Abstract
Sleep-disordered breathing (SDB) links to various cardiovascular diseases (CVDs), but comprehensive insights and detailed profiles remain scarce. This study examined the clinical characteristics of CVD patients complicated by SDB in the enrolled all consecutive 5765 patients who underwent screening tests for SDB during hospitalization from January 2014 to December 2019, using the database of Cardiovascular Medicine, Kurume University Hospital. Desaturation during sleep in SDB was significantly worse in terms of age, sex, daytime resting oxygen saturation (SpO2), systolic blood pressure (SBP), pulse pressure, body mass index (BMI), white blood cell count, hemoglobin A1c, serum creatinine, N-terminal pro-B-type natriuretic peptide (NT-proBNP), cardiothoracic ratio on chest X-ray, and left ventricular ejection fraction on echocardiography (all p-values < 0.001, except for SBP p = 0.002, and NT-proBNP p = 0.004). Coronary artery disease and heart failure, but not pulmonary hypertension, were associated with a higher prevalence and a greater risk of moderate to severe SDB, typically in older patients. Notably, low SpO2 during daytime and elevated BMI were indicative of SDB across all age categories for both sexes. Heatmaps of daytime SpO2 and BMI can effectively predict the severe SDB in patients with CVDs, suggesting the potential for efficient therapeutic interventions for SDB.
Similar content being viewed by others
Introduction
SDB is a condition characterized by abnormal respiratory patterns during sleep, encompassing entities primarily defined by obstructive phenomena -such as repetitive narrowing and closure of the upper airway- as well as disorders marked by neuromuscular output irregularities without significant airway obstruction.1 SDB is linked to a cascade of pathophysiological outcomes including intermittent hypoxia, oxidative stress, sympathetic nervous system activation, and endothelial dysfunction, contributing to the development of hypertension, coronary artery disease, arrhythmias, heart failure, and stroke.2 While normal sleep reduces physiological stress, beneficially impacting the cardiovascular system, SDB disrupts these restorative sleep processes. Both obstructive and central sleep apneas exhibit apneas, hypopneas, and subsequent compensatory hyperpneas, leading to arterial blood gas perturbations, frequent arousals, a shift towards sympathetic dominance, and significant negative intrathoracic pressure fluctuations.2
SDB prevalence is high, with moderate-to-severe obstructive sleep apnea (OSA) affecting an estimated 20% of adult males and 10% of postmenopausal females globally, extending beyond Western populations to include countries such as Japan.3 The worldwide prevalence of OSA affects approximately 1 billion individuals, with rates surpassing 50% in some countries.4 In Japan, an estimated 9.4 million people exhibit an apnea–hypopnea index (AHI) of ≥ 15 events per hour, representing 14.0% of the population aged 30–69 years.3 The adoption of continuous positive airway pressure (CPAP) therapy for OSA is rising sharply, nearing 500,000 users in Japan alone.3
Sleep apnea prevalence is notably higher among older individuals, males, and those with CVDs and lifestyle-related conditions.2,5 However, comprehensive comparisons of SDB prevalence across different CVDs within a specific cohort are lacking. In our clinical practice, we routinely assess for SDB in patients with CVDs admitted to our institution using pulse oximetry. Consequently, this study aimed to investigate the prevalence of SDB among hospitalized patients for CVDs, stratified by age and sex, and to delineate the association between SDB and clinical profiles in each CVD category.
Methods
Study design
This study was a retrospective cohort study using the database of the Department of Cardiovascular Medicine, Kurume University Hospital. We enrolled all consecutive patients, who have taken screening test of SDB by PULSOX®-Me300 (Konica Minolta, Inc., Japan) during hospitalization from January 2014 to December 2019. This oxygen saturation analyzer, PULSOX®-Me300, accurately measures transcutaneous arterial blood oxygen saturation (SpO2) by updating the data every second following the initiation of measurement.
The present study received ethical approval from the Ethics Committee of Kurume University (approval no. 20181). The informed consent was waived due to the retrospective nature and opt-out was used. The study was conducted following the ethical principles outlined in the Declaration of Helsinki.
Study population and protocol
SDB was evaluated using PULSOX®-Me300 in patients, excluding those admitted urgently due to acute illness or those unable to provide consent.
