Abstract
The ST segment elevation myocardial infarction (STEMI) patients tend to experience cardiovascular events following percutaneous coronary intervention (PCI), and the prognosis remains suboptimal. The objective of this investigation was to examine the correlation between the ratio of glycosylated hemoglobin A1c/Apolipoprotein A1 (HbA1c/ApoA-1) and the short-term prognosis in STEMI patients following PCI. This study conducted a retrospective analysis of the clinical data of 182 patients diagnosed with STEMI following PCI and admitted to our hospital between January 2022 and June 2023. The patients were categorized into two groups based on the occurrence of major adverse cardiovascular events (MACEs), and a comparative analysis of baseline characteristics was performed. The significant correlation between HbA1c/ApoA-1 with short-term MACEs in STEMI patients post-PCI were determined through univariate and multivariate logistic regression analysis. Different models and Subgroup analysis demonstrated that HbA1c/ApoA-1 was independent risk factor for MACEs in STEMI patients post-PCI and exhibited high stability. Receiver operating characteristic (ROC) curve and area under curve (AUC) value were utilized to validate the predictive value of HbA1c/ApoA-1 in forecasting outcomes among STEMI patients post-PCI, with an AUC of 0.752 (95% CI: 0.68–0.86), sensitivity of 85.7%, and specificity of 56.8%. Restricted cubic spline (RCS) was employed to evaluate the potential non-linear relationship between HbA1c/ApoA-1 levels and MACEs in STEMI patients post-PCI. Our results demonstrated high and significant correlation between HbA1c/ApoA-1 and short-term prognosis, and indicated that HbA1c/ApoA-1 was independent risk factor for MACEs in STEMI patients following PCI and possessed significant predictive value, facilitating the early identification of high-risk cohorts and the anticipation of MACEs.
Similar content being viewed by others
Introduction
ST-elevation myocardial infarction (STEMI) as a serious type of coronary artery disease (CAD)and stands as a leading cause of mortality and disability. Although percutaneous coronary intervention (PCI) has emerged as a critical intervention for acute myocardial infarction (AMI). Nevertheless, more than 6% of Asian AMI patients still face major adverse cardiovascular events (MACEs) within two years post-PCI1. Studies indicated a close relationship between insulin resistance and the onset and progression of atherosclerotic plaque, substantiating insulin resistance as a significant risk factor for CAD2,3. Insulin resistance disrupts the glucose and lipid metabolism balance, where chronic hyperglycemia from glucose metabolism disorders triggers oxidative stress and inflammation, culminating in cellular damage. Dysregulated lipid metabolism results in abnormal lipid profiles, such as elevated plasma triglycerides, reduced high-density lipoprotein (HDL-C) levels, and the formation of small, dense low-density lipoprotein particles, fostering atherosclerotic plaques’ development2.
Glycosylated hemoglobin A1c (HbA1c) serves as a long-term marker of blood glucose control, minimally affected by acute stress or glucose management, and elevated HbA1c is associated with increased risk of cardiovascular disease4. Irrespective of diabetes status, HbA1c emerges as a pivotal predictor of coronary artery stenosis severity in AMI patient5. Apolipoprotein A1 (ApoA1), the primary apolipoprotein of HDL-C, exerts anti-atherosclerotic effects, with lowered ApoA1 concentrations associated with an escalated risk of atherosclerotic cardiovascular disease6. Glycosylated hemoglobin A1c/Apolipoprotein (A1HbA1c/ApoA-1), an indirect marker of insulin resistance, serves as an accessible atherosclerosis indicator. This ratio also exhibits promise as a cardiovascular risk assessor in acute coronary syndrome (ACS) patients, can independently predict the risk of MACEs in patients with acute ACS. Moreover, an elevated HbA1c/ApoA-1 ratio is associated with a higher risk of MACEs7,8.
