Abstract
The Salas & Gómez, Nazca, and Juan Fernández ridges are among the least studied regions on the planet. Ecological knowledge of deep-sea organisms inhabiting islands and seamounts along these ridges is limited. Using various sources, including published data and in situ information from five scientific expeditions in the region, we analyzed the spatial ecology of Paromola rathbuni and Projasus bahamondei, two mobile species of benthic megafauna. Random Forest models detected different combinations of abiotic variables affecting species distribution. The distribution of P. bahamondei was primarily influenced by dissolved oxygen, longitude (W), and temperature. The distribution of P. rathbuni was mainly explained by longitude (W), followed by depth, temperature, and oxygen. Generalized Linear Models quantified significant effects of low dissolved oxygen and temperature on species presence, influencing their preference for bathymetric ranges (P. bahamondei at 400–500 m, P. rathbuni at 300–400 m). A distribution break to the west of ~ 85°W was confirmed, potentially due to the weakening of the Oxygen Minimum Zone altering oxygen and thermal conditions. This feature could act as a barrier for both species despite their high dispersal potential. Under projected climate change, shifts in latitudinal, longitudinal, and bathymetric distribution patterns are expected for both species.
Similar content being viewed by others
Introduction
In the southeast Pacific (SEP), Salas & Gómez (S&G), Nazca (NZ), and Juan Fernández (JF) ridges are globally recognized as a biodiversity hotspots that high biological and ecological value enhanced by their role as trans-Pacific corridors for numerous migratory species and as nursery areas1,2,3,4. Because of the S&G and NZ ridges unique biodiversity, having the highest levels of endemism known for marine ecosystems and the deepest light-dependent reefs in the world4,5, the Convention on Biological Diversity (CBD) declared this remote high seas region as an Ecologically or Biologically Significant Marine Area (EBSA) in 20144,6. Despite their importance these ridges remain among the least studied regions of the Pacific due to their isolation and the high costs associated with deep-sea research2,4,7.
The S&G and NZ ridges together extend nearly 3,000 km and comprise more than 110 seamounts, whose summits range from 150 to ~ 1,000 m below the surface. These ridges are located in an ultraoligotrophic region, where they represent a biodiversity oases due to the heterogeneity of their geomorphology, which enhances recirculation and local upwelling3,8,9. Along the ridges, oceanographic conditions (temperature, salinity, and oxygen) vary both longitudinally and bathymetrically8. The NZ and JF ridges, as well as the intersection of S&G and NZ, are influenced by the oceanic portion of the Humboldt Current System (HCS), which is characterized by cold subsurface waters, low oxygen levels, and high nutrient content that are typical conditions of the Oxygen Minimum Zone (OMZ)8,10. The western limits of the HCS influence are estimated to be between 82° and 84°W, coinciding with the biogeographic transition zone proposed by Parin et al.11. Further west, the influence of subtropical waters increases, leading to higher temperatures and dissolved oxygen levels but lower nutrient concentrations2,8. The longitudinal and bathymetric gradients of oceanographic conditions may influence community structure along the ridges9,12. For example, the OMZ represents a barrier to the distribution of many benthic megafauna taxa that are intolerant to hypoxic conditions13,14,15,16, whereas species that tolerate hypoxic conditions exhibit physiological and morphological adaptations that enable them to perform their metabolic and biological activities in low-oxygen environments13,17.
Three large decapod crustacean species are common in the mesophotic benthic communities of S&G, NZ, and JF ridges and Desventuradas Islands (east of S&G and NZ intersection): Projasus bahamondei (Decapoda: Achelata), Paromola rathbuni (Decapoda: Brachyura), and Chaceon chilensis (Decapoda: Brachyura)9,11,18,19,20. Projasus bahamondei (Juan Fernández king crab) and P. rathbuni (Chilean jagged lobster) are part of the bycatch fauna in the artisanal C. chilensis fishery, which is carried out using crustacean traps in the Desventuradas Islands and the JF Archipelago18,19. Paromola rathbuni is consumed locally and thus is a secondary interest of artisanal fisheries. In contrast, P. bahamondei has no local market, but its abundance and frequency of bycatch have promoted exploratory fishing studies for other markets19.
There is scarce biogeographic and ecological information (e.g., intra- and interspecific relationships, habitat use) on the benthic communities inhabiting the mesophotic zone of the S&G, NZ, and JF ridges, particularly for P. bahamondei and P. rathbuni. Moreover, their easy identification, available fishery data, and absence in the S&G ridge make these large decapods a useful model for assessing which environmental variables could explain the discontinuity in their longitudinal distribution.
Therefore, the aim of our study is to analyze the spatial distribution and ecology of P. bahamondei and P. rathbuni along the S&G, NZ, and JF ridges. Specifically, we consider two objectives: (i) to model species-environment relationships along the horizontal (longitudinal) and vertical (bathymetric) axes, estimating whether a set of oceanographic variables (temperature, salinity, and dissolved oxygen) could affect the distribution of these species and (ii) to describe the ecology of both species, including their habitat use, habitat characteristics (substrate type, sediment texture, and slope), and ecological associations.
