Abstract
This study aimed to investigate the predictive value of the weight-adjusted waist index (WWI) for osteoporosis in postmenopausal patients with type 2 diabetes mellitus (T2DM). This cross-sectional study included 229 postmenopausal patients with T2DM (mean age 64.53 ± 7.4 years). Collect anthropometric data. Bone mineral density (BMD) of the lumbar spine and femoral necks was measured using dual-energy X-ray absorptiometry. Calculate WWI and Osteoporosis Self-Assessment Tool for Asians (OSTA). Use SPSS 25.0 to analyze data employing binary logistic regression and the receiver operating characteristic (ROC) curve. WWI in osteoporosis group was significantly higher than that in non-osteoporosis group (11.54 ± 0.82 vs. 11.07 ± 0.73, P = 0.000), while the OSTA was significantly lower in osteoporosis group compared to non-osteoporosis group (− 1.40 (− 2.8, 0.40) vs. 0.10 (− 1.45,1.80), P = 0.000). Binary logistic regression analysis indicated that the risk of osteoporosis in WWI ≥ 11.55 group was 3.158 times higher than that in WWI < 11.55 group (95% CI 1.714–5.820, P = 0.000). The risk in OSTA ≤ − 1 group was 3.935 times higher than that in OSTA > − 1 group (95% CI 2.168–7.141, P = 0.000). The area under the ROC curve for OSTA and WWI in predicting the risk of osteoporosis in postmenopausal patients with T2DM aged over 70 was 0.761 and 0.808, respectively, with sensitivities of 0.429 and 0.714. In postmenopausal patients with T2DM, WWI is closely associated with osteoporosis and negatively correlates with BMD. Among postmenopausal T2DM patients aged over 70, WWI may be superior to OSTA in predicting osteoporosis.
Similar content being viewed by others
Introduction
Osteoporosis is a chronic bone disease characterized by decreased bone mass and altered bone microstructure, with age and female gender identified as independent risk factors1,2,3. Diabetes mellitus, another chronic disease associated with aging, has been linked to osteoporosis. Research conducted in Japan indicates that elderly female patients with type 2 diabetes mellitus (T2DM) face the highest risk of bone loss and osteoporosis4. This increased risk is attributed to the sudden decline in estrogen levels and the redistribution of fat in postmenopausal women, which increases the risk of T2DM5 and osteoporosis6. Patients with T2DM often exhibit normal or elevated bone mineral density (BMD), but they face an increased risk of fractures attributed to a deteriorated bone quality7,8. Therefore, it is of profound significance to focus on the high-risk groups of co-morbidity between T2DM and osteoporosis, and to implement early screening and preventative measures for osteoporosis in order to reduce the incidence of osteoporotic fractures.
The current gold standard for osteoporosis diagnosis remains dual-energy X-ray absorptiometry (DXA); however, its accessibility is limited9. The Osteoporosis Self-Assessment Tool for Asians (OSTA) is a straightforward and effective predictive screening tool that estimates osteoporosis risk in postmenopausal women based on age and body weight. Nevertheless, the OSTA employs a limited number of indicators, resulting in insufficient specificity, which diminishes its effectiveness in diagnosing postmenopausal osteoporosis within the Chinese population10,11,12,13. A study by Liu et al. found that the OSTA did not perform well in patients with diabetes, exhibiting a low predictive value and a sensitivity of only 44.8%10.
Osteoporosis and obesity are closely related conditions. Previous studies have suggested that obesity and being overweight may serve as independent protective factors for osteoporosis, a phenomenon often referred to as the ‘obesity paradox’14,15,16,17. Notably, abdominal obesity is more prevalent among Chinese patients with T2DM18, and early research indicated a positive correlation between body mass index (BMI), waist circumference (WC) and BMD19,20,21,22,23,24. However, in recent years, an increasing number of studies have challenged the notion that obesity serves as a protective factor against osteoporosis. Elevated levels of WC and visceral fat area have been found to negatively correlate with BMD25,26, while abdominal obesity has been associated with an increased risk of hip fractures, which poses a significant threat to bone health27,28,29. The weight-adjusted waist index (WWI) is a novel obesity assessment metric proposed by Park et al. in 201830. This index standardizes WC relative to body weight30, thereby combining the advantages of waist circumference while minimizing its association with BMI31. WWI exhibits greater accuracy in evaluating muscle and fat mass compared to conventional metrics, such as BMI and WC, and is capable of reflecting changes in abdominal composition with age, including the loss of muscle mass and the retention or gain of fat mass32. Additionally, several studies have demonstrated the relationship between WWI and bone density or osteoporosis across different age groups33,34,35.