Nonetheless, the full extent of HbA1c/ApoA-1’s role in STEMI patient prognosis post-PCI remains unclear. Thus, this study explores the correlation between HbA1c/ApoA-1 and short-term prognosis in STEMI patients following PCI. And to expound on the predictive potential of the HbA1c/ApoA1 ratio for short-term MACEs in STEMI patients post-PCI, offering valuable predictive capabilities for early MACEs identification and mitigation.
Results
Baseline characteristics of the two groups in the study cohort
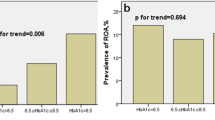
The baseline characteristics of the two groups enrolled in current study were shown in Table 1. Of the 182 patients studied, there were 152 males and 30 females, with an average age of 60.1 ± 12.1 years. Patients in the MACEs group were older than those in the non-MACEs group. The MACEs group exhibited a higher prevalence of diabetes, Killip grades III to IV, multi-vessel coronary artery disease, left ventricular end-diastolic diameter, left ventricular end-systolic diameter (LVESD), amino terminal brain natriuretic peptide precursor(pro-BNP), γ-glutamyl transferase, fasting plasma glucose, HbA1c, and HbA1c/ApoA-1 compared to the non-MACEs group. Conversely, the MACEs group had lower admission left ventricular ejection fraction (LVEF), hemoglobin, hematocrit, and serum albumin levels than the non-MACEs group. Furthermore, a higher proportion of patients in the MACEs group were using β-blockers, angiotensin receptor-neprilysin inhibitor or renin-angiotensin system inhibitor (ARNI/RASi), and sodium-glucose transporter inhibitors (SGLT2) during hospitalization compared to the non-MACEs group (all P < 0.05, Table 1).
Univariate and multi-factor logistic regression analysis of maces influencing factors of STEMI patients after PCI
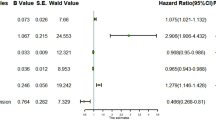
Variables with a significance level of P < 0.05 in Univariate logistic regression were incorporated into the multi-factor logistic regression analysis, The results revealed that HbA1c/ApoA-1 served as an independent risk factor for MACEs in STMEI patients post-PCI. Additionally, other independent predictors encompassed Killip grade III, multivessel coronary artery disease, decreased LVEF, increased of LVESD. The differences exhibited statistical significance (P < 0.05), as shown in Table 2.
Different models and subgroup analysis confirmed the HbA1c/ApoA-1 ratio as an independent risk factor for maces
Different models showed a significant correlation between HbA1c/ApoA-1 and MACEs following PCI in Table 3. Model 1 was a crude model without adjusting for confounding variables. Model 2 adjusted for age and gender. Model 3 adjustments covered gender, age, hypertension, diabetes, cerebral infarction, PCI history, smoking, alcohol consumption, statin usage, and cardiac function classification, confirming the HbA1c/ApoA-1 ratio as an independent predictive marker for MACEs in STEMI patients post-PCI (OR:1.40 ;95%CI :1.13–1.73; P = 0.002) (in Table 3). Subgroup analysis revealed no significant interactions concerning gender, diabetes, smoking history, alcohol usage, Killip grade III, multi-vessel disease, and other subgroup variables (P interaction > 0.05), solidified the study’s result reliability (in Table 4).
The predictive value of HbA1c/ApoA-1 for maces
Receiver operating characteristic (ROC) curve analysis illustrated the robust predictive capacity of HbA1c/ApoA-1 for MACEs in STEMI patients post-PCI, with an area under curve (AUC) of 0.752 (95%CI: 0.68–0.86) and corresponding sensitivity and specificity values of 85.7% and 56.8%, respectively. HbA1c/ApoA-1 ratio remained a reliable predictor for MACEs occurrence in STMEI patients post-PCI. As shown in Table 5; Fig. 1.