Of the 8628 patients enrolled in the study, 5765 were evaluable for 3% Oxygen Desaturation Index (ODI) using PULSOX®-Me300. They ranged in age from 13 to 100 years (mean 66.5 ± 14.4 years), with 3523 (61.1%) males and 2242 (38.9%) females. Patients were classified no SDB (3% ODI < 5), mild SDB (5 ≤ 3% ODI < 15), moderate SDB (15 ≤ 3% ODI < 30), and severe SDB (30 ≤ 3% ODI).6
Considering the well-documented prevalence of SDB in older populations, we stratified patients into age groups of under 60, 60 s, 70 s, and 80 s, and conducted subsequent analyses by sex within each age category. We assessed the prevalence and severity of SDB across various CVDs to identify those most significantly impacted by SDB.
Data collection
Data were collected from the database, which consists of baseline demographic data such as age, sex, height, body weight, SpO2 in the awake state as daily clinical practice, systolic blood pressure, diastolic blood pressure, and pulse rate. BMI was calculated as weight (kilograms) divided by the square of height (square meters) as an index of obesity.
Blood samples were obtained from the antecubital vein and measured at a commercially available laboratory in Kurume University Hospital (white blood cell count, hemoglobin, hemoglobin A1c, serum creatinine, serum sodium, NT-proBNP). Also, cardiothoracic ratio on plain chest x-ray and left ventricular ejection fraction (LVEF) measured using the 2D modified Simpson’s method were obtained.
We, 4 expert cardiologists, classified CVDs as coronary artery disease, arrhythmia, heart failure, valvular heart disease, pulmonary hypertensive heart failure, and others. In this study, we defined the classified CVDs as the primary diagnosis based on the chief complaint leading to hospitalization. For example, if a patient with old myocardial infarction was hospitalized for heart failure, heart failure was recorded as the primary diagnosis.
Coronary artery disease included chronic stable angina, asymptomatic myocardial ischemia, acute coronary syndrome, previous myocardial infarction, previous coronary revascularization, coronary spastic angina, and nonocclusive coronary atherosclerosis. Arrhythmia was the primary clinical diagnosis, with patients admitted for atrial and/or ventricular arrhythmias, including atrial fibrillation and ventricular tachycardia. Heart failure is a complex clinical syndrome resulting from structural and functional abnormalities of the heart. The clinical diagnosis of heart failure was defined as a patient presenting with heart failure symptoms due to some cardiac disease. We classified the heart failure patients into heart failure with reduced ejection fraction (HFrEF, LVEF < 40%), heart failure with mildly reduced ejection fraction (HFmrEF, 40 ≤ LVEF < 50%), and heart failure with preserved ejection fraction (HFpEF, LVEF > 50%). Valvular heart disease was diagnosed as the primary clinical diagnosis in patients admitted due to abnormalities of the aortic, mitral, tricuspid, or pulmonary valves (e.g., aortic stenosis, aortic regurgitation, mitral regurgitation). Pulmonary hypertension was diagnosed when the mean pulmonary arterial pressure at rest increased by 25 mmHg or more due to pulmonary arterial disease and chronic thromboembolic disease; pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension were also diagnosed. Diseases other than these five categories were classified as others.
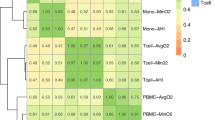
Heatmap
The heatmap illustrates the prevalence (%) of severe SDB patients and the relationship between BMI and SpO2. BMI data is divided into deciles, and SpO2 is segmented into ranges from 90 to 97 with increments of 1. The shading on the heatmap uses red to indicate the highest occurrence frequency and green to denote the lowest occurrence frequency.
Statistical analysis
Data were presented mean ± standard deviation (SD). Chi-square (χ2) tests were utilized to analyze categorical parameters and assess differences between groups. The mean values of the parameters, stratified by the SDB severity, were compared using analysis of variance (ANOVA). Univariate regression analysis, employing linear regression, was utilized to explore the associations between variables. Factors deemed significant in the univariate analysis were further evaluated through multiple regression analysis. This method enabled the sequential addition or removal of predictors based on their statistical significance, allowing for the determination of their independent contributions to the dependent variable. Variableness that maintained statistical significance in the ultimate model were recognized as having an independent association with the dependent variable. The Mantel–Haenszel test was employed to analyze the SDB severity across various CVDs. Significant differences in SDB severity were observed among types of CVDs, stratified by the entire cohort and by sex. P-values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 23.0 (IBM Inc., Chicago, IL, USA).