This study aimed to investigate whether WWI, an assessment indicator closely associated with abdominal obesity, could serve as a predictive screening indicator for osteoporosis in postmenopausal women with T2DM. We selected postmenopausal female patients with T2DM who were admitted to the hospital, with the objective of evaluating the effectiveness of WWI compared to OSTA in predicting the risk of developing osteoporosis across various age groups.
Material and methods
Study design and participants
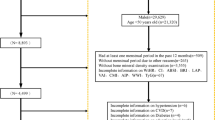
This study is a small sample cross-sectional study. Data comes from the database of hospitalized patients with T2DM in the Department of Endocrinology at the First Hospital of Qinhuangdao from September 2020 to September 2022.
The inclusion criteria encompassed postmenopausal women diagnosed with T2DM who possessed basic communication skills, comprehension abilities and full behavioral capacity. Additionally, they had relatively stable and standardized physical activity levels and dietary calcium intake. Exclusion criteria included patients with a history of severe physical impairment, such as uncorrected acute complications of diabetes mellitus, acute cerebrovascular disease, gastrointestinal bleeding, acute renal failure, severe hepatic abnormalities and severe osteoarthritic or neuromuscular disorders that impact activities of daily living. Additionally, patients with acute infectious diseases, malignant tumors, inflammatory diseases, a history of endocrine disorders affecting bone metabolism (e.g., thyroid disease, hypopituitarism, Addison’s disease, Cushing’s syndrome, pituitary adenomas, parathyroid disease or hypogonadism) and patients taking medications that influence bone metabolism were also excluded. This study also excluded sedentary and bedridden patients and populations with missing data. This study finally involved a total of 229 postmenopausal patients with T2DM.
Data collection
In this study, the general information, anthropometric data and laboratory data of the enrolled patients were collected. Collect the age, history of diabetes, history of smoking and history of drinking of each patient. Anthropometric data include height, weight, WC, systolic blood pressure (SBP), diastolic blood pressure (DBP) and BMD. These data were measured by well-trained medical staff. BMI was calculated by dividing weight (kg) by the square of height (m). WWI was calculated by dividing the waist circumference (cm) by the square root of weight (kg). OSTA was calculated based on the formula: (weight (kg) − age(years)) × 0.2. BMD of the lumbar spine and bilateral femoral necks was measured using DXA equipment from MEDILINK, France.
Laboratory data was obtained from the hospital information system. Blood tests were performed after at least 9 h of fasting. Glycosylated hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) were measured by direct methods on an automated biochemical analyzer (Hitachi LST008 Tokyo, Japan).
Diagnostic criteria
The diagnostic criteria for T2DM adhered to the 1999 World Health Organization (WHO) criteria36. The diagnostic criteria for osteoporosis adhered to the WHO diagnostic criteria37: T-scores ≤ − 2.5 were defined as osteoporosis, − 2.5 < T-scores ≤ − 1.0 were defined as osteopenia, T-scores > -1.0 were defined as normal BMD. Patients with osteopenia and normal BMD are considered non-osteoporosis.
Groups
Based on the diagnostic criteria for BMD, the population was categorized into osteoporosis and non-osteoporosis groups, allowing for a comparison of indicators between these two groups. The analysis was further stratified by WWI tertiles to examine the relationship between WWI and both BMD and the risk of osteoporosis. Additionally, the population was divided by age groups to investigate the correlation between WWI and BMD of each site.
Statistical analysis
Continuous variables that follow a normal distribution are expressed as mean ± standard deviation (x̄ ± s), and comparisons between two groups are conducted using the t-test. In contrast, continuous variables that do not adhere to a normal distribution are expressed as median (interquartile range) [M (P25, P75)], and comparisons between groups are conducted using the rank sum test. Categorical variables are expressed as counts, frequencies or percentages (%), and the chi-square test is utilized for comparisons between groups. Binary logistic regression was employed to analyze the factors influencing osteoporosis in postmenopausal patients with T2DM. The receiver operating characteristic (ROC) curve for the subjects was plotted, and the area under the curve (AUC) was utilized to assess the predictive capability of WWI and OSTA regarding the risk of osteoporosis in postmenopausal T2DM patients over the age of 70. All statistical analyses were conducted using SPSS version 25.0(SPSS Inc., Chicago, IL, USA), with differences considered statistically significant at P < 0.05.