Nonlinear relationship between HbA1c/ApoA-1 and maces
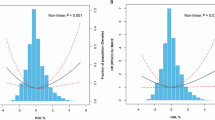
Restricted cubic spline (RCS) curve analysis showed a statistically significant nonlinear association (P nonlinearity = 0.023) between HbA1c/ApoA-1 and the occurrence of MACEs. The inflection point of the RCS curve was identified at HbA1c/ApoA-1 = 6.5. Subsequently, the dataset was stratified based on this inflection point, leading to the conduct of distinct logistic regression analyses for each segment. The results indicated that for HbA1c/ApoA-1 < 6.5, (OR: 3.95; 95%CI: 1.56–9.99, P = 0.004), Conversely, for HbA1c/ApoA-1 ≥ 6.5, (OR: 1.47; 95%CI: 0.96–2.26, P = 0.075). as shown in Fig. 2.
Discussion
This study retrospectively examined the influence of HbA1c/ApoA1 on the occurrence of MACEs in STEMI patients following PCI. The results indicated that the level of HbA1c/ APOA-1 in patients with MACEs was higher than that in patients without MACES. Adjusted for gender, age, hypertension, diabetes, cerebral infarction, PCI history, smoking, and other factors, elevated HbA1c/ApoA-1 levels remained an independent risk factor for MACE occurrence in this patient cohort, demonstrating predictive capability. There is statistically significant nonlinear association between HbA1c/ApoA-1 and the occurrence of MACEs.
Primary PCI has been shown to be an effective reperfusion therapy for STEMI to restore coronary artery blood flow and has contributed to reducing mortality rates, Nevertheless, the long-term prognosis of STEMI patients post-PCI remains suboptimal. primarily due to cardiovascular events occurring predominantly within the initial month following PCI11. Our study demonstrated that HbA1c/ApoA-1 can serve as a predictive factor for MACEs in patients with STEMI following PCI. It can be used to early identification and mitigation of the occurrence of MACEs.
Dysregulated glucose and lipid metabolism alongside insulin resistance are prominent factors heightening atherosclerosis risks, and diabetes can lead to myocardial ischemia and even myocardial infarction, contributing to cardiac structural changes and heart failure development12. Research indicates that Elevated HbA1c levels are associated with heightened cardiovascular disease risks in diabetic individuals and even non-diabetic individuals, correlating with increased overall mortality and cardiovascular ailments13,14. Furthermore, in non-diabetic individuals, HbA1c levels are positively correlated with the extent of coronary artery stenosis in AMI patients15,16. Thus, irrespective of diabetes status, HbA1c is strongly linked to poor prognosis of patients5,17. Yi Lao et al. examined 173 postmenopausal patients with ACS and diabetes, elevated HbA1c levels are associated with a higher incidence of MACEs post-PCI18. Kang Yi et al. analyzed 461 ACS patients and observed a relationship between HbA1c levels and the occurrence of in-hospital MACEs in myocardial infarction patients19. The reasons may be as follows, that high blood sugar levels can negatively impact endothelial function by reducing nitric oxide release and increasing superoxide production in the blood vessel wall20. And can promote the proliferation and migration of vascular smooth muscle cells, ultimately leading to vascular neointimal proliferation and in-stent restenosis after stent implantation21,22,23,24. Additionally, hyperglycemia, reflecting impaired insulin secretion, can trigger lipolysis and elevate circulating free fatty acids. Excessive free fatty acids may increase myocardial oxygen consumption, exert toxic effects on the myocardium, and result in ventricular arrhythmias and reduced myocardial contractility 25,26,27.