Results
Modeling the species-environmental relationships
The combinations of variables that allowed predicting the distribution of P. bahamondei and P. rathbuni through the S&G, NZ, and JF ridges (Figs. 1 and 2) differed between species. In general, the variables that most contribute to predicting the presence or absence of both species are dissolved oxygen, temperature, and longitude, but their importance within the ordering differs between species. The combined effects of geographic/geologic and physico-chemical variables explained 95% of the P. bahamondei distribution. The abiotic variables that contributed to high accuracy of the RF model (pseudo-R2 = 0.95) and low error rate (OOB = 4.93%) were dissolved oxygen, longitude (W), and temperature (mean decrease accuracy > 35), whereas, depth and latitude (S) had a secondary contribution (< 20 with mean decrease accuracy > 30) for the model (Fig. 3a,b).
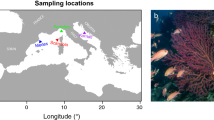
Distribution and presence of the target crustacean species, Projasus bahamondei (A–C) and Paromola rathbuni (D–F). Representative habitats where the species were observed in situ along the Salas and Gómez, Nazca and Juan Fernández ridges are shown. Green dots indicate presence, red crosses indicate absence. Map generated using ArcMap (version 10.8) and GEBCO and NCEI base maps (British Oceanographic Data Centre).
Projasus bahamondei: Accumulated frequency of Projasus bahamondei, as a function of dissolved oxygen (µM), temperature (°C), longitude (°W) and depth (m). Paromola rathbuni: Accumulated frequency of Paromola rathbuni, as a function of dissolved oxygen (µM), temperature (°C), longitude (°W) and depth (m). The red vertical line indicates the upper limit of the OMZ (2 ml L–1 = 89.2 µM).
Model outputs for Random Forest classification method. The oceanographic variables (temperature, dissolved oxygen and salinity) and geographic variables (Longitude W, Latitude S, depth) are hierarchically ordered indicating the most important effects on the distribution of Projasus bahamondei (a, b) and Paromola rathbuni (c, d). Mean Decrease Accuracy is a metric that evaluates the importance of each variable. Mean Decrease Gini is a measure of the impurity of decision tree nodes; higher values indicate that the variable is important for reducing the impurity of the nodes.
The set of predictive variables that explained 93% the presence of the P. rathbuni and contributed to high accuracy of the model (pseudo-R2 = 0.93) and low error rate (OOB = 6.92%) were the combination of longitude (W) and depth (mean decrease accuracy > 35), whereas temperature and dissolved oxygen had a secondarily contribution (< 25 with mean decrease accuracy > 32) (Fig. 3c,d).
The best-fitting GLM (AIC = 84.83, R2 = 0.61) for the effects of physico-chemical variables on P. bahamondei explained 62% of the variance for presence data. The high predictive performance of the model (AUC = 0.97) indicates a high ability to discriminate between presence and absence data (Table 1). The presence of P. bahamondei was significantly (p < 0.001) affected by dissolved oxygen (low oxygen) and temperature (Table 1). Similarly, the best-fitting GLM for P. rathbuni (AIC = 152.8, R2 = 0.37) explained 37% of the variance for presence data, including a high predictive model performance (AUC = 0.83) (Table 2). The presence of P. rathbuni was significantly affected by low dissolved oxygen (p < 0.001) and low temperature (p < 0.001) (Table 2).
The best-fitting GLM (AIC = 122.4; R2 = 0.54) for the effects of geographic/geologic variables on P. bahamondei explained 54% of the variance for presence data, with high predictive model performance (AUC = 0.95). The presence of P. bahamondei was significantly (p < 0.001) affected by longitude and depth (Table 1). The best GLM (AIC = 155.6, R2 = 0.32) explained 32% of variance for P. rathbuni presence data, with high predictive performance (AUC = 0.82) (Table 2). The presence of P. rathbuni was significantly affected by longitude (p < 0.001), depth (p = 0.003), and latitude (p = 0.008) (Table 2).
Habitat use and ecology in mobile deep-sea decapods
The target crustacean species were associated with two habitats types (Fig. 1): (i) Hard substrate, with sharp relief, including areas interspersed with patches of coarse sand and large rock formations, dominated by small sponges, lace corals (Stylaster sp.), and sea pens (Scleroptilum sp.). These habitats featured steep slopes with cracks providing shelter to numerous fish species (e.g., Synaphobranchidae, Lotella fernandeziana and Scorpaena thompsoni). (ii) Soft substrate, with little relief, composed of coarse sand and thanatocoenosis (foraminifera deposits and pteropod shells). This habitat was inhabited by Ceriantharidae and Hormathiidae anemones, sea pens (Protoptilum sp.), and various fish species (e.g., Notopogon sp., Parapercis sp., and Squalus mitsukurii).
The Chilean jagged lobster (P. bahamondei) was mostly associated with hard substrate and sharp relief (i.e., habitat) at depths of 150 to 608 m. Notably, during most dives, this species was observed forming aggregations of > 10 individuals on vertical walls or in caves (Fig. 1a–c). Conversely, the Juan Fernandez king crab (P. rathbuni) was found in both habitats, (i) hard (rocky) and (ii) soft (sandy) substrate, at depths of 50 to 400 m. This species was observed to be disaggregated as small groups of 2 to 3 individuals (Fig. 1d–f).