Ethic declarations
This study was approved by the Ethics Committee of the First Hospital of Qinhuangdao and was conducted according to the Helsinki Declaration guidelines, including all relevant details. Due to the retrospective nature of the study, the Ethics Committee of the First Hospital of Qinhuangdao waived the need of obtaining informed consent.
Results
Characteristics of the subjects in osteoporosis and non-osteoporosis groups
In this study, we selected 229 eligible postmenopausal patients with T2DM, who were hospitalized and had a mean age of 64.53 ± 7.4 years. Among these patients, 79 (34.50%) were diagnosed with osteoporosis. The age and WWI of the osteoporosis group were significantly higher than those of the non-osteoporosis group (P < 0.05), while the OSTA was significantly lower in the osteoporosis group compared to the non-osteoporosis group (P < 0.05), as presented in Table 1. No statistically significant differences were observed in other indicators between the two groups (P > 0.05).
Association between WWI and the risk of osteoporosis
In the tertile subgroups of WWI, as the WWI increased from low to high, there was a corresponding decrease in BMD of the lumbar spine, right femoral neck and left femoral neck (P < 0.05). Concurrently, the detection rate of osteoporosis increased in conjunction with the rise in WWI, with these differences being statistically significant (21.1% vs. 29.9% vs. 52.6%, X2 = 17.869, P = 0.000), as shown in Table 2.
WWI and BMD were analyzed across different age subgroups, as illustrated in Fig. 1. In group T (total population), WWI exhibited a negative correlation with lumbar spine BMD (r = − 0.218, P = 0.002), right femoral neck BMD (r = − 0.171, P = 0.015) and left femoral neck BMD (r = − 0.149, P = 0.035). In group A (aged 50–60), no correlation was found between WWI and both lumbar spine BMD and femoral neck BMD (P > 0.05). In group B (aged 60–70), a negative correlation was observed between WWI and lumbar spine BMD (r = − 0.237, P = 0.015), while no correlation was noted with femoral neck BMD (P > 0.05). In group C (aged over 70), WWI exhibited a negative correlation with lumbar spine BMD (r = − 0.450, P = 0.019), right femoral neck BMD (r = − 0.427, P = 0.026) and left femoral neck BMD (r = − 0.480, P = 0.011).
Comparison of the predictive efficacy of WWI and OSTA in assessing the risk of osteoporosis
A binary logistic regression analysis was conducted, where the dependent variable was defined as the presence of osteoporosis (no osteoporosis = 0; osteoporosis = 1). The independent variables included age, WWI stratification (WWI < 11.55 = 0; WWI ≥ 11.55 = 1) and OSTA stratification (OSTA > − 1 = 0; OSTA ≤ − 1 = 1). The results indicated that the risk of osteoporosis was 3.158 times higher (95% CI 1.714–5.820, P = 0.000) in the WWI ≥ 11.55 group compared to the WWI < 11.55 group. Additionally, the risk was 3.935 times higher (95% CI 2.168–7.141, P = 0.000) in the OSTA ≤ − 1 group compared to the OSTA > − 1 group (Table 3).
ROC curves were plotted using the presence of osteoporosis as the status variable (no osteoporosis = 0; osteoporosis = 1), with WWI and OSTA serving as the test variables (Fig. 2). The results indicated that the AUC for OSTA and WWI in predicting the occurrence of osteoporosis in postmenopausal patients with T2DM aged over 70 years was 0.761 (95% CI 0.636–0.886) and 0.808 (95% CI 0.688–0.927), respectively, with sensitivities of 0.429 and 0.714 (Table 4).
Discussion
This cross-sectional study included 229 postmenopausal patients with T2DM. Our findings indicate a detection rate of 34.5% for osteoporosis among postmenopausal patients with T2DM. The prevalence of postmenopausal osteoporosis varies significantly, ranging from 10.75 to 32.1%38,39,40,41, which can be attributed to differences in population characteristics and diagnostic methods.