Both ApoA-1 and HDL-C exhibit protective effects against atherosclerosis. HDL-C, serving as a carrier of plasma cholesterol, possesses inherent anti-atherosclerotic and anti-inflammatory properties, yet may become dysfunctional in the presence of systemic and vascular inflammation, thereby promoting proatherogenic and proinflammatory responses28,29. ApoA-1 is one of the earliest discovered apolipoproteins and primary protein constituent of HDL-C30,31. ApoA-1 plays a crucial role in regulating cholesterol transport and metabolism by facilitating intracellular cholesterol efflux and transferring cholesterol from peripheral tissues to the liver for eventual excretion into the intestine via bile. It exhibits anti-atherosclerosis and cardiovascular disease properties32,33. The promotion of HDL-C synthesis by ApoA-1 is inversely correlated with vascular atherosclerosis and stenosis, making it a significant factor in atherosclerosis resistance. Moreover, ApoA-1 exhibits anti-inflammatory, anti-apoptotic, anti-thrombotic, and cardioprotective properties, reducing the possibility of cardiovascular diseases, including myocardial infarction34,35. Furthermore, a large cohort study indicated that apoA-1 can serve as indicators of coronary heart disease risk and targets for lipid-lowering therapy36. Glucose metabolism disorders can lead to more complex lipid metabolism abnormalities. A high-glucose environment, oxidative stress, and inflammation causing structural and functional impairments in mature ApoA-1, diminishing its regulatory effect on cholesterol metabolism and exacerbating atherosclerosis37,38,39. A comprehensive study revealed that Lower ApoA-1 levels have been linked to insulin resistance in individuals with impaired glucose tolerance40. Contrarily, ApoA-1 can enhance glycemic control in type 2 diabetes patients41, primarily through elevation of plasma insulin levels, improving pancreatic β-cell function, and enhancing insulin sensitivity for optimized glucose uptake in muscle and cardiac tissues 42,43,44,45.
A study involving 467 patients with ACS has identified the ratio of HbA1c/ApoA-1 as an independent predictor of all-cause mortality and MACEs in ACS patients, demonstrating significant prognostic value8,8. Nevertheless, the utility of HbA1c/ApoA-1 as an independent prognostic indicator in STEMI patients following PCI remains uncertain. Our investigation revealed higher HbA1c/ApoA-1 levels were observed in the MACEs group compared to non-MACEs individuals in STEMI patients post-PCI, thereby validating its role as a predictive risk factor for short-term MACEs. These findings align with previous studies; however, contrary to prior research, we observed a non-linear relationship between HbA1c/ApoA-1 and short-term MACEs in STEMI patients post-PCI. This discrepancy may be attributed to variations in population distribution, living environment, genetic characteristics, baseline attributes, and model calibration. The diverse factors influencing the progression towards clinical endpoints across different models warrant attention. Nonetheless, these observations hold significant implications for early identification of individuals at risk of experiencing MACEs.Although this study presents valuable insights, several limitations should be acknowledged. Residual confounders may impact observational study outcomes, and limitations in conducting time-dependent analyses due to dynamic HbA1c and ApoA-1 levels are evident as only baseline values were obtained. Additionally, the single-center design, small sample size, and short follow-up period call for caution in generalizing the findings. Further validation through larger multi-center studies is therefore recommended to strengthen the study conclusions.
Method
Study population
The research study received approval from the ethics committee of The Second Affiliated Hospital of Xi’an Jiao Tong University (2023083), and informed consent was obtained from all patients. A total of 182 consecutive STEMI patients undergoing PCI at the hospital between January 2022 and December 2023 were included in the analysis. The inclusion criteria were: (1) aged at least 18 years old, (2) diagnosed with STEMI and were undergoing PCI, in accordance with the diagnostic criteria of the 2019 Guidelines for the Diagnosis and Treatment of acute STEMI Infarction of the Chinese Society of Cardiology9, (3) all PCI procedures and medications administrations adhered to relevant guidelines, and complete clinical data were required for inclusion10. The exclusion criteria were: (1) patients with severe hepatic and renal insufficiency; (2) patients with malignant tumors and severe infectious diseases; (3) patients who underwent plain balloon angioplasty or coronary angiography without stent placement, and those lost to follow-up post-discharge were excluded. Cardiologists confirmed the medical histories and diagnoses of all patients, and perioperative and postoperative treatments were administered based on the attending cardiologist’s discretion.