Results
Characterization of sleep-disordered breathing in patients with cardiovascular diseases
A total of consecutive 8628 patients were enrolled in this study. Of them, 2,863 were excluded due to the lack of available PULSOX®-Me300 data, often resulting from emergency or acute care hospitalizations, and 5,765 patients were included. The age distribution of the included patients ranged from 13 to 100 years, with a mean age of 66.5 ± 14.4 years. The cohort comprised 3,523 (61.1%) males and 2,242 (38.9%) females (Table 1). Notably, significant sex differences were observed across all profiles except for pulse pressure and NT-proBNP levels, underscoring the importance of sex-specific evaluations to elucidate the characteristics of SDB.
Patients were categorized into 4 groups based on SDB severity: no SDB, mild SDB, moderate SDB, and severe SDB. Consistent with prior findings, more severe SDB was predominantly observed in older males (Tables 1 and 2). Remarkably, SpO2 upon awakening demonstrated a significant decline with increasing SDB severity (no SDB; 95.1 ± 1.9%, mild SDB; 94.0 ± 2.0%, moderate SDB; 93.2 ± 2.1%, and severe SDB; 91.9 ± 3.3%, P < 0.001, Table 2). Furthermore, patients with more severe SDB exhibited higher pulse pressure, increased BMI, elevated white blood cell counts, reduced hemoglobin levels, higher HbA1c, elevated serum creatinine and NT-proBNP levels, greater instances of cardiomegaly, and decreased LVEF (Table 2).
Subsequent analyses focused on sex-differences revealed that both males and females with more severe SDB were older, had lower SpO2 at awakening, higher pulse pressure, larger BMI, higher WBC counts, increased HbA1c, elevated serum creatinine and NT-proBNP levels, and more frequent cardiomegaly (Supplementary Table S1). Among males, more severe SDB was significantly associated with reduced LVEF, but not in females (Supplementary Table S1).
To further investigate age-related differences, the cohort was stratified by age groups -under 60, 60 s, 70 s, and over 80- as well as by sex, facilitating a more nuanced analysis of the interplay between age, sex, and SDB severity.
Analysis of sleep-disordered breathing severity across age groups in all enrolled patients
In examining SDB severity across various age groups, analyses were performed for combined sexes, males alone, and females alone, yielding consistent findings. Across these groups, more severe forms of SDB were invariably linked with lower SpO2 upon awakening and increased BMI (Supplementary Tables S2, S3, S4, S5). Additionally, elevated HbA1c levels correlated with SDB severity in all demographics, except female at the age of 70 years or older. The association of cardiomegaly and decreased LVEF with SDB was more pronounced in males than in females (Supplementary Tables S2, S3, S4, S5).
Association between cardiovascular diseases and sleep-disordered breathing in the enrolled population
Within the studied cohort, the prevalence of CVDs was highest for coronary artery disease at 28.8%, followed by arrhythmias at 25.9%, heart failure at 22.1%, valvular heart disease at 6.3%, pulmonary hypertension at 5.8%, and other conditions at 11.1%. Among males, coronary artery disease prevalence increased to 34.7%, with subsequent prevalences for arrhythmias, heart failure, valvular heart disease, pulmonary hypertension, and other conditions aligning with the overall trends. Conversely, in females, arrhythmias were most common at 24.6%, followed by heart failure, coronary artery disease, pulmonary hypertension, valvular heart disease, and other conditions, displaying a unique distribution pattern (Fig. 1).
Given the significant correlation between SDB severity and factors such as sex, age, SpO2 at awakening, BMI, and HbA1c levels, we explored the prevalence trends of underlying CVDs in relation to SDB. Valvular heart disease patients were generally older, the lowest SpO2 levels were observed in pulmonary hypertension, the highest BMI was noted in coronary artery disease, and elevated HbA1c levels were most prevalent among patients with coronary artery disease (Table 3, Supplementary Table S6).
