Abstract
This article introduces a near-field microwave sensor based on a monopole antenna for breast tumor detection. The designed antenna operates at 2 GHz and is miniaturized by 37% through the etching of two pairs of rectangular slots along its edges, achieving a compact footprint of 0.28λg × 0.42λg (where λg is the guided wavelength at 2 GHz). To enhance gain, a partially reflective surface (PRS) is positioned behind the radiating monopole, increasing the antenna gain from 2.15 to 7 dBi. The monopole antenna is fabricated using Rogers RT5880 (0.787 mm thickness), while the PRS is made from Rogers TMM13i (3.8 mm thickness). The proposed antenna exhibits high radiation efficiency (95–99%) across the 1.9–2.1 GHz bandwidth. A 3D artificial female breast equivalent phantom is developed to evaluate the sensor’s performance. The PRS-enabled antenna is analyzed in terms of reflected and transmitted power variations at different distances from the phantom. Simulation and experimental results confirm that integrating PRS improves the sensor’s sensitivity and its ability to differentiate between healthy and malignant tissues. Furthermore, specific absorption rate (SAR) analysis indicates that an input power of 50 mW meets SAR safety standards. The proposed antenna is compact, simple, and a safer alternative to X-rays, providing a portable solution for effective breast tumor detection.
Similar content being viewed by others
Introduction
Breast cancer stands as a significant cause of female mortality worldwide1,2,3. Detecting breast cancer early significantly diminishes mortality rates, enhancing recover prospects4,5,6. According to current findings, identification of tumors at an initial stage, typically under 5 mm in size, indicates a 97% likelihood of recovery. Prioritizing early detection, not only boosts survival rates but also reduces the necessity for drastic measures like mastectomy7,8,9. A mastectomy is a surgical procedure to remove one or both breasts, typically to treat or prevent breast cancer. Traditional techniques like X-ray mammography, ultrasound, and MRI have downsides such as breast compression, ionizing radiation exposure, high costs, and limited accuracy for specific breast tissue types. Researchers are currently exploring microwave radiation-based systems as alternatives to existing techniques10,11,12,13. These systems offer several advantages, including lower cost, higher accuracy, and safety from high-energy harmful radiation of X-rays. For instance, microwave imaging (MWI) is a cost-effective, safe, and suitable for wearable or portable devices. Unlike mammography, MWI does not require breast compression or prolonged confinement, potentially offering a more comfortable experience for patients. Moreover, MWI has emerged as a promising alternative, addressing the limitations of ionizing radiation and breast compression14,15,16,17. Additionally, it is expected to be more cost-effective, as microwave equipment is significantly less expensive compared to MRI systems. These characteristics suggest that MWI could enhance patient adherence in future applications.
Although, MWI remains an investigational technique, it shows promising potential for non-invasive breast assessment by detecting variations in dielectric properties such as conductivity and permittivity, to effectively distinguish between healthy and malignant tissues18,19. Further studies are needed to evaluate MWI’s clinical efficiency and diagnostic accuracy relative to established imaging methods. Research indicates that tumors often exhibit variations in dielectric properties, which may be influenced by factors such as water content, cellular density, and tissue heterogeneity. However, these properties can vary significantly across patients and tumor types20. Such variability underscores the importance of further investigation into MWI’s diagnostic capabilities in diverse clinical scenarios. The antennas utilized in radar-based imaging systems are typically characterized by several key features, a wide impedance bandwidth, compact size, cost-effective manufacturing, and efficient power coupling to the breast tissues.
Developing a compact breast cancer detection system with the ability to detect deep-seated tumors is highly desirable. While lower frequencies provide greater penetration depth, they require larger sensor sizes, whereas higher frequencies offer finer range resolution but suffer from increased signal attenuation. Numerous efforts have been made to develop compact antennas for microwave breast imaging21. One notable advancement is the design of a compact bowtie antenna22 operating within the 2–4 GHz frequency range. In this study, the antenna is integrated with a breast phantom composed solely of fat tissues. Another development involves a broadband monopole antenna23 covering 3.1–10.6 GHz, though its focus is limited to specific absorption rate (SAR) calculations, varying tumor size and location. Additionally, a two-element dielectric resonator antenna (DRA) sensor array24 operating at 6.5 GHz has been introduced for breast tumor detection. However, this study employs a homogeneous phantom with only a fat layer, resulting in minimal sensitivity to tumor presence. Moreover, in25, the effectiveness of wide-slot antennas and stacked patch antennas for breast tumor detection is compared. Both antennas are cavity-mounted to improve radiation performance, but their bulky size and the use of a fat-tissue phantom remain limitations. A common challenge in breast cancer detection technologies is that compact near-field antenna sensors typically exhibit lower gain and efficiency, reducing their tumor detection sensitivity. In recent years, partially reflective surfaces (PRS)41,42 have gained popularity for enhancing antenna performance, improving bandwidth, gain, and directive radiation patterns while maintaining low cross-polarization26,27. For instance, in26, a Fabry–Perot cavity-backed antenna achieves a gain enhancement of up to 8 dBi, while in27, a large 230 mm × 240 mm partially reflective sheet placed in front of the antenna boosts gain up to 14 dBi through multiple reflections. Similarly, in28,29,30, high-gain antennas are designed using periodic unit-cell arrays arranged in a 2D lattice, combined with basic radiators such as patch, slot, or dipole antennas to further enhance performance.