Our study found that, within the tertile subgroups of WWI, BMD of the lumbar spine, right femoral neck and left femoral neck in postmenopausal T2DM patients gradually decreased with increasing WWI, while the detection rate of osteoporosis correspondingly rose. A significant negative correlation was observed between WWI and both lumbar spine and femoral neck BMD, with this relationship being more pronounced in postmenopausal T2DM patients over 70 years. This aligns with results from several studies utilizing the NHANES and KNHANES databases16,33,34,35,42. In the correlation analysis between WWI and BMD across different age subgroups, a negative correlation was observed between WWI and BMD of both the lumbar spine and femoral neck only in the age group of over 70 years. In contrast, no correlation was found in the age group of 50–60 years. This finding suggests a significant relationship between WWI and aging, indicating a potential non-linear relationship between WWI and the risk of osteoporosis. A cross-sectional study conducted by Li et al.14 reported that no association was detected between WWI and osteoporosis when WWI < 11.14 cm/√kg. However, a significant positive correlation was identified between WWI and an increased risk of osteoporosis when WWI ≥ 11.14 cm/√kg, as well as a non-linear relationship between WWI and osteoporosis within the T2DM population. Additionally, a study involving individuals aged 65 years and older in the United States34 revealed a U-shaped relationship between WWI and osteoporosis. Our further logistic regression analysis confirmed that a WWI exceeding 11.55 cm/√kg significantly increased the risk of osteoporosis by 3.158-fold compared to the group with a WWI below 11.55 cm/√kg. This finding is in proximity to the cut-off value of 11.14 cm/√kg reported in the study by Li et al.14.
We found a 3.935-fold increased risk of developing osteoporosis in logistic regression analysis for subjects with an OSTA below − 1. However, the ROC curve revealed that the AUC of the OSTA for predicting osteoporosis risk in postmenopausal individuals with T2DM over the age of 70 was 0.761. Despite the limited sample size of postmenopausal T2DM patients aged over 70, the AUC of the WWI was 0.808. This indicates a higher sensitivity compared to the OSTA, suggesting that the WWI may serve as a more effective screening tool for predicting the risk of osteoporosis in postmenopausal patients with T2DM.
To analyze the possible reasons for the differences between these two screening metrics, we first selected postmenopausal T2DM patients for our study. This population is significantly impacted by the rapid decline in physiological estrogen levels, which affects overall body composition including bone, muscle and fat. Consequently, it is essential to consider the effects of fat and muscle composition on bone mass when assessing it. In contrast, OSTA focuses solely on age and body weight, failing to differentiate between fat and muscle mass and lacking the ability to interpret their relationship with bone metabolism and bone mass. Secondly, abdominal obesity is a common somatotype among patients with T2DM18, particularly among the elderly population in China43. WWI serves as an effective indicator for assessing the presence of abdominal obesity by standardizing WC relative to body weight. This approach combines the advantages of WC while minimizing the association with BMI31, thereby reflecting the complex condition of sarcopenic obesity14,42. Existing literature indicates a positive correlation between WWI and body fat mass, alongside a negative correlation with muscle mass32,42. Elevated WWI suggest that individuals with larger WC possess higher fat mass and lower muscle mass compared to those with the same weight but smaller WC. Furthermore, WWI demonstrates minimal variation across different racial groups, making it broadly applicable in multiracial contexts14.
In our study, we found that the negative correlation between WWI and BMD in the postmenopausal population with T2DM was more pronounced in individuals over 70 years. We inferred that this phenomenon may be attributed to the influence of abdominal obesity on bone metabolism, which leads to a reduction in bone mass; furthermore, abdominal obesity is more prevalent among the elderly population43. However, disease states and sensitive indicators may vary across different populations. Osteoporosis is influenced by a wide range of etiological factors and risk factors. As an indicator related to abdominal obesity, WWI considers only two parameters: WC and body weight30. Consequently, its applicability may be limited across diverse populations. Nevertheless, the validity of the WWI in younger age groups has been established33,44. Therefore, further studies are necessary to assess the sensitivity and applicability of the WWI in non-diabetic populations. Additionally, more appropriate cut-off value of WWI should be investigated. The strength of the present study lies in its focus on the relationship between WWI and the risk of osteoporosis in postmenopausal patients with T2DM, an area that has been underexplored in previous studies, which predominantly focused on bone health in the general population. However, this study also has certain limitations. Firstly, being a cross-sectional retrospective study, it failed to reveal a causal relationship between WWI and the risk of osteoporosis. Secondly, this study was conducted at a single center with a small sample size, which limited the ability to determine a more appropriate cut-off value for WWI. Thirdly, the population of this study was restricted to hospitalized postmenopausal individuals with T2DM, and no healthy control group was included. Consequently, we will continue to expand our sample size, particularly among the non-diabetic population aged over 70 years, to obtain clearer statistical evidence of the superiority of WWI over OSTA in screening for osteoporosis. Additionally, further prospective studies and causal inference analyses are necessary to corroborate our findings.