Data collection and divide into groups
Data on the demographic characteristics, clinical, and laboratory information of all patients were extracted from the hospital information system, comprising details such as demographic profiles, laboratory results, and PCI-related specifics. Collected patient clinical data included gender, age, medical history (hypertension, diabetes, cerebral infarction, and prior PCI, smoking and drinking ), Killip cardiac function grade, preoperative TIMI blood flow grade, number of coronary artery lesions, systolic/diastolic blood pressure, left ventricular ejection fraction, left ventricular end-diastolic diameter, left ventricular end-systolic diameter, and comprehensive venous blood analyses upon hospital admission covering blood tests, amino terminal brain natriuretic peptide precursor, and hypersensitive troponin T. Laboratory assessments, including liver and kidney function evaluations, lipid profiles, fasting glucose levels, HbA1c, and other parameters, were conducted on the second day of admission, alongside in-hospital medication administration. 2) Short-term prognosis: The occurrence of MACEs within six months post-discharge were recorded, MACEs comprising cardiac death, recurrent myocardial infarction, target vessel revascularization, recurrent angina pectoris, heart failure, and non-fatal stroke. Patients were categorized based on MACE occurrence into a good prognosis group (n = 132, no MACEs) and a poor prognosis group (n = 50, MACEs present).
Statistical analysis
Statistical analyses were conducted using SPSS version 27.0. Normally distributed measurement data were presented as mean ± standard deviation (SD), with group comparisons analyzed using the t-test. Non-normally distributed data were expressed as the median (interquartile range), and intergroup comparisons were performed using the rank-sum test. Categorical variables were assessed using chi-square or Fisher’s exact tests and presented as frequencies and percentages. whereas comparisons of categorical variables were conducted by using chi-square or Fisher’s exact test presented as frequencies and percentages. Variables with a significance level of P < 0.05 in single-factor logistic regression were incorporated into the multi-factor logistic regression analysis, adjusting for age, gender, smoking, alcohol consumption, hypertension, diabetes, previous cerebral infarction, Killip cardiac function grade, and statin usage. Subgroup analyses were conducted to validate the findings. Moreover, the ROC curve and AUC value were utilized to assess the predictive capacity of HbA1c/ApoA-1 for MACEs occurrence. The RCS model illustrated a non-linear correlation between HbA1c/ApoA-1 ratio and MACE incidence, analyzed using R version 4.2.2 software. A significance level of P < 0.05 was considered statistically significant.
Conclusions
In conclusion, HbA1c/ApoA-1, which can be readily obtained without additional cost, serves as an independent risk factor for short-term MACEs in patients with STEMI following PCI. It offers significant predictive value, enabling the early identification of high-risk groups and anticipation of MACE occurrences.
Data availability
All data generated or analyzed during this study are included in this published article.
Change history
02 July 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-08172-2
References
Zhang, S. Antithrombotic management and long-term outcomes following percutaneous coronary intervention for acute coronary syndrome in Asia. Int. J. Cardiol. 310, 16–22. https://doi.org/10.1016/j.ijcard.2020.01.008 (2020).
Hill, M. A. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119, 154766. https://doi.org/10.1016/j.metabol.2021.154766 (2021).
Ormazabal, V. et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17 (1), 122. https://doi.org/10.1186/s12933-018-0762-4 (2018). PMID: 30170598; PMCID: PMC6119242].
McAlister, F. A. et al. Association between glycated haemoglobin levels and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease: a secondary analysis of the TECOS randomized clinical trial[J]. Eur. J. Heart Fail. 22 (11), 2026–2203 (2020).
She, J. et al. Hemoglobin A1c is associated with severity of coronary artery stenosis but not with long term clinical outcomes in diabetic and nondiabetic patients with acute myocardial infarction undergoing primary angioplasty. Cardiovasc. Diabetol. 16 (1), 97. https://doi.org/10.1186/s12933-017-0578-7 (2017). PMID: 28789650; PMCID: PMC5549379.