Projasus bahamondei was only found east of 84.2°W, with 50% of the records around 82°W and some records along the continental margin of Chile. This lobster was observed predominantly within the OMZ, where 75% of the records occurred in hypoxic conditions (< 2 mL L− 1 [< 89 µM]) at 150–500 m and within a narrow thermic range of 6–9 °C (Fig. 2). In contrast, P. rathbuni was distributed east of 85°W, with 50% of their records occurring at 84°W. A bout 50% of P. rathbuni records were registered under low oxygen conditions (< 2 mL L− 1), below 300 m depth, and at temperatures < 10 °C (Fig. 2). The presence of epibionts on the body of P. bahamondei and P. rathbuni was observed (see Fig. S1). Live cirripedians, including species such as Poecilasma spp., Solidobalanus nascanus, and Verruca scrippsae, were recorded attached to the antennae, cephalothorax, abdomen, and appendages of P. bahamondei and the carapace of P. rathbuni. Among the dominant epibionts inhabiting the studied crustaceans, Poecilasma spp. was particularly prevalent, with numbers exceeding 20 individuals on P. bahamondei cephalothorax. Interestingly, the presence of these cirripedians species was exclusively restricted to crustaceans collected on the NZ Ridge within the Nazca-Desventuradas Marine Park at the intersection of NZ and S&G and on the Desventuradas; individuals collected from the continental shelf of Chile and Juan Fernandez Archipelago were devoid of epibionts.
Discussion
Our findings reveal that the target species are prevalent near the western extent of HCS at ~ 82–84 W11, located at the intersection of the S&G and NZ ridges, in areas with hypoxic conditions and colder temperatures, where higher concentrations of food should be available. RF and GLM models provided complementary evidence that indicates the presence of the OMZ on the NZ, S&G, and JF ridges is influencing the spatial and bathymetric distribution of both species, but more strongly for P. bahamondei. According to models, and that ~ 75% of observations occurred under hypoxic conditions, oxygen concentration is the most significant factor explaining the presence of P. bahamondei (R2= 0.61, GLM); the probability of finding P. bahamondei increases as dissolved oxygen concentration decreases. Thus, its longitudinal distribution in the NZ and S&G ridges does not extend beyond ~ 85°S, where the influence of the OMZ weakens and oxygen concentration exceeds 2 mL L− 18,9. Its latitudinal and bathymetric distribution is also strongly influenced by the extent of the OMZ (Fig. 3). The association of P. rathbuni with the OMZ is not as strong since only 50% of the observations occurred under hypoxic conditions and temperatures below 10 °C. The low model variance (R2 = 0.37, GLM) suggests that other factors, in addition to the presence of the OMZ, may also shape the distribution of P. rathbuni.
The OMZ acts as an exclusion zone for many metazoan species lacking physiological and morphological adaptations to hypoxic environments. However, for OMZ residents, it provides a refuge, reduced predation, and a food source for detritivorous and generalist organisms12,13. In the S&G Ridge, the accumulation of organic material and water column productivity decrease from east to west21,22. Consequently, food availability declines sharply as the OMZ weakens, which could have significant impacts on populations of large organisms such as P. bahamondei and P. rathbuni.
In biogeographic terms, the discontinuity in the distribution of both decapods west of ~ 85°W (see Fig. 2) aligns with the presence of the biogeographic transition zone described by Parin et al.11 between 82°–84°W11,23, which generally separates Indo-Pacific benthic species to the west and temperate-origin fauna to the east24. West of the transition zone, the size of benthic megafauna tends to be smaller, suggesting that the size of potential prey would also be smaller and less abundant, whereas towards the east, prey would be larger and more available11,25,26.
Although both crustaceans are OMZ residents and are expected to have low metabolic rates, they are large-sized species in terms of volume, and require a higher total energy intake (food) compared to smaller decapods. Information on the dietary components of these decapods is limited with only one study on P. bahamondei. Báez et al.26 described the gastrointestinal contents of P. bahamondei, which included sediments and Thioploca sp. bacteria, foraminifera, gastropod mollusks, crustacean remains and eggs, shrimp, and fish from the family Gonostomatidae26. The presence of Thioploca sp. bacteria, a genus associated with hypoxic environments, suggests that P. bahamondei may feed in low-oxygen environments, such as those found in certain seamounts of the Nazca Ridge and at the S&G/NZ intersection26,27. The high incidence of foraminifera and crustacean remains suggests both detritivorous and carnivorous/scavenger feeding behaviors, indicating a generalist feeding habit, which is common among OMZ residents. Further insights on dietary habits of these species can be gained from dietary records for congeners such as P. cuvieri, a species from the Mediterranean Sea28,29. The gastrointestinal contents of P. cuvieri include chondrichthyan and teleost fish, pteropods, echinoids, decapod remains, and a small amount of foraminifera, along with detritus, as indicated by the high δ15N values observed30,31,32. Thus congeners also exhibit a generalist feeding habit, i.e., combination of detritivorous and carnivorous/scavenger feeding behaviors, common among OMZ residents.
Mobile deep-sea decapods under different habitat characteristics
The benthic observations of the target species obtained from these expeditions allowed the description of the preferred habitat of each species and their ecological associations along the three ridges of the SEP. The lobster P. bahamondei was frequently associated with hard substrates and sharp relief, with a distribution across a wide depth range (150 to 608 m) and being more frequent and abundant on steep slopes (aggregations of > 10 individuals observed), similar to what was reported by Retamal and Arana20, Arana19, and Tapia-Guerra et al.25. In contrast, the Juan Fernández crab, P. rathbuni, was observed more frequently on sandy sediments (low roughness, flat or smoothed slope) and secondarily on rocky habitats (low roughness, vertical slope) within a narrower depth range (50 to 400 m). While both species were occasionally observed sharing habitat, each species is predominant in different habitats. Thus, slope, roughness, and sediment type may influence the presence and aggregation of these two deep-sea decapod species.