In this article, a PRS-based monopole antenna-sensor is designed to operate at a frequency of 2 GHz within operational bandwidth of 1.9–2.1 GHz. To achieve a compact-size antenna, four rectangular slots are etched on the non-radiating edges of the patch, leads to 37% reduction in antenna size. The placement of a reflective surface significantly enhances the monopole antenna’s gain from 2.1 to 7 dBi. The PRS is made of sub-wave length unit cells positioned behind the antenna to improve the antenna gain leading to expand sensor’s sensitivity in detecting breast tumors significantly. A heterogeneous female breast equivalent phantom is modeled using CST Microwave Studio and later realistic phantom is fabricated for validation. The proposed antenna sensor demonstrates good sensitivity, achieving high directivity and efficiency. In free space, it provides a gain of 7 dBi, while in a breast phantom environment, it yields a gain of 2.2 dBi.
Antenna sensor design
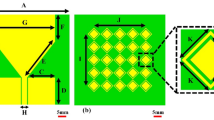
The monopole antenna is designed with dimensions including a width wp and an overall length of lp + S with partial ground plane. The dimensions of monopole antenna are calculated using Eq. (1) operating at a frequency of 2.65 GHz, as shown in Fig. 1a. The ground plane of monopole antenna as shown in Fig. 1b. The operating frequency of the antenna is further adjusted to 2 GHz by etching four rectangular slots of length \(\left({l}_{s}\right)\) of 0.2λg (where λg is the guide wavelength at 2.65 GHz) along the length edges of the monopole patch as shown in Fig. 1c. The proposed slotted monopole antenna achieves a miniaturization of 37%, offers a fractional bandwidth of 20%, and provides a gain of 2.1 dBi in the operating frequency band 1.83–2.17 GHz. The operating frequency of the monopole antenna excited in its dominant mode is calculated using the following Eq. (1).
Design evolution: (a) Top view of Monopole antenna with W = 50, L = 50, \({w}_{p}\) = \({w{\prime}}_{p}\)= 31.5, \({l}_{p}\) = \({l{\prime}}_{p}\) = 23, \({w}_{i}\) = 0.5, S = 23, (b) Bottom view with \({w}_{g}\) = 28.8, \({l}_{g}\) = 8.5, (c) Slotted monopole antenna \({w}_{s}\) = 1, \({l}_{s}\) = 13.6,\({w}_{f}\) = 2.6 (units: mm).
Figure 2a. demonstrate the S11 versus frequency plots before and after incorporating rectangular slots. It reveals that, without slots the monopole radiator resonates at 2.65 GHz in its dominant mode. Carving four rectangular slots along the length of the antenna current, leading to miniaturization and causing the dominant mode to resonates at 2 GHz.
Design of partially reflective surface
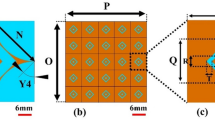
The unit cell is designed by firstly opting a square patch of dimensions lu × wu mm2 (i.e. 0.22λg × 0.22λg) and later by etching a ring of width gu around the boundary of the patch. The unit cell is designed and fabricated on Rogers TMM13i material of thickness 3.8 mm, a dielectric constant of 12.2, and a loss tangent (tan δ) of 0. 0019. The unit cell’s dimensions26,27 are tuned meticulously to resonate at 2 GHz, as shown in Fig. 2b. The S-parameters of unit cells demonstrating reflection coefficient (S11) and transmission coefficient (S21), for the proposed unit cell. A 4 × 4 order of unit cells43,44,45 with a uniform gap of g between any two-unit cells is created, with overall dimensions of 50 mm × 50 mm, as shown in Fig. 2c.
This array of unit cells is positioned behind the proposed monopole antenna at approximately distance of 0.27λg (where λg = \(c/f\times \sqrt{{\varepsilon }_{reff}}\), and \({\varepsilon }_{reff}={\varepsilon }_{reff}\) of radiator +\({\varepsilon }_{reff}\) of PRS)31,39. Figure 3a shows the proposed design performance with and without PRS in terms of bandwidth. By placing the PRS at this distance, it enhances the directional characteristics of the antenna, increasing its forward gain. The reflector helps to focus the radiated energy in the desired direction (i.e. broadside) by reflecting and redirecting the electromagnetic waves in the frequency band of 1.9–2.1 GHz. Moreover, it improves the front-to-back ratio of the antenna by significantly reducing the amount of radiation in the direction opposite the intended beam. Figure 3b presents the gain and efficiency characteristics of the proposed antenna sensor. Upon integrating the PRS, the sensor exhibits an increased gain nearly 7 dBi, while achieving an efficiency of up to 99%. The incorporation of the PRS leads to a 12% decrease in the fractional bandwidth of the sensor. The 4 × 4 grid creates a symmetric structure that promotes a balanced and uniform reflection pattern.