Further analysis of CVD prevalence within SDB categories revealed that, in all patients, coronary artery disease was the most prevalent, followed by arrhythmia and heart failure in mild SDB cases. In moderate and severe SDB categories, coronary artery disease remained predominant, with heart failure and arrhythmia following in prevalence (Table 4, Fig. 2). For males, the prevalence pattern of underlying CVDs mirrored the overall findings. However, for females, arrhythmia and heart failure were the most prevalent, succeeded by coronary artery disease, pulmonary hypertension, and valvular heart disease. In moderate to severe SDB, heart failure took precedence, followed by coronary artery disease, arrhythmia, valvular heart disease, and pulmonary hypertension (Table 4, Fig. 2).
Lastly, the prevalence of SDB within each CVD category was assessed (Table 4, Fig. 3). In coronary artery disease, 30% of patients exhibited no SDB, nearly half had mild SDB, 20% moderate SDB, and 5–6% severe SDB). For arrhythmia, a combination of no and mild SDB was observed in 80% of cases. In heart failure and valvular heart disease, no SDB was observed in 20–30% of cases, nearly half presented with mild SDB, 20% with moderate SDB, and less than 10% with severe SDB. In pulmonary hypertension, over half of the patients exhibited no SDB, with 40% displaying mild SDB (Table 4, Fig. 3).
Heatmaps of oxygen saturation levels and body mass index in relation to the severity of sleep-disordered breathing
As shown in Fig. 4, the heatmaps illustrate the relationship between SpO2 upon awaking and BMI, with the color gradient indicating the percentage of severe SDB cases (fewer severe cases in green, more severe cases in red). It visually demonstrates that lower awake SpO2 and higher BMI are associated with an increased percentage of severe SDB cases (Fig. 4).
Heatmaps of Oxygen Saturation Levels and Body Mass Index in Relation to the Severity of Sleep-Disordered Breathing. The x-axis represents BMI, categorized into various ranges, while the y-axis represents SpO2 levels upon awakening. The color intensity indicates the percentage of severe SDB, with green representing fewer severe SDB cases and red indicating more severe cases. As the color transitions from green to red, the percentage of severe SDB increases, visually emphasizing that patients with elevated BMI and reduced SpO2 are at significant risk for severe SDB.
Discussion
To our knowledge, this is the first study to juxtapose the severities of SDB across a spectrum of CVDs utilizing a singular database. Notably, we discovered that patients with more severe SDB exhibited lower SpO2 upon awakening, specifically within the context of CVDs. The combination of higher BMI and lower SpO2 levels upon awakening can predict the presence of severe SDB. The other primary findings of our investigation can be encapsulated as follows: (1) Mirroring the general population, being male and of advanced age emerged as risk factors for SDB among CVD patients. (2) The analysis was stratified by sex and age to furnish a granular understanding of SDB susceptibility. (3) Across both sex and age brackets, a correlation was established between the severity of SDB and both hypoxia saturation at awakening and BMI. (4) Elevated HbA1c levels were associated with SDB in females in their 70 s and those aged 80 and above. (5) An increased cardiothoracic ratio, as assessed via chest radiography, was linked with heightened SDB severity among all male cohorts and females over 80 years of age. Furthermore, reduced LVEF, as determined by echocardiography, was associated with SDB across all male groups and females in their 70 s. The overarching theme underscores a strong correlation between traditionally adverse clinical parameters and the presence of SDB.
Oxygen saturation upon awakening: a potential predictor of SDB severity
In our analysis, the average SpO2 at awakening was 93.2 ± 2.1%. Sex-specific analysis revealed that males had an average SpO2 of 93.4 ± 2.0% and females 92.7 ± 2.3% upon awakening, with both showing moderate to severe SDB. Our findings indicate that lower daytime SpO2 is associated with more severe SDB, a trend observable regardless of sex or age. Thus, SpO2 upon awakening may serve as a reliable marker for SDB, especially in control participants with CVDs undergoing hospital-based diagnostic and therapeutic procedures.