The presence and absence of epibionts such as the barnacle Poecilasma cf. crassa on the cephalothorax of both species may provide information about the connectivity between biogeographical zones of the SEP. This barnacle has been observed along the S&G Ridge on other brachyurans: Chaceon chilensis, Hispidolambrus mirnovovi, Platepistoma balssii, and Cyrtomaia spp. (Personal Observations), as well as on the target species of this study, indicating that it does not have a host preference. However, this epibiont is absent from the carapaces of P. bahamondei and P. rathbuni on the JF Ridge. Poecilasma cf. crassa has been described as a species with a circumtropical distribution and high dispersal potential33, so its absence on the JF Ridge (1,000 km south of the S&G/NZ) likely reflects a physiological limitation to the temperate conditions of the Juan Fernández Ecoregion rather than the dispersal potential of its planktonic phase or the absence of other hosts. Similarly, the absence of P. bahamondei and P. rathbuni west of 85°W is likely due to limitations in environmental tolerances (increased temperature and dissolved oxygen) rather than dispersal capacity in the larval or adult stage, as both species are present on the JF Ridge (1,000 km south of S&G/NZ). The family Palinuridae is considered to have high dispersal potential (> 6 months), which could explain the distribution of P. bahamondei on the Chilean continental shelf and out to the intersection of the NZ and S&G ridges and the JF Ridge. In contrast, the family Homolidae is also described as having high dispersal potential, but its planktonic phase lasts six times less than that of Palinuridae (30 days)29,34, which could explain the absence of P. rathbuni on the continental shelf of Chile.
Considering the results obtained and the projected climate change scenarios for this part of the SEP8, where alterations in temperature and oxygen conditions are expected, it is plausible that the geographic and bathymetric distribution patterns of P. bahamondei and P. rathbuni may change in the future. In particular, changes in the intensity and extent of the OMZ could affect the spatial distribution of these species along the three ridges studied.
Conclusions
The distribution of both species is likely constrained by the contrasting oceanographic conditions of the biogeographic transition zone located between 82° and 85°W, where the confluence of subtropical water masses and temperate waters to the east occurs, including the weakening and disappearance of the OMZ influence. These conditions could limit the connectivity of larvae and adults to the west, possibly beyond their environmental tolerance ranges, preventing them from expanding westward despite their dispersal potential and the presence of preferred habitat. Given the mobility of adults, their generalist feeding habits, and dispersal potential, it is expected that under climate change conditions, including changes in oxygen and temperature, there could be expansions/contractions in the distribution of both species, depending on the direction of the projected change.
Methods
Study area
The S&G and NZ ridges are two adjacent chains of volcanic seamounts located in the southeastern Pacific, extending 3,000 km off the coast of Chile4,7,9. Rapa Nui (Easter Island) and Motu Motiro Hiva (Salas & Gómez Islet) in the west are the only summits that emerge from the S&G and NZ ridges7. To the east of the S&G and NZ intersection are the Desventuradas Islands (San Félix and Sand Ambrosio) and further south lies the JF ridge, which has ~ 15 seamounts and three emergent summits forming an archipelago composed of two islands (Robinson Crusoe, Alejandro Selkirk) and islets (Santa Clara)5.
The scientific information collected from these oceanic ridges comes mainly from Russian expeditions conducted between 1973 and 198711. Additional information arises from campaigns led by Chile, including the CIMAR 5 (1999), CIMAR 6 (2000), CIMAR 22 (2016) cruises, as well as expeditions by National Geographic and Oceana (2013). More recently, information was collected from the EPIC campaign (2019) in collaboration with JAMSTEC (Japan)2 and the Schmidt Ocean Institute (SOI) expeditions aboard the R/V Falkor (too) in collaboration with ESMOI (Chile) and The University of Texas Rio Grande Valley (USA). These expeditions surveyed the three ridges at depths ranging from 150 to 4,500 m, with greater spatial distribution that the early expeditions, which were preliminary on the high sea (Fig. S2).
Oceanographic conditions, circulation and water masses
The S&G, NZ, and JF ridges are located on the eastern edge of the SEP anticyclonic gyre, which is formed by the South Pacific Current to the east, the South Equatorial Current to the west and the Humboldt Current to the north10. Towards the east, the oceanographic conditions are highly influenced by the HCS, evidenced by low temperature, high nutrients concentration, and low dissolved oxygen concentration at the sub-surface level8,10,35. Towards the west, within the Easter Island ecoregion36, the waters extending from Motu Motiro Hiva (Salas & Gómez islet) to Rapa Nui are characterized by increasing temperature and dissolved oxygen concentration and decreasing nutrient concentration8,9. Oligotrophic conditions promote high water clarity, allowing the passage of light at greater depths than in other areas, resulting in the presence of mesophotic communities (e.g. crustose coralline algae) at depths of > 300 m on the S&G ridge1,4,8.
