Abstract
Urge urinary incontinence (UUI) poses diagnostic challenges due to subjective assessments and limited biomarkers. This study aims to enhance accuracy by integrating skin sympathetic nerve activity (SKNA) with urine biomarkers. A prospective analysis included 36 UUI and 36 non-UUI participants. All participants underwent measurements of SKNA and evaluations of nine urine biomarkers, both with and without urinary creatinine correction. Logistic regression and support vector machine with L1 penalty were applied to SKNA and urine biomarker measurements. Six-fold stratified cross-validation ensuring equitable distribution of UUI positive and negative samples was adopted. Nonzero model weights were extracted to identify most relevant biomarkers. Elevated SKNA and calibrated urine biomarkers were observed in UUI participants. Calibrated urinary biomarkers alone achieves better accuracy than using raw biomarkers. Integration of SKNA and calibrated biomarkers demonstrated superior diagnostic performance for UUI (AUC = 0.80 ± 0.07; sensitivity = 0.72, specificity = 0.83) compared to using SKNA alone, raw or calibrated urine biomarkers alone, and alternative combinations. Baseline SKNA, calibrated MCP-1, MIP-1β, and IP-10 emerged as promising biomarkers. In conclusion, combining SKNA and urinary creatinine-normalized biomarkers yielded the highest diagnostic accuracy for UUI. This study proposes an innovative diagnostic algorithm, advancing UUI diagnostics by integrating autonomic function parameters and urine biomarker analysis.
Similar content being viewed by others
Introduction
Overactive bladder (OAB), a constellation of storage lower urinary tract symptoms (LUTS) characterized by urinary urgency, with or without urge urgency incontinence (UUI), presents a diagnostic challenge relying on subjective assessments such as medical history, patient bother, and expectations1. Despite the incorporation of questionnaires to assist physicians in gauging the severity of storage LUTS and understanding patient perspectives2, these tools remain inherently subjective and susceptible to variations based on patients’ interpretation of symptoms.
Among the OAB subtypes, UUI is particularly severe and burdensome. Prevalence estimates for UUI vary widely, ranging from 1.8 to 30.5% in European populations, 1.7 to 36.4% in US populations, and 1.5 to 15.2% in Asian populations3. The economic burden of UUI is substantial, with an annual cost estimate of € 7 billion in the United Kingdom4, while a US cost-of-illness study reported a total cost of $66 billion5. The global impact of UUI underscores the urgency of implementing public health initiatives and clinical management programs to enhance awareness, encourage early diagnosis, and improve overall management.
Recognizing the limitations of current diagnostic approaches and the need for specific biomarkers for UUI, this study emphasizes the importance of developing objective and more accurate diagnostic tools. Although several urine biomarkers have been proposed, their utility is hindered by low sensitivity and specificity6,7, prompting the 2023 EAU guideline to suggest not routinely using urinary biomarkers in the diagnosis and management of lower urinary tract (LUT) diseases8. This highlights the necessity for further research and the development of improved predictive biomarkers.
Moreover, prior studies assessing the diagnostic capabilities of urine biomarkers in diagnosing LUTS typically relied on initial groupings to construct Receiver Operating Characteristic (ROC) curves for assessing the diagnostic value of each cytokine or developing combination algorithms6,7,8,9,10,11. However, these studies exhibited two limitations: (1) rigorous validation on the predictive power is often limited. For example, the area under the ROC is reported, but without dissecting the data as a cross-validation procedure nor having an independent holdout test set, which could lead to poor generalizability in real-world clinical practice; (2) heterogeneity in the utilization of urine creatinine (Cre) correction for assessing the efficacy of urine biomarkers, with some studies implementing correction while others did not, introducing inconsistency. Thus, addressing these limitations constitutes an additional focal point of our current study.
Building on our prior research using “NeuECG” method instead of heart rate variability (HRV) to measure OAB patients, we identified significantly elevated skin sympathetic nerve activity (SKNA) in OAB patients compared to controls, suggesting its potential as a diagnostic biomarker13. Based on this foundation, our present study seeks to investigate the incorporation of SKNA with urine biomarkers both with and without Cre correction, employing two distinct algorithms. The aim is to develop a novel diagnostic algorithm for UUI, with the innovative intention of enhancing diagnostic accuracy for this condition.