Conclusion
The results of this study demonstrate that in postmenopausal patients with T2DM, WWI is closely associated with osteoporosis and negatively correlates with BMD. Furthermore, among postmenopausal T2DM patients over 70 years, WWI may be more effective than OSTA in predicting osteoporosis. We anticipate that WWI will serve as a predictive screening indicator for osteoporosis, enabling early intervention to mitigate its adverse consequences.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- DBP:
-
Diastolic blood pressure
- DXA:
-
Dual-energy X-ray absorptiometry
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Glycosylated hemoglobin A1c
- HDL-C:
-
High density lipoprotein cholesterol
- LDL-C:
-
Low density lipoprotein cholesterol
- OSTA:
-
Osteoporosis Self-Assessment Tool for Asians
- ROC:
-
Receiver operating characteristic curve
- SBP:
-
Systolic blood pressure
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- T2DM:
-
Type 2 diabetes mellitus
- WC:
-
Waist circumference
- WHO:
-
World Health Organization
- WWI:
-
Weight-adjusted waist index
References
Yoshimura, N. et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: The research on osteoarthritis/osteoporosis against disability study. J. Bone Miner. Metab. 27(5), 620–628. https://doi.org/10.1007/s00774-009-0080-8 (2009).
Horii, C. et al. Differences in prevalence and associated factors between mild and severe vertebral fractures in Japanese men and women: The third survey of the ROAD study. J. Bone Miner. Metab. 37(5), 844–853. https://doi.org/10.1007/s00774-018-0981-5 (2019).
Huang, R. et al. Increased glycemic variability evaluated by continuous glucose monitoring is associated with osteoporosis in type 2 diabetic patients. Front. Endocrinol. (Lausanne). 13, 861131. https://doi.org/10.3389/fendo.2022.861131 (2022).
Yamamoto, Y. et al. Age and sex are excellent predictors of bone complications in patients with type 2 diabetes with no history of osteoporotic fracture or treatment for osteoporosis. J. Int. Med. Res. 52(5), 3000605241246743. https://doi.org/10.1177/03000605241246743 (2024).
De Paoli, M. & Werstuck, G. H. Role of estrogen in type 1 and type 2 diabetes mellitus: A review of clinical and preclinical data. Can. J. Diabetes. 44(5), 448–452. https://doi.org/10.1016/j.jcjd.2020.01.003 (2020).
Lu, L. & Tian, L. Postmenopausal osteoporosis coexisting with sarcopenia: The role and mechanisms of estrogen. J. Endocrinol. 259(1), e230116. https://doi.org/10.1530/JOE-23-0116 (2023).
Vigevano, F. et al. In men with obesity, t2dm is associated with poor trabecular microarchitecture and bone strength and low bone turnover. J. Clin. Endocrinol. Metab. 106(5), 1362–1376. https://doi.org/10.1210/clinem/dgab061 (2021).
Zhang, Y. S., Zheng, Y. D., Yuan, Y., Chen, S. C. & Xie, B. C. Effects of anti-diabetic drugs on fracture risk: A systematic review and network meta-analysis. Front. Endocrinol. (Lausanne). 12, 735824. https://doi.org/10.3389/fendo.2021.735824 (2021).
Han, W. et al. Association between lumbar bone mineral density and serum uric acid in postmenopausal women: A cross-sectional study of healthy Chinese population. Arch. Osteoporos. 12(1), 50. https://doi.org/10.1007/s11657-017-0345-0 (2017).
Liu, Z. et al. Evaluation of singh index and osteoporosis self-assessment tool for asians as risk assessment tools of hip fracture in patients with type 2 diabetes mellitus. J. Orthop. Surg. Res. 12(1), 37. https://doi.org/10.1186/s13018-017-0539-6 (2017).
Zha, X. Y., Hu, Y., Pang, X. N., Chang, G. L. & Li, L. Diagnostic value of osteoporosis self-assessment tool for Asians (OSTA) and quantitative bone ultrasound (QUS) in detecting high-risk populations for osteoporosis among elderly Chinese men. J. Bone Miner. Metab. 33(2), 230–238. https://doi.org/10.1007/s00774-014-0587-5 (2015).