Over the S&G ridge (~ 25–26°S) the confluence of several water masses occurs along two axes: bathymetric and longitudinal. In the surface layer (between 0 and 200 m), the Subtropical Water (STW) is situated to the west, while the Sub-Antarctic Water (SAW) is dominating to the east8. Below 200 m the presence of the Equatorial Subsurface Water (ESSW) characterized by low levels of dissolved oxygen (O2 < 44.6 µmol/L), high salinity (34.9 PSU) and high nutrients concentrations8,10, is associated with the core of the OMZ. To the east the OMZ reaches values less than 4.5 µmol/L at 200 m but increases its depth towards the west, reaching 380 m, before disappearing west of 82°W8,9,35.
Deep-sea megafauna under study
In general, species of family Palinuridae possess a long larval phase comprising from 11 to 17 planktonic larval stages (phyllosoma and puerulus), with high dispersal potential due to an estimated planktonic duration of 6 to 11 months37 during which time they can travel large distances with high dispersal potential38. Projasus bahamondei is distributed along the NZ ridge and the eastern portion of the S&G Ridge, mainly east of ~ 84°W11, where it has been highly commercially exploited by industrial vessels operating in international waters off the coast of Peru, mainly between 20°00′ S–24°00′ S and 79°50′ W–84°50′ W19. It has also been found in the JF Ridge19 and Desventuradas islands (286–400 m). The presence of P. bahamondei is registered occasionally in the continental margin of Chile20 (Retamal and Arana, 2000) off Iquique (170–550 m), central coast of Valparaíso39, and off Huasco (28°28′ S) and Constitución (35°20′ S)19,20,40. On the Chilean continental shelf, P. bahamondei is incidentally caught, mainly in trawl fisheries targeting Heterocarpus reedi (nylon shrimp), Grimothea johni (yellow squat lobster) and Grimothea monodon (red squat lobster)41. This lobster species has been described in both sandy and rocky bottom habitats, typically on the summits of seamounts11,19,25.
Paromola rathbuni is endemic to the ridges of the SEP, being described from the JF Ridge42,43 to the Desventuradas Islands, with a bathymetric distribution between 100 and 300 m and in association with muddy or sandy bottoms19,25,43,44. It is incidentally captured as bycatch in the artisanal fisheries targeting Chaceon chilensis in the JF Ridge45,46.
Scientific cruises: operational characteristics
The operational information of the onboard equipment, sampling procedure, and the data collected during the EPIC 2019, CIMAR 22, FKt240108, FKt240224, FKt240708 cruises, as well the analysis of video records and samples processing on laboratory, are described in the supplementary material. Geographical and bathymetric presence, ecological observations, and habitat characteristics of the studied decapod crustaceans were obtained from the observation of benthic videos recorded during each cruise (Fig. 2S; see details in the supplementary material). The habitat was characterized by substrate type (e.g., rock, silty sediments, mixed substrate, coarse sand), “modifier” elements (e.g., biological communities, sedimentation, and bioturbation), and associated fauna following Greene et al.47 and Tapia-Guerra et al.25.
Sampling and data collection
Oceanographic data
The measurements of temperature (°C), salinity (PSU), and dissolved oxygen (µmol/L) were collected in situ during four of the five cruises (EPIC 2019, CIMAR 22, FKt240108, FKt240224, and FKt240708). For the CIMAR 22 cruise and due to logistical constraints (non-calibrated CTD), the abiotic variables (temperature, salinity, and dissolved oxygen) were obtained from Mecho et al.9, whose processed data from CSIRO Atlas of Regional Seas Climatology 2009 database. The metadata detailing the equipment deployed during the five cruises are provided in Table 3 and the oceanographic variables measured in situ are described in Table 4. Additional details on the oceanographic variables are available in Table S1. The metadata characterizing the ecology and habitat features associated with the target species (P. bahamondei and P. rathbuni) are summarized in Table 5 and further detailed in Table S2.
Biological and ecology data
The benthic specimens collected during the five cruises were preliminarily sorted, counted, photographed, labeled, and preserved in 95% ethanol aboard the vessels. Subsequently, the samples were transferred to the Sala de Colecciones Biológicas at Universidad Católica del Norte (SCBUCN), where they were taxonomically identified to the lowest possible taxon, asigned an identification code, and cataloged. The identification of the target species was confirmed using the description guides for P. rathbuni20,24,44,48 and P. bahamondei19,24,48,49.
A bibliographic review of P. bahamondei and P. rathbuni was conducted to complement data on presence, distribution, and ecology. The review included data from previous expeditions, artisanal fishing data, online databases, and scientific articles (Fig. S3). For each record, the collected data included year, station, latitude, longitude, depth, species, subsystem type, and bibliographic source (see Tables S1, S3).
Statistical modeling
Data modeling was performed using RStudio software, version 4.3.350. The Random Forest (RF) method (one model per species) was applied for classification and regression51,52 to estimate whether ensembles of physicochemical variables (bottom temperature, dissolved oxygen, and salinity) and geographic/geologic variables (longitude [W], latitude [S], and depth) significantly influence the distribution of P. bahamondei and P. rathbuni. The RF allows the most important abiotic (predictive) variables to be hierarchically ordered to explain the distribution of each species separately. Model accuracy (pseudo-R2) was evaluated, and the model error rate (out-of-bag error, OOB), which represents the prediction error that occurs when RF takes into account a set of variables that were not selected, was calculated account51,52. The number of variables selected in each partition of each RF tree was two, the default value. The mean decrease in accuracy per variable was calculated, applying random permutations, to estimate the relative importance of the predictive variables. The mean decrease in the Gini coefficient was calculated to measure how each variable contributes to the homogeneity of the nodes and leaves in the resulting RF models. If the variable is useful, it tends to split mixed labeled nodes into pure single class nodes. Splitting by a permuted variable tends neither to increase nor decrease node purities. The RF analysis was run using the “randomForest” package version 4.6.1252.