Patients and methods
Study design and participants
This prospective study involved 36 participants with UUI and 36 without UUI, all of whom provided written informed consent. The non-UUI group refers to participants who exhibit storage lower urinary tract symptoms but do not experience UUI. This study received approval from the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(II)-20220265). All research was performed in accordance with relevant guidelines and regulations. Participants were asked to complete the validated OAB-related questionnaires, including the OAB symptom score questionnaires14, International Prostate Symptom Score15, and Urinary Sensation Scale16. UUI diagnosis followed the International Continence Society’s definition17. Exclusion criteria encompassed age ≤ 18, pregnancy, urinary tract infections, neurological disorders, cardiovascular disease, medication or management for UUI, history of uterine, cervical, vaginal, or urethral cancer, history of cyclophosphamide use, any type of chemical cystitis, and benign or malignant bladder tumors.
Primary and secondary outcomes
The primary outcome involved assessing autonomic function and urine biomarkers, both calibrated (with Cre correction) and raw (without Cre correction), between groups, while the secondary outcome aimed to develop a novel diagnostic algorithm for UUI utilizing autonomic function, raw and calibrated urine biomarkers, and their combination. Further exploration sought to identify the most effective combinations that enhance diagnostic capabilities.
Measurement of autonomic function
Autonomic function was evaluated between 10:00 a.m. and 2:00 p.m. in the supine position in a temperature- and moisture-controlled research area, using the noninvasive “neuECG” method, which analyzed average skin sympathetic nerve activity (SKNA) during baseline, stress, and recovery phases with a 5-min recording in each phase, which have been reported in detail in the previous study13,18,19. The sympathetic reserve, calculated as the ratio between stress and baseline SKNA, was defined20.
Measurement of urine biomarkers
Urine was self-voided when the subjects reported a full bladder sensation. Fresh midstream urine samples underwent centrifugation (at 5000 rpm, 10 min, at 4 °C), and the resulting cell pellet and supernatant were stored at − 80 °C. Biomarker analyses involved measuring nine cytokines and chemokines using a commercially available Milliplex® Human cytokine/chemokine magnetic bead-based panel kit (Millipore, Darmstadt, Germany). The study measured a total of 9 analytes of interest, including TNFα, IL-6, IL-8, IL-10, IL-15, IL-1β, MCP-1, MIP-1β and IP-10 were measured using the multiplex kit with the catalog number HCYTA-60K-09. The following laboratory procedures for the quantification of these targeted analytes were performed similarly to those studies by Jiang et al.10,11.
To ensure consistency and account for variations in urine concentration, all measured urine biomarkers were subjected to normalization. This involved the use of urinary creatinine as a reference, and the results of urine biomarker-to-creatinine ratio were presented as the calibrated values. Both the raw and calibrated biomarker values were considered in the subsequent data analysis, providing a comprehensive view of the urinary biomarker profiles.
Statistical analysis
Group differences were analyzed using the chi-square test for categorical variables or analyses of variance for continuous variables. In addition, we applied logistic regression (logreg) and support vector machine (SVM), both with L1 penalty to encourage sparsity, to the autonomic function and urine biomarker measurements. These two sets of features were concatenated and standardized as the input to the models. We used the model output on the probability of each sample being UUI as the model scores, and reported the performance in terms of area under the ROC curve (AUC) and the sensitivity targeting 80% specificity. sixfold stratified cross-validation was adopted to evaluate the models, in which we split the samples in to 6 folds randomly, while ensuring the UUI positive and UUI negative samples are distributed equally.
Both logistic regression and SVM with L1 penalty have a regularization parameter that encourages sparsity. These hyperparameters were selected by grid search using fivefold cross-validation on the training set. And the selected hyperparameters were used to fit a single model on the training set. The nonzero model weights were extracted for further discussion, and viewed as the most relevant biomarkers.
Sample size estimation and power calculation
With a two-sided type I error of 5%, a standard deviation of 0.24 for SKNA value (obtained from a previous study)13, and an approximate UUI/non-UUI participant ratio of 1:1, a total sample size of 48 participants (24 per group) was estimated to achieve 80% statistical power in detecting an effect size of 0.2 for the SKNA value between UUI and non-UUI groups.
Results
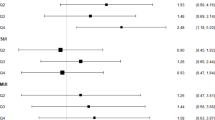
Baseline characteristics between UUI and non-UUI group were presented in Table 1. Significantly increased calibrated MCP-1, calibrated MIP-1β, raw MIP-1β, baseline SKNA and recovery SKNA were found in UUI than those in controls (Table 2). Table 3 and Fig. 1 present the performance measures of featured diagnostic tests for UUI, evaluating SKNA, raw biomarkers, and calibrated biomarkers both individually and in combination. The combined diagnostic test of SKNA-related measurements and calibrated urine biomarkers demonstrated the best performance in diagnosing UUI using SVM (AUC: 0.80, Fig. 1; sensitivity: 72.2%, specificity: 83.3%, positive predictive value: 81.3%, and negative predictive value: 75.0%, Table 3). This highlights the potential of this combined diagnostic test as a robust tool for diagnosing UUI when high specificity is essential.