Lu, C., Chen, D., Cai, Y. & Wei, S. Concordane of OSTA and lumbar spine BMD by DXA in identifying risk of osteoporosis. J. Orthop. Surg. Res. 1, 14. https://doi.org/10.1186/1749-799X-1-14 (2006).
Yang, Y. et al. Validation of an osteoporosis self-assessment tool to identify primary osteoporosis and new osteoporotic vertebral fractures in postmenopausal Chinese women in Beijing. BMC Musculoskelet. Disord. 14, 271. https://doi.org/10.1186/1471-2474-14-271 (2013).
Pan, R. & Li, Y. The association of weight-adjusted waist index with the risk of osteoporosis in patients with type 2 diabetes: A cross-sectional study. J. Orthop. Surg. Res. 19(1), 518. https://doi.org/10.1186/s13018-024-04991-7 (2024).
Salamat, M. R., Salamat, A. H. & Janghorbani, M. Association between obesity and bone mineral density by gender and menopausal status. Endocrinol. Metab. (Seoul). 31(4), 547–558. https://doi.org/10.3803/EnM.2016.31.4.547 (2016).
Zhang, Y. et al. Associations between weight-adjusted waist index and bone mineral density: Results of a nationwide survey. BMC Endocr. Disord. 23(1), 162. https://doi.org/10.1186/s12902-023-01418-y (2023).
Tao, J., Zhang, Y., Tan, C. & Tan, W. Associations between weight-adjusted waist index and fractures: A population-based study. J. Orthop. Surg. Res. 18(1), 290. https://doi.org/10.1186/s13018-023-03776-8 (2023).
Liu, D. et al. Effects of exercise intervention on type 2 diabetes patients with abdominal obesity and low thigh circumference (EXTEND): study protocol for a randomized controlled trial. Front. Endocrinol. (Lausanne). 13, 937264. https://doi.org/10.3389/fendo.2022.937264 (2022).
Gadde, K. M., Martin, C. K., Berthoud, H. R. & Heymsfield, S. B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 71(1), 69–84. https://doi.org/10.1016/j.jacc.2017.11.011 (2018).
Sampaio, L. G., Marques, J., Petterle, R. R., Moreira, C. A. & Borba, V. Z. C. Association between fractures and traditional risk factors for osteoporosis and low bone mineral density in patients with obesity. Arch. Endocrinol. Metab. 65(2), 152–163. https://doi.org/10.20945/2359-3997000000331 (2021).
Li, Y. Association between obesity and bone mineral density in middle-aged adults. J. Orthop. Surg. Res. 17(1), 268. https://doi.org/10.1186/s13018-022-03161-x (2022).
Zhang, Y. & Pu, J. The saturation effect of obesity on bone mineral density for older people: The NHANES 2017–2020. Front. Endocrinol. (Lausanne). 13, 883862. https://doi.org/10.3389/fendo.2022.883862 (2022).
Julian, V. et al. Does the severity of obesity influence bone density, geometry and strength in adolescents?. Pediatr. Obes. 16(12), e12826. https://doi.org/10.1111/ijpo.12826 (2021).
Zhang, L. et al. Association of body compositions and bone mineral density in Chinese children and adolescents: Compositional data analysis. Biomed.Res. Int. 2021, 1904343. https://doi.org/10.1155/2021/1904343 (2021).
Piñar-Gutierrez, A., García-Fontana, C., García-Fontana, B. & Muñoz-Torres, M. Obesity and bone health: A complex relationship. Int. J. Mol. Sci. 23(15), 8303. https://doi.org/10.3390/ijms23158303 (2022).
Yin, Z., Yan, H., Yu, Y. & Liu, Y. Different associations between waist circumference and bone mineral density stratified by gender, age, and body mass index. BMC Musculoskelet. Disord. 23(1), 786. https://doi.org/10.1186/s12891-022-05736-5 (2022).
Søgaard, A. J. et al. Abdominal obesity increases the risk of hip fracture. A population-based study of 43,000 women and men aged 60–79 years followed for 8 years. Cohort of Norway. J. Intern. Med. 277(3), 306–317. https://doi.org/10.1111/joim.12230 (2015).
Vala, C. H. et al. Risk for hip fracture before and after total knee replacement in Sweden. Osteoporos Int. 31(5), 887–895. https://doi.org/10.1007/s00198-019-05241-x (2020).