Generalized Linear Models (GLM) were conducted to model the functional relationship between the independent variables (environmental and geographic) and the dependent variable (presence/absence), as well as to quantify how each variable directly or indirectly affects the probability of presence/absence for each species. Two GLMs were run; one including physico-chemical variables and the other containing geographic/geologic variables as independent (explanatory) variables. Prior to GLM runs, a multiple correlation analysis between abiotic variables and a multicollinearity between independent variables was evaluated using the Variance Inflation Factor (VIF; Table S4), a useful measure to detect multicollinearity between variables within a correlation matrix (Library Car, version 3.1-2)53. Highly correlated abiotic variables (r > 0.5) and with high collinearity (VIF > 5) were excluded as simultaneous explanatory variables to build the GLMs. The correlation between abiotic variables was not significant r < 0.5, except for salinity and temperature correlation at both data sets (P. rathbuni: r = 0.58; P. bahamondei: r = 0.53; Table S4). In turn, low collinearity between abiotic variables was detected within the correlation matrix for both data sets (P. rathbuni: VIF < 1.25; P. bahamondei: VIF < 2.33; Table S4). The frequentist statistical approach was used to fit the GLM method54. The MuMIn package (version 1.48.455) was used to select the best-performing model from a set of candidate models. The GLMs were built by applying the binomial family with a logit link function. The Akaike Information Criterion (AIC) was used to identify the best model. The goodness of fit for the best model was evaluated to provide an indication of the proportion of variance explained by the model. The predictive model performance was assessed using the Area Under the Curve (AUC), which is a measure of the model’s ability to discriminate between presence and absence observations, of the Receiver Operating Characteristic (ROC) curve with the pROC package (version 1.18.556).
To create graphs and visual results, the “ggplot2” data package version 3.4.357 was used.
Data availability
All data generated or analysed during this study are included in the manuscript and its supplementary information files.
References
Easton, E. E. et al. in Chile and the Salas Y Gómez Ridge, Vol. 12, 477–490 (eds Mesophotic Coral, E., Loya, Y., Puglise, K. A. & Bridge, T. C. L.) (Springer International Publishing, 2019).
Gaymer, C. F. et al. Research advances and conservation needs for the protection of the Salas y Gómez and Nazca ridges: A natural and cultural heritage hotspot in the southeastern Pacific ocean. Mar. Policy. 171, 106453 (2025).
Rogers, A. D. The biology of seamounts: 25 years on, in Advances in Marine Biology, Vol. 79, 137–224 (Elsevier, 2018).
Wagner, D. et al. The Salas y Gómez and Nazca ridges: A review of the importance, opportunities and challenges for protecting a global diversity hotspot on the high seas. Mar. Policy. 126, 104377 (2021).
Friedlander, A. M. et al. Marine biodiversity in Juan Fernández and desventuradas Islands, Chile: Global endemism hotspots. PLOS ONE. 11, e0145059 (2016).
Gálvez, M. Description of area meeting CBD’s criteria in the Eastern tropical and temperate Pacific region. Area: Salas y Gómez and Nazca ridges. Conv Biol. Divers. 6, 1–6 (2012).
Gálvez-Larach, M. Montes submarinos de Nazca y Salas y Gómez: Una revisión Para El Manejo y conservación. Lat. Am. J. Aquat. Res. 37, 479–500 (2009).
Dewitte, B. et al. Understanding the impact of climate change on the oceanic circulation in the Chilean Island ecoregions. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 232–252 (2021).
Mecho, A. et al. Environmental drivers of mesophotic echinoderm assemblages of the southeastern Pacific ocean. Front. Mar. Sci. 8, 574780 (2021).
Fuenzalida, R., Schneider, W., Blanco, J. L., Garcés-Vargas, J. & Bravo, L. Sistema de Corrientes Chile-Perú y Masas de Agua entre caldera e Isla de Pascua. Cienc. Tecnol. Mar. 30, 5–16 (2007).
Parin, N. V., Mironov, A. N. & Nesis, K. N. Biology of the Nazca and Sala y Gómez submarine ridges, an outpost of the Indo-West Pacific fauna in the Eastern Pacific Ocean: Composition and distribution of the fauna, its communities and history. Adv. Mar. Biol. 32, 145–242 (1997).
Gallo, N. D. & Levin, L. A. Fish ecology and evolution in the world’s oxygen minimum zones and implications of ocean deoxygenation. Adv. Mar. Biol. 74, 117–198 (2016).
Levin, L. Oxygen minimum zone Benthos: Adaptation and community response to hypoxia. Oceanogr. Mar. Biol. Annu. Rev. 41, 1–45 (2003).
Gallardo, V. A. et al. Macrobenthic zonation caused by the oxygen minimum zone on the shelf and slope off central Chile. Deep Sea Res. Part. II Top. Stud. Oceanogr. 51, 2475–2490 (2004).
Sellanes, J., Neira, C., Quiroga, E. & Teixido, N. Diversity patterns along and across the Chilean margin: A continental slope encompassing oxygen gradients and methane seep benthic habitats. Mar. Ecol. 31, 111–124 (2010).