Hypertension, obesity, and sleep-disordered breathing
The relationship between systolic and diastolic blood pressures, as well as pulse pressure, and SDB appeared to be weak in our study.7,8,9 Although there is a well-documented link between hypertension and SDB in previous reports, such an association was not evident in our cohort, probably because our participants with hypertension were already under treatment with antihypertensive medications. Consequently, the therapeutic control of hypertension might obscure the detection of any direct association between blood pressure and SDB.
In contrast, a significant correlation between pulse rate and SDB was observed; however, it was limited to the overall group under 59 years of age and specifically among males in this age group. Our findings suggest that an elevated heart rate is associated with the severity of SDB in the younger age groups. It is important to note that the pulse rate measurements referenced in this study were taken upon awakening and at rest, as previously reported10.
Consistent with these findings,11,12 our study demonstrated that higher BMI levels were associated with greater severity of SDB across all demographic groups. This relationship was evident regardless of sex or age, underscoring the strong influence of obesity on SDB severity. As shown in Fig. 4, the combination of BMI and daytime SpO2 may predict the severe SDB.
Blood test and sleep-disordered breathing
In our analysis, white blood cell counts showed correlations with SDB across all age groups, except males aged over 80. Among females, significant correlations were observed only in those under 59 years. Conversely, when age was not considered in the analysis, a correlation was evident. While previous studies have suggested a link between WBC count and SDB, our findings indicate that white blood cell count may not serve as a reliable indicator when categorized by age group. Given the variability of this association across different age brackets,13 a nuanced analysis that takes into account both sex and age is recommended for a more accurate assessment.
The relationship between hemoglobin levels and SDB in our study was weak. Although SDB can lead to polycythemia vera due to hypoxia-induced erythrocytosis,14,15,16 hemoglobin levels may not be a dependable marker for SDB, particularly in patients with CVDs who often exhibit anemia as a consequence of their underlying condition.
Regarding HbA1c, a strong correlation with SDB was identified in most groups, except for females over 70 and males over 80. The association between diabetes mellitus and SDB, as demonstrated in numerous studies,17,18,19 was also reflected in our study of patients with CVDs.
The correlation between serum creatinine levels and SDB was confirmed in the overall analysis;20,21 however, when segmented by age group, the correlation persisted only in males under 59 years and females under 69 years. This discrepancy might be attributed to the inclusion of dialysis patients in our study, many of whom had impaired renal function secondary to CVDs. Consequently, serum creatinine may not be a reliable marker in elderly patients with CVDs.
Concerning NT-proBNP, the overall analysis showed a correlation, particularly in females under 59 and males in their 60 s. Although continuous positive airway pressure therapy for sleep apnea has been reported to reduce NT-proBNP levels in some studies, others have found no association.22,23 The variability observed in our study, which included patients with CVDs who generally have elevated baseline NT-proBNP levels, suggests that NT-proBNP may not be a definitive indicator in the presence of CVDs.
Cardiac function and sleep-disordered breathing
The cardiothoracic ratio, as measured by chest X-ray, demonstrated significant correlations with SDB across all male age groups and in all female age groups, with the exception of those aged 60–79 years. Notably, this correlation persisted in both males and females aged over 80 years, a demographic in which few parameters were found to correlate with SDB. Several studies have identified a link between obstructive sleep apnea and cardiac hypertrophy,24,25 suggesting that the cardiothoracic ratio could serve as a reliable indicator of SDB.
Furthermore, a relationship between reduced LVEF and the severity of sleep apnea has been documented.26,27 In our study, a decreased LVEF, as determined by echocardiography, was associated with SDB in all male age groups. Among females, this association was observed specifically in those in their 70 s. Previous research has shown a correlation between SDB and decreased LVEF in healthy individuals, indicating that LVEF could be a pertinent indicator of SDB severity, particularly in males with CVDs.
Perspective of disease-specific profiles
Our analysis revealed that factors such as sex, age, SpO2 upon awakening, BMI, and HbA1c levels are associated with the severity of SDB. However, when examining the impact of specific CVDs, heart failure and coronary artery disease emerged as conditions with a higher prevalence and severity of SDB compared to others. Specifically, valvular heart disease, typically linked with older age, and pulmonary hypertension, associated with lower SpO2 levels upon awakening, exhibited lower incidences and severities of SDB. These findings suggest that coronary artery disease and heart failure might be particularly susceptible to the development and exacerbation of SDB.