Since utilizing a combined autonomic and calibrated biomarker with an SVM model achieved the best accuracy, we further investigated which parameters are important in this model. Notably, Baseline SKNA, calibrated MCP-1, calibrated MIP-1β, and calibrated IP-10 carry higher weights in this model (Table 4) and are considered important biomarkers for UUI.
Discussion
This study presents a comprehensive assessment of various parameters related to autonomic function, urinary biomarkers, and their integration to enhance the diagnostic accuracy of UUI. The incorporation of SKNA alongside creatinine-normalized urine biomarkers has unveiled a promising feature set, demonstrating superior performance in both AUC and sensitivity at high specificity levels. This combination presents itself as a potential effective diagnostic algorithm for UUI, the most severe type of OAB, emphasizing the significance of developing precise and reliable diagnostic tools for clinical practice.
It is important to phenotype OAB, particularly distinguishing between OAB wet (UUI) and OAB dry. In our study, we found that the integrated diagnostic algorithm comprising SKNA and normalized urine biomarkers primarily aims to improve the accuracy of diagnosing the presence of UUI. Although our findings predominantly focus on diagnostic accuracy rather than severity, this article opens the opportunity to explore possible etiology and mechanisms behind UUI, serving as a foundation for future research inquiries. Understanding the underlying mechanisms contributing to UUI symptoms could also inform tailored treatment strategies. Moreover, UUI remains a highly subjective complaint for patients, especially among the older population commonly affected by OAB, who may struggle to articulate their symptoms clearly. We believe our study has the potential to provide first-line physicians with a more precise understanding of their patients’ conditions, ultimately leading to improved patient care.
Evaluation of autonomic function and urine biomarker analysis provides a non-invasive and convenient approach to important clinical diagnostic information in those UUI patients. In this study, we assessed the ability of several potential OAB urinary biomarkers including TNFα, IL-6, IL-8, IL-10, IL-15, IL-1β, MCP-1, MIP-1β and IP-10. The decision to include these biomarkers was driven by previous research indicating their elevated levels in OAB patients and their potential to differentiate various OAB subtypes10,11,12. Notably, we depart from the limitations of previous studies that focused on single putative urine OAB biomarkers and univariate statistical approaches (i.e. each biomarker was considered discretely and independent of participants’ confounders or other biomarkers), recognizing their sensitivity and specificity issues6,7,21. By considering multiple biomarkers simultaneously and integrating autonomic function parameters, our approach aims to address the complexities of OAB, offering a more nuanced and individualized diagnostic algorithm.
OAB is a complex disorder with urgency as a primary symptom, and UUI often identified as the most bothersome manifestation. While the pathogenesis of OAB remains incompletely understood and several subtypes of OAB should be distinguished22,23, the causes of OAB symptoms are often believed to be as a dysfunction of the autonomic nervous system24. Despite the widespread use of HRV to evaluate ANS function, its limitations in accuracy and temporal resolution, causing the results of studies on HRV in patients with OAB are conflicting and inconsistent25,26,27,28, prompted our exploration of alternative measures13. Building on our previous finding of significantly elevated SKNA in OAB patients by using “NeuECG” method13 and elevated pre-treatment levels of SKNA in predicting resistance to oral monotherapy in the treatment of OAB29, we extended this investigation to severe OAB cases, particularly UUI, which incurs significant economic burden. The integration of urine biomarkers with SKNA presents an opportunity to develop a more objective and rapid diagnostic algorithm, effectively identifying severe and quality of life-impacting UUI cases.
The debate surrounding the normalization of urine biomarkers with creatinine remains ongoing. While normalization against creatinine concentration is proposed to mitigate differences dependent on urinary osmolality, the literature on urine biomarkers related to storage LUTS reveals a divergence in practices—some studies advocate for normalization12, while others do not10,11. In the current study, we adopted both approaches and observed that calibrated urinary biomarkers alone exhibit superior predictive capability for UUI compared to the use of raw urinary biomarkers alone. Additionally, the combination of autonomic function parameters with calibrated urinary biomarkers surpasses the predictive ability achieved by combining autonomic function parameters with raw biomarkers for UUI. Consequently, we recommend that future discussions on this topic prioritize the use of urinary creatinine-normalized biomarkers over raw urinary biomarkers, given their enhanced predictive performance in the context of UUI.