Gallardo, M. Á. et al. Reproductive patterns in demersal crustaceans from the upper boundary of the OMZ off north-central Chile. Cont. Shelf Res. 141, 26–37 (2017).
Gallardo, M. Á. et al. Life on the edge: Incubation behaviour and physiological performance of squat lobsters in oxygen-minimum conditions. Mar. Ecol. Prog Ser. 623, 51–70 (2019).
Ahumada, M. & Arana, P. Pesca Artesanal de Cangrejo Dorado (Chaceon chilensis) En El Archipiélago de Juan Fernández, Chile. Lat Am. J. Aquat. Res. 37, 285–296 (2009).
Arana, P. M. Chilean Jagged lobster, Projasus bahamondei, in the southeastern Pacific Ocean: Current state of knowledge. Lat. Am. J. Aquat. Res. 42, 1–17 (2014).
Retamal, M. A. & Arana, P. M. Descripción y Distribución de Cinco crustáceos Decápodos recolectados En Aguas profundas En Torno a Las Islas Robinson crusoe y Santa Clara (Archipiélago de Juan Fernández, Chile). Investig .Mar. 28 (2000).
González, C. E., Escribano, R., Bode, A. & Schneider, W. Zooplankton taxonomic and trophic community structure across biogeochemical regions in the Eastern South Pacific. Front. Mar. Sci. 5, 498 (2019).
Von Dassow, P. & Collado Fabbri, S. Biological oceanography, biogeochemical cycles, and pelagic ecosystem functioning of the East central South Pacific Gyre: Focus on easter Island and Salas y Gomez Island. Lat. Am. J. Aquat. Res. 42, 703–742 (2014).
Mironov, A. & Detinova, N. Bottom fauna of the Nazca and Sala y Gomez ridges, in Plankton and benthos from the Nazca and Sala y Gomez Submarine Ridges 269–278 (1990).
Retamal, M. A. & Moyano, H. I. Zoogeography of Chilean marine and freshwater decapod crustaceans. Lat. Am. J. Aquat. Res. 38, 302–328 (2010).
Tapia-Guerra, J. M. et al. First description of deep benthic habitats and communities of oceanic Islands and seamounts of the Nazca desventuradas Marine park, Chile. Sci. Rep. 11, 6209 (2021).
Báez, P., Riquelme, C. & Weirborn, J. Contenidos gástricos de La Langosta de Valparaíso Projasus bahamondei George, 1976 (Crustacea: Decapada: Palinuridae) de Los Montes submarinos Del cordón Nazca. Bol. Mus. Nac. Hist. Nat. Chile. 54, 71–79 (2005).
Gallardo, V. A., Carrasco, F. D., Roa, R. & Cañete, J. I. Ecological patterns in the benthic macrobiota across the continental shelf off central Chile. Ophelia 40, 167–188 (1995).
Capezzuto, F. et al. Occurrence and behaviour of Paromola cuvieri (Crustacea, Decapoda) in the Santa Maria Di leuca cold-water coral community (Mediterranean Sea). Deep Sea Res. Part. Oceanogr. Res. Pap. 59, 1–7 (2012).
Capezzuto, F., D’Onghia, G., Carluccio, A. & Maiorano, P. Distribution and life strategy of the deep-sea crab Paromola cuvieri (Risso, 1816) (Brachyura, Homolidae) in the central mediterranean sea over twenty-five years. Deep Sea Res. Part. Oceanogr. Res. Pap. 196, 104002 (2023).
Cartes, J. E. Diets of deep-sea brachyuran crabs in the Western Mediterranean sea. Mar. Biol. 117, 449–457 (1993).
Fanelli, E. et al. Food web structure of the epibenthic and infaunal invertebrates on the Catalan slope (NW Mediterranean): Evidence from δ13C and δ15N analysis. Deep Sea Res. Part. Oceanogr. Res. Pap. 58, 98–109 (2011).
Papiol, V., Cartes, J. E., Fanelli, E. & Rumolo, P. Food web structure and seasonality of slope megafauna in the NW mediterranean elucidated by stable isotopes: Relationship with available food sources. J. Sea Res. 77, 53–69 (2013).
González, J. A., Shalaeva, K., Martín-García, L., Lorenzo, J. M. & Southward, A. J. First account on deep-sea stalked barnacles from the Canary Islands (NE Atlantic), with an updated checklist of the cirripedia, Thoracica and their hosts in the area. Crustaceana 90, 1575–1597 (2017).
Triay-Portella, R. et al. Reproductive pattern and egg development of the deep-sea crab Paromola cuvieri (Brachyura, Homolidae) around the Canary Islands (NE Atlantic). Deep Sea Res. Part. Oceanogr. Res. Pap. 85, 1–14 (2014).
Pizarro-Koch, M. et al. Seasonal variability of the Southern tip of the oxygen minimum zone in the Eastern South Pacific (30°–38°S): A modeling study. J. Geophys. Res. Oceans. 124, 8574–8604 (2019).
Spalding, M. D. et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 57, 573–583 (2007).
Rivera, J. & Mujica, A. Larvas phyllosoma (Decapoda, Palinuridae y Scyllaridae) de Las Islas Oceánicas Chilenas. Investig Mar. 32 (2004).