Karjalainen, M. K. et al. Apolipoprotein A-I concentrations and risk of coronary artery disease: A Mendelian randomization study. Atherosclerosis 299, 56–63. https://doi.org/10.1016/j.atherosclerosis.2020.02.002 (2020 Apr). .Epub 2020 Feb 14. PMID: 32113648.
Wang, Y. J. et al. Value of glycosylated hemoglobin A1c and Apolipoprotein A-1 ratio on predicting outcome of patients with acute coronary syndrome[J]. Zhong Hua Xin Xue Guan Bing Za Zhi. 51 (1), 38–44 (2023).
Song, F. et al. The role of the plasma glycosylated hemoglobin A1c/Apolipoprotein A-l ratio in predicting cardiovascular outcomes in acute coronary syndrome. Nutr. Metab. Cardiovasc. Dis. 31 (2), 570–578 (2021). Epub 2020 Oct 19.
Chinese Medical Association Cardiovascular Diseases Branch. Editorial board of Chinese journal of cardiovascular diseases. Guidelines for the diagnosis and treatment of acute ST-Elevation myocardial infarction (2019). Chin. J. Cardiovasc. Dis. 47 (10), 766–783 (2019).
Neumann, F. J. et al. ESC/EACTS Guidelines on myocardial revascularization [published correction appears in Eur Heart J. 2019;40(37):3096. (2018). https://doi.org/10.1093/eurheartj/ehz507]. Eur Heart J. 2019;40(2):87–165. doi:10.1093/eurheartj/ehy394.
Brainin, P. et al. Early systolic lengthening in patients with ST-Segment elevation myocardial infarction: A novel predictor of cardiovascular events. J. Am. Heart Assoc. 9 (3), e013835. https://doi.org/10.1161/JAHA.119.013835 (2020).
DunlaySM et al. Type 2 diabetes mellitus and heart failure: A scientific statement from the American heart association and the heart failure society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update [published correction appears in circulation. 2019;140(12):e692. Doi: 10.1161/CIR.0000000000000735]. Circulation 140 (7), e294–e324. https://doi.org/10.1161/CIR.0000000000000691 (2019).
Guenther et al. Glycated hemoglobin predicts All-Cause, cardiovascular, and Cancer mortality in people without a history of diabetes undergoing coronary angiography. Diabetes Care Vol. 34, 1355–1361. https://doi.org/10.2337/dc10-2010 (2011).
Selvin, E. et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. New. Engl. J. Med. Vol. 362, 800–811. https://doi.org/10.1056/NEJMoa0908359 (2010).
Haring, R. et al. Glycated hemoglobin as a marker of subclinical atherosclerosis and cardiac remodeling among non-diabetic adults from the general population. Diabetes Res. Clin. Pract. 105 (3), 416–423. https://doi.org/10.1016/j.diabres.2014.05.004 (2014).
Rossello, X. et al. Glycated hemoglobin and subclinical atherosclerosis in people without diabetes. J. Am. Coll. Cardiol. 77 (22), 2777–2791. https://doi.org/10.1016/j.jacc.2021.03.3358 (2021).
Shahid, M. et al. Prognostic value of hyperglycemia on admission on In- hospital outcomes in patients presenting with ST-elevation myocardial infarction. Cureus 12 (2), e7024. https://doi.org/10.7759/cureus.7024 (2020). PMID: 32211260; PMCID: PMC7081956.
Lao Yi et al. Prognostic Value of Hemoglobin A1c Levels in Postmenopausal Diabetic Patients Undergoing Percutaneous Coronary Intervention (PCI) for Acute Coronary Syndrome. Medical science monitor: international medical journal of experimental and clinical research vol. 24 9399–9405. 27 Dec. (2018). https://doi.org/10.12659/MSM.912108
Kang, Y. et al. Predictive value of glycosylated hemoglobin concentration on in-hospital prognosis of patients with acute coronary syndrome. Ling Nan J. Cardiovasc. Dis. 26 (6), 619–624 (2020). issn.1007 9688.2020.06.01.