Several limitations were present in this study. Firstly, the sample size in the study groups was relatively small. However, it is important to note that the collection of cases underwent prior size calculation, and during the calculation of the AUC, validation was implemented in current study to bring the AUC values closer to real-world scenarios. Despite these efforts, future investigations should prioritize the enrollment of a larger cohort to enhance statistical power and generalizability. Secondly, the majority of study participants were women, introducing a potential gender bias. To mitigate this limitation, future studies should strive for a more balanced representation of genders to ensure the generalizability of findings. Thirdly, the potential for intra-individual variation and the presence of other systemic inflammatory diseases and comorbidities may have confounded the assessment of autonomic function and urine cytokine levels. Nevertheless, diligent efforts were made to exclude diseases that could induce sympathetic nervous system excitation. Furthermore, we acknowledge that we did not control for variables such as diet and activity level during urine collection. However, our urine biomarker collection method was based on established protocols from previous research exploring urinary biomarkers in OAB10,11. We also employed a calibration approach by normalizing urine biomarkers against creatinine levels to minimize interference. Additionally, we did not discuss the menopausal and menstruating status, which may influence urinary and autonomic function. However, in our study, there were no statistically significant age differences between the UUI and non-UUI groups. Therefore, our findings still provide valuable clinical insights. Future research endeavors should continue to address these limitations by conducting more comprehensive and well-designed studies with larger sample sizes and diverse participant demographics.
Conclusion
Our studies found that using calibrated urinary biomarkers alone achieves better accuracy in UUI than using raw urinary biomarkers. The combination of SKNA and urinary creatinine-normalized biomarkers achieved the best diagnostic accuracy in UUI. Baseline SKNA, calibrated MCP-1, calibrated MIP-1b, and calibrated IP-10 are potential biomarkers for UUI. Our study advances the understanding of UUI diagnostics by proposing an innovative diagnostic algorithm that integrates autonomic function parameters and urine biomarker analysis.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abrams, P. et al. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Am. J. Obstet. Gynecol. 187(1), 116–126 (2002).
Holroyd-Leduc, J. M., Tannenbaum, C., Thorpe, K. E. & Straus, S. E. What type of urinary incontinence does this woman have?. JAMA 299(12), 1446–1456 (2008).
Milsom, I. et al. Global prevalence and economic burden of urgency urinary incontinence: A systematic review. Eur. Urol. 65(1), 79–95 (2014).
Irwin, D. E. et al. The economic impact of overactive bladder syndrome in six Western countries. BJU Int. 103(2), 202–209 (2009).
Ganz, M. L. et al. Economic costs of overactive bladder in the United States. Urology 75(3), 526–532 (2010).
Wróbel, A. F. et al. Novel biomarkers of overactive bladder syndrome. Ginekol. Pol. 88(10), 568–573 (2017).
Tsiapakidou, S., Apostolidis, A., Pantazis, K., Grimbizis, G. F. & Mikos, T. The use of urinary biomarkers in the diagnosis of overactive bladder in female patients. A systematic review and meta-analysis. Int. Urogynecol. J. 32(12), 3143–3155 (2021).
EAU Guidelines. Edn. presented at the EAU Annual Congress Milan 2023. ISBN 978-94-92671-19-6.
Rada, M. P. et al. The profile of urinary biomarkers in overactive bladder. Neurourol. Urodyn. 39(8), 2305–2313. https://doi.org/10.1002/nau.24487 (2020).
Jiang, Y. H., Jhang, J. F., Wu, Y. H. & Kuo, H. C. Investigating urine biomarkers in detrusor underactivity and detrusor overactivity with detrusor underactivity patients. Biomedicines. 11(4), 1191 (2023).
Jiang, Y. H. et al. Urine biomarkers in ESSIC type 2 interstitial cystitis/bladder pain syndrome and overactive bladder with developing a novel diagnostic algorithm. Sci. Rep. 11, 914 (2021).
Firouzmand, S., Ajori, L. & Young, J. S. New participant stratification and combination of urinary biomarkers and confounders could improve diagnostic accuracy for overactive bladder. Sci. Rep. 10(1), 3085. https://doi.org/10.1038/s41598-020-59973-6 (2020).
Chen, Y. C. et al. Skin sympathetic nerve activity as a potential biomarker for overactive bladder. World J. Urol. https://doi.org/10.1007/s00345-023-04376-1 (2023).
Hung, M. J. et al. Development and validation of the Chinese Overactive Bladder Symptom Score for assessing overactive bladder syndrome in a RESORT study. J. Formos Med. Assoc. 112(5), 276–282. https://doi.org/10.1016/j.jfma.2011.09.020 (2013).
Choi, E. P., Lam, C. L. & Chin, W. Y. Validation of the International Prostate Symptom Score in Chinese males and females with lower urinary tract symptoms. Health Qual. Life Outcomes. 12, 1 (2014).