Weersing, K. & Toonen, R. Population genetics, larval dispersal, and connectivity in marine systems. Mar. Ecol. Prog. Ser. 393, 1–12 (2009).
Báez, P. & Weinborn, J. Proposición de Semicultivo Para El Recurso Langostas Marinas de Chile, 295–314 (Universidad del Norte, 1988).
Retamal, M. A. Los Decápodos de Chile. Proy. Desarro. Docencia vicerrectoría académica dir. Docencia Univ. Concepc 256 (1994).
Arana, P. et al. Evaluación de stock y Distribución de La Langosta y El Cangrejo Dorado En El Archipiélago de Juan Fernández. Inf. Final Estud Pontif Univ. Católica Valparaíso 272006, 257 (2006).
Castilla, J. C. Islas Oceánicas Chilenas: Conocimiento Científico Y Necesidades De Investigación (Ediciones Universidad Católica de Chile, 1987).
Rozbaczylo, N. & Castilla, J. C. Invertebrados Marinos del Archipiélago de Juan Fernández, in Islas Oceánicas Chilenas: Conocimiento Científico y Necesidades de Investigaciones (Ediciones Universidad Católica de Chile (1987).
Zarenkov, N. A. & Decapods Stenopodidea, brachyura, Anomura) of the underwater Nazca and Salas y Gómez ridges. Trans. PP Shirshov Inst. Oceanol. Mosc. USSR 124, 218–244 (1990).
Arana, P. M. Pesca exploratoria Con Trampas Alrededor de Las Islas Robinson crusoe y Santa Clara, Archipiélago de Juan Fernández, Chile. Investig. Mar. 28 (2000).
Ernst, B. et al. Pesquerías de crustáceos Del Archipiélago de Juan Fernández. Boletín de difusión. Programa de Seguimiento de Las principales Pesquerías Nacionales, Año 2020. Inst. Fom Pesq IFOP 1–42 (2021).
Greene, H. G. et al. A classification scheme for deep seafloor habitats. Oceanol. Acta. 22, 663–678 (1999).
Retamal, M. A. & Arana, P. M. Record of stomatopods and decapods, including descriptions of the species of commercial interest from the submarine rises and surrounding waters of the Chilean oceanic Islands (southeastern Pacific Ocean). Lat. Am. J. Aquat. Res. 44, 16–33 (2016).
George, R. W. A new species of spiny lobster, Projasus bahamondei (Palinuridae ‘Silentes’), from the South East Pacific Region. Crustaceana 30, 27–32 (1976).
RStudio Team. RStudio: Integrated Development Environment for R. RStudio (PBC, 2023).
Breiman, L. Random forest. Mach. Learn. 45 5–32 (2001).
Breiman, L. & Cutler, A. Package ‘RandomForest’: Breiman and Cutler’s Random Forests for Classification and Regression (2015).
Fox, J., Sanford, W. & Price, B. Package ‘Car’. Companion to Applied Regression (2024).
Inchausti, P. Chapter 11: Further issues involved in the modeling of counts, in Statistical Modeling with R: A Dual Frequentist and Bayesian Approach for Life Scientists 271–292 (Oxford University, 2022).
Barton, K. Package ‘MuMIn’: Multi-Model Inference (2024).
Robin, X. et al. Package ‘pROC’: Display and Analyze ROC Curves (2023).
Wickham, H. et al. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. 3.5.1 https://doi.org/10.32614/CRAN.package.ggplot2 (2007).
Acknowledgements
For their assistance at sea, we would like to thank the Captain and crew of AGS Cabo de Hornos (CIMAR 22 cruise), R/V Mirai (EPIC cruise), R/V Falkor (too) (Schmidt Ocean Institute, FKt240108, FKt 240224, FKt240708 cruises), and the scientific personnel participating in and leading these expeditions. This article contains partial results of M.F.Z. degree thesis. Funding was provided by ANID-ATE 220044 BiodUCCT, FONDEQUIP EQM150109, FONDECYT 1241386, Conservation International on behalf of Blue Nature Alliance (CI-115211), and The Nippon Foundation-Nekton Ocean Census Programme. J.M.T acknowledges ANID Subdirección de Capital Humano Doctorado Nacional 2022-21220674. R.V. thanks Comisión Central de DT-UdelaR, SNI-ANII, and PEDECIBA-Biología, Uruguay. We thanks Erin E. Easton PhD to developing the proposal, securing funding for participation in the CIMAR 22 and FKt240224 cruises, and completing the English review.
Author information
Authors and Affiliations
Contributions
Maximiliano Fernández-Zúñiga: Conceptualization, data analysis, writing and editing—first draft, writing—supplementary material. Rodolfo Vögler: Conceptualization, results, discussion, writing and editing—first draft, Writing—supplementary material. Jan M. Tapia-Guerra: Writing and editing—first draft, Writing—supplementary material. Edition—figures, tables and maps. Participated in data collection. María de los Ángeles Gallardo: Original idea, participated in data collection, data analysis, conceptualization, results, discussion, writing and editing. Javier Sellanes: Devised the project, participated in data collection, conceptualization, writing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Zúñiga, M., Vögler, R., Gallardo, M. et al. Spatial ecology of two emblematic deep-sea crustaceans in the Salas y Gómez, Nazca and Juan Fernández ridges Southeast Pacific. Sci Rep 15, 14538 (2025). https://doi.org/10.1038/s41598-025-98820-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98820-4