Timmer, J. R. et al. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation Vol. 124 (6), 704–711. https://doi.org/10.1161/CIRCULATIONAHA.110.985911 (2011).
Jakubiak, G. K. et al. Pathogenesis and clinical significance of In-Stent restenosis in patients with diabetes. Int. J. Environ. Res. Public. Health. 18 (22), 11970. https://doi.org/10.3390/ijerph182211970 (2021). PMID: 34831726; PMCID: PMC8617716.
Nabati, M. et al. Alterations in echocardiographic left ventricular function after percutaneous coronary stenting in diabetic Pa tients with isolated severe proximal left anterior descending artery steno Sis. Indian Heart J. Vol. 69 (2), 146–150. https://doi.org/10.1016/j.ihj.2016.08.004 (2017).
Tran, H. A. et al. The effect of previous coronary artery stenting on short- and intermediate-term outcome after surgical Revas cularization in patients with diabetes mellitus. J. Thorac. Cardiovasc. Surg. 138 (2), 316–323. https://doi.org/10.1016/j.jtcvs.2009.03.004 (2009).
Germing, A. et al. Coronary artery stenting in diabetes mellitus –unfavourable clinical outcome due to increased rate of myocardial ischemia and percutaneous interventions. Eur. J. Med. Res. 7 (6), 265–270 (2002).
Xi, G. et al. Hyperglycemia enhances IGF-I-stimulated Src activation via increasing Nox4-derived reactive oxygen species in a PKCζ-dependent manner in vascular smooth muscle cells. Diabetes Vol. 61 (1), 104–113. https://doi.org/10.2337/db11-0990 (2012).
Babes, E. E. et al. Acute coronary syndromes in diabetic patients, outcome, revascularization, and antithrombotic therapy. Biomed. Pharmacotherapy = Biomedecine Pharmacotherapie. 148, 112772. https://doi.org/10.1016/j.biopha.2022.112772 (2022).
Oliver, M. F. Sudden cardiac death: the lost fatty acid hypothesis. QJM 99 (10), 701–709. https://doi.org/10.1093/qjmed/hcl084 (2006).
Jomard, A. & Osto, E. High Density Lipoproteins: Metabolism, Function, and Therapeutic Potential. Frontiers in cardiovascular medicine vol. 7 39. 31 Mar. (2020). https://doi.org/10.3389/fcvm.2020.00039
Salazar, J. et al. Dysfunctional High-Density lipoprotein: an innovative target for proteomics and lipidomics. Cholesterol 2015 (2015): 296417. https://doi.org/10.1155/2015/296417
Menzel, H. J. et al. One-step screening method for the polymorphism of apolipoproteins A-I, A-II, and A-IV. J. Lipid Res. Vol. 23 (6), 915–922 (1982).
Rhee, E. J. et al. The HDL cholesterol/apolipoprotein A-I ratio: an indicator of cardiovascular disease. Current opinion in endocrinology, diabetes, and obesity vol. 24,2 : 148–153. (2017). https://doi.org/10.1097/MED.0000000000000315
Tanaka, S. et al. Apr. High-density lipoproteins during sepsis: from bench to bedside. Critical care (London, England) vol. 24,1 134. 7 (2020). https://doi.org/10.1186/s13054-020-02860-3
Chen et al. Relationship between HDL-cholesterol and Apolipoprotein A1 and the severity of coronary artery disease. [J]. J. Qingdao Univ. (Medical Sciences). 59 (5), 745–748 (2023).
Karthikeyan, G. et al. Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: an analysis from the INTERHEART study. J. Am. Coll. Cardiol. Vol. 53 (3), 244–253. 10.1016/j. jacc.2008.09.041 (2009).