Coyne, K. S., Margolis, M. K., Hsieh, R., Vats, V. & Chapple, C. R. Validation of the urinary sensation scale (USS). Neurourol. Urodyn. 30(3), 360–365 (2011).
Haylen, B. T. et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 21(1), 5–26. https://doi.org/10.1007/s00192-009-0976-9 (2010).
Kusayama, T. et al. Simultaneous noninvasive recording of electrocardiogram and skin sympathetic nerve activity (neuECG). Nat. Protoc. 15(5), 1853–1877 (2020).
Kop, W. J. et al. Effects of acute mental stress and exercise on T-wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation 109(15), 1864–1869. https://doi.org/10.1161/01.CIR.0000124726.72615.60 (2004).
Gratze, G., Mayer, H. & Skrabal, F. Sympathetic reserve, serum potassium, and orthostatic intolerance after endurance exercise and implications for neurocardiogenic syncope. Eur. Heart J. 29, 1531–1541 (2008).
Robotti, E., Manfredi, M. & Marengo, E. Biomarkers discovery through multivariate statistical methods: A review of recently developed methods and applications in proteomics. J. Proteom. Bioinform. S3, 003 (2014).
Apostolidis, A. et al. Can we create a valid treatment algorithm for patients with drug resistant overactive bladder (OAB) syndrome or detrusor overactivity (DO)? Results from a think tank (ICI-RS 2015). Neurourol. Urodyn. 36(4), 882–893 (2017).
Chen, Y. C., Chen, H. W. & Kuo, H. C. Bladder neck incompetence could be an etiology of overactive bladder syndrome in women with stress urinary incontinence after anti-incontinence surgery: Insights from transrectal sonography. World J. Urol. 41(11), 3083–3089 (2023).
Piętak, P. A. & Rechberger, T. Overactive bladder as a dysfunction of the autonomic nervous system—A narrative review. Eur. J. Obstet. Gynecol. Reprod. Biol. 271, 102–107. https://doi.org/10.1016/j.ejogrb.2022.01.022 (2022).
Kim, J. C. et al. Alteration of autonomic function in female urinary incontinence. Int. Neurourol. J. 14, 232–237 (2010).
Im, H. W., Kim, M. D., Kim, J. C. & Choi, J. B. Autonomous nervous system activity in women with detrusor overactivity. Korean J. Urol. 51(3), 183–186 (2010).
Ben-Dror, I., Weissman, A., Leurer, M. K., Eldor-Itskovitz, J. & Lowenstein, L. Alterations of heart rate variability in women with overactive bladder syndrome. Int. Urogynecol. J. 23(8), 1081–1086 (2012).
Billman, G. E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 4, 26 (2013).
Chen, Y. C. et al. Higher skin sympathetic nerve activity as a potential predictor of overactive bladder in females refractory to oral monotherapy. Kaohsiung J. Med. Sci. 40(11), 1020–1028 (2024).
Acknowledgements
The authors appreciate the generous support from the Health and the Welfare Ministry to Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung Medical University Hospital at Kaohsiung Medical University and Kaohsiung Medical University Regenerative Medicine and Cell Therapy Research Center.
Funding
This research was funded partly by the National Science and Technology Council of Taiwan (Grant number: NSTC 112-2314-B-037-130 and NSTC 113-2314-B-037-028) and Kaohsiung Medical University Hospital (Grant number: KMUH-111-1M48 and KMUH113-3R53). The funders had no role in study design, data collection and analysis, and decision to publish.
Author information
Authors and Affiliations
Contributions
Y.C.C., H.W.C., and W.C.T. conceptualized and designed the study, acquired data, and analyzed and interpreted it. Y.C.C. drafted the manuscript. Y.C.C., H.W.C., and T.Y.L. critically revised the manuscript for important intellectual content. T.Y.L. and C.H.L. performed statistical analysis. Y.C.C. and W.C.T. obtained funding. Y.C.C., H.W.C., Y.S.J., Y.P.L., S.L.C., and W.C.T. provided administrative, technical, and material support. Y.S.J. and W.J.W. supervised the study. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(II)-20220265).
Consent to participate
All participants provided written informed consent.
Consent for publication
The manuscript does not contain any individual personal data in any form. All authors reviewed and approved the final version of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, YC., Chen, HW., Liu, TY. et al. Combination of skin sympathetic nerve activity and urine biomarkers in improving diagnostic accuracy for urge urinary incontinence. Sci Rep 15, 14117 (2025). https://doi.org/10.1038/s41598-025-98889-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98889-x