Sadeghi, O., Saneei, P., Nasiri, M., Larijani, B. & Esmaillzadeh, A. Abdominal obesity and risk of hip fracture: A systematic review and meta-analysis of prospective studies. Adv. Nutr. 8(5), 728–738. https://doi.org/10.3945/an.117.015545 (2017).
Park, Y., Kim, N. H., Kwon, T. Y. & Kim, S. G. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci. Rep. 8(1), 16753. https://doi.org/10.1038/s41598-018-35073-4 (2018).
Ye, J. et al. Association between the weight-adjusted waist index and stroke: A cross-sectional study. BMC Public Health 23(1), 1689. https://doi.org/10.1186/s12889-023-16621-8 (2023).
Kim, N. H., Park, Y., Kim, N. H. & Kim, S. G. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 50(3), 780–786. https://doi.org/10.1093/ageing/afaa208 (2021).
Wang, X., Yang, S., He, G. & Xie, L. The association between weight-adjusted-waist index and total bone mineral density in adolescents: NHANES 2011–2018. Front. Endocrinol (Lausanne). 14, 1191501. https://doi.org/10.3389/fendo.2023.1191501 (2023).
Lin, Y. et al. Relationship between weight-adjusted waist index and osteoporosis in the senile in the United States from the national health and nutrition examination survey, 2017–2020. J. Clin. Densitom. 26(2), 101361. https://doi.org/10.1016/j.jocd.2023.02.002 (2023).
Guo, M. et al. The relationship between weight-adjusted-waist index and total bone mineral density in adults aged 20–59. Front. Endocrinol. (Lausanne). 14, 1281396. https://doi.org/10.3389/fendo.2023.1281396 (2023).
Drouin, P. et al. Diagnostic et classification du diabète sucré: Les nouveaux critères [Diagnosis and classification of diabetes mellitus: the new criteria]. Diabetes Metab. 25(1), 72–83 (1999).
Kanis, J. A., Melton, L. J. 3rd., Christiansen, C., Johnston, C. C. & Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 9(8), 1137–1141. https://doi.org/10.1002/jbmr.5650090802 (1994).
Wang, L. et al. Prevalence of osteoporosis and fracture in china: the china osteoporosis prevalence study. JAMA Netw Open. 4(8), e2121106. https://doi.org/10.1001/jamanetworkopen.2021.21106 (2021).
Lu, Y. C. et al. Prevalence of osteoporosis and low bone mass in older chinese population based on bone mineral density at multiple skeletal sites. Sci. Rep. 6, 25206. https://doi.org/10.1038/srep25206 (2016).
Wright, N. C. et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 29(11), 2520–2526. https://doi.org/10.1002/jbmr.2269 (2014).
Irani, A. D., Poorolajal, J., Khalilian, A., Esmailnasab, N. & Cheraghi, Z. Prevalence of osteoporosis in Iran: A meta-analysis. J. Res. Med. Sci. 18(9), 759–766 (2013).
Kim, K. J., Son, S., Kim, K. J., Kim, S. G. & Kim, N. H. Weight-adjusted waist as an integrated index for fat, muscle and bone health in adults. J. Cachexia Sarcopenia Muscle 14(5), 2196–2203. https://doi.org/10.1002/jcsm.13302 (2023).
Liu, C. et al. Hyperhomocysteinemia increases risk of metabolic syndrome and cardiovascular death in an elderly Chinese community population of a 7-year follow-up study. Front. Cardiovasc. Med. 8, 811670. https://doi.org/10.3389/fcvm.2021.811670 (2022).
Ma, G. et al. Association between weight-adjusted waist index and bone mineral density in adolescents. Sci. Rep. 14(1), 16509. https://doi.org/10.1038/s41598-024-66565-1 (2024).
Acknowledgements
We are deeply grateful to all the participants in this study.
Author information
Authors and Affiliations
Contributions
GD, LZ and JL wrote the main manuscript text and prepared Tables 1-4 and Figs. 1 and 2. JL, XW and LS collected and analyzed the data. DF, NW and LL contributed to the study design. BL and FY organized the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, G., Zeng, L., Lan, J. et al. Weight-adjusted waist index as a new predictor of osteoporosis in postmenopausal patients with T2DM. Sci Rep 15, 14427 (2025). https://doi.org/10.1038/s41598-025-99098-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99098-2