S28. https://doi.org/10.1016/j.gaitpost.2023.07.037 (2023).
Gather, K. S. et al. Functional electrical stimulation in adult patients with cerebral palsy and foot drop-outcome in gait. Prosthet. Orthot. Int. https://doi.org/10.1097/pxr.0000000000000424 (2024).
Plummer, P. & Eskes, G. Measuring treatment effects on dual-task performance: a framework for research and clinical practice. Front. Hum. Neurosci. 9. https://doi.org/10.3389/fnhum.2015.00225 (2015).
Abernethy, B. Dual-task methodology and motor skills research: some applications and methodological constraints. J. Hum. Mov. Stud. 14, 101–132 (1988).
Spanò, B. et al. The effect of Dual-Task Motor-Cognitive training in adults with neurological diseases who are at risk of falling. Brain Sci. 12 https://doi.org/10.3390/brainsci12091207 (2022).
Montero-Odasso, M. et al. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 9, 41. https://doi.org/10.1186/1471-2318-9-41 (2009).
Chen, A., Kirkland, M. C., Wadden, K. P., Wallack, E. M. & Ploughman, M. Reliability of gait and dual-task measures in multiple sclerosis. Gait Posture. 78, 19–25. https://doi.org/10.1016/j.gaitpost.2020.03.004 (2020).
Kirkland, M. C., Wallack, E. M., Rancourt, S. N. & Ploughman, M. Comparing Three Dual-Task Methods and the Relationship to Physical and Cognitive Impairment in People with Multiple Sclerosis and Controls. Multiple Sclerosis International 1–7, (2015). https://doi.org/10.1155/2015/650645 (2015).
Davis, R. B., Õunpuu, S., Tyburski, D. & Gage, J. R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 10, 575–587. https://doi.org/10.1016/0167-9457(91)90046-Z (1991).
Kadaba, M. P., Ramakrishnan, H. K. & Wootten, M. E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 8, 383–392. https://doi.org/10.1002/jor.1100080310 (1990).
Kadaba, M. P. et al. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J. Orthop. Res. 7, 849–860. https://doi.org/10.1002/jor.1100070611 (1989).
Downer, M. B., Kirkland, M. C., Wallack, E. M. & Ploughman, M. Walking impairs cognitive performance among people with multiple sclerosis but not controls. Hum. Mov. Sci. 49, 124–131. https://doi.org/10.1016/j.humov.2016.06.010 (2016).
Berenpas, F., Geurts, A., Keijsers, N. & Weerdesteyn, V. Benefits of implanted peroneal functional electrical stimulation for continual gait adaptations in people with ‘drop foot’ due to chronic stroke. Hum. Mov. Sci. 83, 102953. https://doi.org/10.1016/j.humov.2022.102953 (2022).
Berenpas, F. et al. Surplus value of implanted peroneal functional electrical stimulation over ankle-foot orthosis for gait adaptability in people with foot drop after stroke. Gait Posture. 71, 157–162. https://doi.org/10.1016/j.gaitpost.2019.04.020 (2019).
van Swigchem, R., van Duijnhoven, H. J. R., den Boer, J., Geurts, A. C. & Weerdesteyn, V. Effect of peroneal electrical stimulation versus an Ankle-Foot orthosis on obstacle avoidance ability in people with Stroke-Related foot drop. Phys. Ther. 92, 398–406. https://doi.org/10.2522/ptj.20100405 (2012).
Marsden, J., Stevenson, V., McFadden, C., Swain, I. & Taylor, P. The effects of functional electrical stimulation on walking in hereditary and spontaneous spastic paraparesis. Neuromodulation: Technol. Neural Interface. 16, 256–260. https://doi.org/10.1111/j.1525-1403.2012.00494.x (2013).
Drake, R. et al. Ankle-foot orthoses improve walking but do not reduce dual-task costs after stroke. Top. Stroke Rehabil. 28, 463–473. https://doi.org/10.1080/10749357.2020.1834271 (2021).
Van der Linden, M. L., Hazlewood, M. E., Hillman, S. J. & Robb, J. E. Functional electrical stimulation to the dorsiflexors and quadriceps in children with cerebral palsy. Pediatr. Phys. Ther. 20, 23–29. https://doi.org/10.1097/PEP.0b013e31815f39c9 (2008).
Gandolla, M. et al. Re-thinking the role of motor cortex: Context-sensitive motor outputs? NeuroImage 91, 366–374. https://doi.org/10.1016/j.neuroimage.2014.01.011 (2014).
Young, W. Electrical stimulation and motor recovery. Cell Transplant. 24, 429–446. https://doi.org/10.3727/096368915x686904 (2015).
Abdullahi, A., Wong, T. W. L. & Ng, S. S. M. Variation in the rate of recovery in motor function between the upper and lower limbs in patients with stroke: some proposed hypotheses and their implications for research and practice. Front. Neurol. 14, 1225924. https://doi.org/10.3389/fneur.2023.1225924 (2023).
Baudendistel, S. T., Haussler, A. M., Rawson, K. S. & Earhart, G. M. Minimal clinically important differences of Spatiotemporal gait variables in Parkinson disease. Gait Posture. 108, 257–263. https://doi.org/10.1016/j.gaitpost.2023.11.016 (2024).
Perera, S., Mody, S. H., Woodman, R. C. & Studenski, S. A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 54, 743–749. https://doi.org/10.1111/j.1532-5415.2006.00701.x (2006).
Funding
Open Access funding enabled and organized by Projekt DEAL.
This research has been funded by the German Research Foundation (DFG) within the project LokoAssist: Seamless integration of assistance systems for natural locomotion of humans (GRK 2761).
Author information
Authors and Affiliations
Contributions
N.B. conducted the study in collaboration with A.S. and C.W. from the Technical Orthopaedics Department. The study was designed by N.B. in close collaboration with A.S. and C.W., under the supervision of D.H. and M.A. for technical feasibility and biomechanical assessments; F.N. and H.F. for neuropsychological aspects; and C.P. and S.I.W. for clinical applicability. Data acquisition and analysis were performed by N.B., D.H., and J.R. N.B. drafted the manuscript, which was finalised with contributions from D.H., M.A., J.R., F.N., H.F., and S.I.W. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bleichner, N., Heitzmann, D., Raynaud, J. et al. Effects of functional electrical stimulation on cognition rate and gait in neurological patients during single- and dual-task walking. Sci Rep 15, 13557 (2025). https://doi.org/10.1038/s41598-025-98755-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98755-w