Clinical implication
The clinical significance of this study lies in identifying profiles that would warrant further examination with polysomnography. After screening of SDB, we normally recommend polysomnography. However, if risk stratification works well in patients with CVDs, CPAP intervention can be performed earlier. Further, if low oxygen saturation during daytime and elevated BMI were indicative of SDB even after CPAP intervention, it would be clinically beneficial.
Limitations
This study has several limitations. First, the focus is exclusively on patients with CVDs, rather than the general population. Consequently, many findings reported for the general population may not be directly applicable or relevant to our study. Second, the findings are pertinent primarily to hospitalized patients undergoing evaluation and treatment for CVDs, limiting the applicability of our results to this specific patient group. Moreover, the study groups included a mix of conditions -coronary artery disease, arrhythmias, heart failure, and pulmonary hypertension- without a detailed categorization or background analysis. Future research should aim to dissect and validate findings by individually analyzing each disease type for a more precise understanding. Third, SDB encompasses two distinct conditions: OSA and CSA, each with unique mechanisms. Our methodology, relying on PULSOX®-Me, was not able to differentiate between these types, a significant limitation given the importance of distinguishing between OSA and CSA for accurate diagnosis and management. Forth, we do not have enough data regarding lifestyle-related diseases such as hypertension, dyslipidemia, diabetes, smoking, and chronic kidney or lung disease, as well as the presence of atrial fibrillation. Fifth, we do not have data on clearer clinical indicators that are strongly associated with SDB in the mixed pathological conditions in both coronary artery disease and heart failure, which should be clarifised in the future studies. Sixth, SDB in patients with pulmonary hypertension was milder, contrary to our expectations. We will examine more details regarding pulmonary hemodynamics and SDB as our next study.
Conclusions
The current study elucidates the clinical profiles of CVD patients exhibiting more severe forms of SDB. Our disease-specific analysis revealed that coronary artery disease and heart failure are associated with a higher prevalence and a greater risk of moderate to severe SDB compared to valvular heart diseases, typically in older patients. Notably, low oxygen saturation during daytime and elevated BMI were indicative of SDB across all age categories for both sexes.
Data availability
The data underlying this article will be shared with others for the purpose of reproducing results or replicating procedures upon reasonable request to the corresponding author, participant to institutional and ethics committee approval.
Abbreviations
- AHI:
-
Apnea-hypopnea index
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CPAP:
-
Continuous positive airway pressure
- CVDs:
-
Cardiovascular diseases
- HbA1c:
-
Hemoglobin A1c
- LVEF:
-
Left ventricular ejection fraction
- NT-proBNP:
-
N-terminal pro B-type natriuretic peptide
- ODI:
-
Oxygen desaturation index
- OSA:
-
Obstructive sleep apnea
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- SDB:
-
Sleep-disordered breathing.
- SpO2 :
-
Transcutaneous arterial blood oxygen saturation
References
Cowie, M. R., Linz, D., Redline, S., Somers, V. K. & Simonds, A. K. Sleep disordered breathing and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 78, 608–624 (2021).
Javaheri, S. et al. Sleep Apnea: types, mechanisms, and clinical cardiovascular consequences. J. Am. Coll. Cardiol. 69, 841–858 (2017).
Akashiba, T. et al. Sleep Apnea syndrome (SAS) clinical practice guidelines 2020. Respir. Investig. 60, 3–32 (2022).
Benjafield, A. V. et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir. Med. 7, 687–698 (2019).
Heinzer, R. et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir. Med. 3, 310–318 (2015).
Kapur, V. K. et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep Apnea: an American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 13, 479–504 (2017).
Peppard, P. E., Young, T., Palta, M. & Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl. J. Med. 342, 1378–1384 (2000).
Nieto, F. J. et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283, 1829–1836 (2000).
Bixler, E. O. et al. Association of hypertension and sleep-disordered breathing. Arch. Intern. Med. 160, 2289–2295 (2000).
Kikuchi, T. et al. Relationship between sleep disordered breathing and heart rate turbulence in non-obese subjects. Heart Vessels 34, 1801–1810 (2019).