Shioji, K. et al. An association analysis between ApoA1 polymorphisms and the high-density lipoprotein (HDL) cholesterol level and myocardial infarction (MI) in Japanese. J. Hum. Genet. Vol. 49, 433–439. https://doi.org/10.1007/s10038-004-0172-1 (2004).
Walldius, G. et al. Dec. Long-term risk of a major cardiovascular event by ApoB, apoA-1, and the apoB/apoA-1 ratio-Experience from the Swedish AMORIS cohort: A cohort study. PLoS medicine 18,12 e1003853. 1 https://doi.org/10.1371/journal. (2021). Pmed.1003853.
Kashyap Sangeeta, R. et al. Glycation reduces the stability of ApoAI and increases HDL dysfunction in Diet-Controlled type 2 diabetes. J. Clin. Endocrinol. Metabolism Vol. 103 (2), 388–396. https://doi.org/10.1210/jc.2017-01551 (2018).
Navab, M. et al. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nature reviews. Cardiology vol. 8,4 : 222 – 32. (2011). https://doi.org/10.1038/nrcardio.2010.222
Rosenson, R. S. et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Reviews Cardiol. Vol. 13 (1), 48–60. https://doi.org/10.1038/nrcardio.2015.124 (2016).
Feng, X. M. et al. Apr. Low apoA-I is associated with insulin resistance in patients with impaired glucose tolerance: a cross-sectional study. Lipids in health and disease vol. 16,1 69. 4 (2017). https://doi.org/10.1186/s12944-017-0446-1
Menon, V. et al. Effect of CETP Inhibition with Eva cetrapib in patients with diabetes mellitus enrolled in the ACCELERATE trial. BMJ Open. Diabetes Res. Care Vol. 8 (1), e000943. https://doi.org/10.1136/bmjdrc-2019-000943 (2020).
Stenkula Karin, G. et al. Single injections of apoA-I acutely improve in vivo glucose tolerance in insulin-resistant mice. Diabetologia Vol. 57 (4), 797–800. https://doi.org/10.1007/s00125-014-3162-7 (2014).
Domingo-Espin, J. et al. Dual actions of Apolipoprotein A-I on glucose- stimulated insulin secretion and insulin-Independent peripheral tissue glucose uptake lead to increased heart and skeletal muscle glucose disposal. Diabetes 65,7, 1838–1848. https://doi.org/10.2337/db15-1493 (2016).
Cochran, B. J. et al. In vivo PET imaging with [(18)F]FDG to explain improved glucose uptake in an apolipoprotein A-I treated mouse model of diabetes. Diabetologia vol. 59,9 (2016): 1977-84. https://doi.org/10.1007/s00125-016-3993-5
Cochran, B. J. et al. APOA1: a Protein with Multiple Therapeutic Functions. Current atherosclerosis reports vol. 23,3 11. 16 Feb. 2021, doi:10.1007/s11883-021-00906-7.
Author information
Authors and Affiliations
Contributions
Jin Wei and Wei Jang designed the study. Danni Li wrote the manuscript. Danni Li, Yuyu Sun , and Jie Han collected, analyzed and interpreted the data. Chen Guo, Linying Xia, Jiahao Dou and Jie Deng reviewed, edited, and approved the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the name of the author Danni Li which was incorrectly given as Dani Li.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, D., Sun, Y., Han, J. et al. The relationship of glycosylated hemoglobin A1c and Apolipoprotein A‑1 ratio on short-term prognosis in STEMI patients following PCI: a retrospective study. Sci Rep 15, 14110 (2025). https://doi.org/10.1038/s41598-025-99003-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99003-x
Keywords
This article is cited by
-
Dual-pathway model of IL-6 and HSP22/HSP27 dynamics predicts post-PCI MACE in STEMI patients
BMC Cardiovascular Disorders (2025)