Young, T., Skatrud, J. & Peppard, P. E. Risk factors for obstructive sleep apnea in adults. JAMA 291, 2013–2016 (2004).
Peppard, P. E. et al. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 177, 1006–1014 (2013).
Freire, A. X., Kadaria, D., Avecillas, J. F., Murillo, L. C. & Yataco, J. C. Obstructive sleep apnea and immunity: relationship of lymphocyte count and apnea hypopnea index. South Med. J. 103, 771–774 (2010).
Li, N. et al. Nocturnal mean oxygen saturation is associated with secondary Polycythemia in young adults with obstructive sleep Apnea. Especially in Men. Nat. Sci. Sleep 11, 377–386 (2019).
Pathak, R., Giri, S., Karmacharya, P. & Aryal, M. R. Obstructive sleep apnea syndrome and secondary polycythemia: analysis of the nationwide inpatient sample. Sleep Med. 16, 205–206 (2015).
Zhang, X. B., Zeng, Y. M., Zeng, H. Q., Zhang, H. P. & Wang, H. L. Erythropoietin levels in patients with sleep apnea: a meta-analysis. Eur. Arch. Otorhinolaryngol. 274, 2505–2512 (2017).
Reichmuth, K. J., Austin, D., Skatrud, J. B. & Young, T. Association of sleep apnea and type II diabetes: a population-based study. Am. J. Respir. Crit. Care Med. 172, 1590–1595 (2005).
Muraki, I., Wada, H. & Tanigawa, T. Sleep apnea and type 2 diabetes. J. Diabetes Investig. 9, 991–997 (2018).
Tasali, E., Mokhlesi, B. & Van Cauter, E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest 133, 496–506 (2008).
Hwu, D. W., Lin, K. D., Lin, K. C., Lee, Y. J. & Chang, Y. H. The association of obstructive sleep apnea and renal outcomes-a systematic review and meta-analysis. BMC Nephrol. 18, 313 (2017).
Umbro, I., Fabiani, V., Fabiani, M., Angelico, F. & Del Ben, M. A systematic review on the association between obstructive sleep apnea and chronic kidney disease. Sleep Med. Rev. 53, 101337 (2020).
Seyis, S., Usalan, A. K., Rencuzogullari, I., Kurmus, O. & Gungen, A. C. The effects of continuous positive airway pressure on premature ventricular contractions and ventricular wall stress in patients with heart failure and sleep Apnea. Can Respir. J. 2018, 2027061 (2018).
Wu, Q. et al. Efficacy of continuous positive airway pressure on NT-pro-BNP in obstructive sleep apnea patients: a meta-analysis. BMC Pulm Med. 23, 260 (2023).
Cuspidi, C. et al. Obstructive sleep apnoea syndrome and left ventricular hypertrophy: a meta-analysis of echocardiographic studies. J. Hypertens 38, 1640–1649 (2020).
Dursunoglu, D. et al. Impact of obstructive sleep apnoea on left ventricular mass and global function. Eur. Respir. J. 26, 283–288 (2005).
Yu, L. et al. Left ventricular remodeling and dysfunction in obstructive sleep apnea : Systematic review and meta-analysis. Herz 45, 726–738 (2020).
Jin, S. et al. Subclinical left ventricular myocardial dysfunction in patients with obstructive sleep apnea syndrome: insights from noninvasive left ventricular myocardial work analysis. BMC Cardiovasc. Disord. 22, 552 (2022).
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Koutatsu_Shimozono: Conceptualization, Methodology, Formal analysis, Data Curation, and Writing-Original draft preparation. Hisashi_Adachi: Statistics. Shoichiro_Nohara: Methodology, Investigation, Writing-Review and editing. Tatsuhiro_Shibata: Methodology, Data Curation, and Formal analysis. Yoichi_Sugiyama: Investigation. Yoshihiro_Fukumoto: Project administration, Conceptualization, Writing-Review and editing.
Corresponding author
Ethics declarations
Competing interests
Nothing to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shimozono, K., Adachi, H., Nohara, S. et al. Sleep-disordered breathing profiles in patients with cardiovascular diseases: Kurume SDB-CVD study. Sci Rep 15, 16521 (2025). https://doi.org/10.1038/s41598-025-99064-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99064-y