This symmetry helps the antenna maintain a stable and consistent radiation pattern, which is essential for detecting tumors accurately in breast tissue by avoiding any unintended lobes or nulls in the radiation field. As shown in Fig. 4a, the single antenna emits an omnidirectional radiation pattern with a gain of 2.15 dBi. In contrast, when equipped with PRS as illustrated in Fig. 4b, the antenna’s gain is enhanced by 4.85 dBi, resulting in a unidirectional radiation pattern. In Fig. 4c the top metallic layer of both the patch and PRS, displaying only the scalar electric field (E-field) at 2 GHz to focus on understanding the magnitude of the E-field distribution. Although, it is chosen to illustrate the scalar field here, it is also possible to analyze the vector E-field at this frequency to observed amplitude as well as phase for a more comprehensive view.
Antenna- sensor performance over heterogeneous breast phantom
To assess the efficiency of the suggested sensor identifying breast tumor cells, the antenna-sensor is simulated using CST Microwave Studio 2022 near a 3D heterogeneous hemispherical breast phantom mimicking female breast. A hemispherical phantom is fashioned with four distinct layers: skin, fat, fibro glandular, and tumor. The dielectric characteristics of the breast tissues at 2 GHz operating frequency utilized in this study are outlined in Table 1, sourced from references35,36.
The dielectric properties including permittivity (εr) and conductivity (σ) are analyzed at 2 GHz using a one-pole Cole–Cole model as referenced in15,35. In all simulation studies, the tumor’s location within the fat layer is kept consistent and the tumor’s radius is maintained fixed of 8 mm. Fig. 5 illustrates the S-parameters variation with placement of the antenna-radiator without PRS at a distance p mm, from a breast phantom. Figure 5a shows the S11 variation in operating frequency band without tumor while Fig. 5b shows it with tumor. The thickness of skin and fat layers of heterogeneous phantom has been considered 4 mm and 16 mm, while the radius of the fibro-gland layer and tumor are considered 20 mm and 8 mm, respectively. The S-parameters are obtained for both conditions of healthy tissues and tissues containing malignant tumors as mentioned in Table 2. Figure 5c shows the variation in S-parameters at a distance of 2 mm from the phantom. It shows, the reflection loss of around -15 dB at an operating frequency of 2.024 GHz in healthy case (i.e. without tumor) while a small deviation of 1 dB between reflection loss (S11) and (S22) at 2.024 GHz in the case malignant tumor. Similarly, at 6 mm, in healthy case, the reflection loss of around − 15 dB at an operating frequency of 2.028 GHz while in tumor case there is 1 dB variation at 2.028 GHz, as depicted in Fig. 5d. Moreover, at 10 mm, in healthy case, the reflection loss of around − 15.5 dB at an operating frequency of 2.11 GHz while in tumor case there is − 16.2 dB variation in reflection losses at port1 and port2 at 2.11 GHz, as depicted in Fig. 5e. It is quite evident in Fig. 5 at different distances of sensor from phantom that the sensitivity of detection of tumors is limited. These figures demonstrate a minor variation in S-parameters with tumor presence while small variation in frequency can be observed at different distances from phantom.
The similar analysis is performed by placing antenna-sensor with PRS near to the phantom to detect the presence of tumor. The respective breast equivalent heterogenous phantoms are shown in Fig. 6a and b, respectively with healthy case and malignant case. When the proposed antenna-sensor is placed at 2 mm distance from phantom in healthy tissues both S11 and S22 are observed around − 38 dB and operating frequency of 1.99 GHz, as mentioned in Table 3.
However, in the presence of tumor the S11 is observed of − 32.5 dB while S22 is observed of − 38.8 dB at 1.986 GHz shown in Fig. 6c. Similarly, when both antenna sensors moved away to 6 mm from breast, in healthy case both S11/S22 are observed around − 50 dB with resonating frequency of 2.02 GHz while in presence of tumor, the S11 is observed of − 28.6 dB and S22 is observed of − 47.1 dB at 2.02 GHz shown in Fig. 6d. Further, when both antenna- sensors are moved at 10 mm from the phantom, in healthy case the S11/S22 are observed of − 26.5 dB at 2.04 GHz while in the presence of tumor the S11 is observed of − 22.2 dB and S22 is observed of − 26.4 dB at 2.04 GHz shown in Fig. 6e. From the S-parameters study, it can be concluded that the resonating frequency varies primarily with the location of the antenna sensors, while significant differences in the magnitude of S11 and S22 values indicate the presence of a tumor. The use of PRS greatly enhances the sensor’s ability to detect contrasts in dielectric properties between healthy and malignant tissues, leading to successful tumor detection. With PRS, the antenna sensor’s sensitivity is notably improved, achieving a gain increase from 0.931 to 2.95 dBi when close to the breast phantom.
SAR evaluation and other important parameters
The specific absorption rate (SAR) is calculated to quantify the rate at which the human body absorbs electromagnetic energy16. According to the IEEE C95.1 standard32, the SAR must not exceed 1.6 W/kg for 1 gm mass of tissue to adhere to safety guidelines for radiation exposure. The SAR is assessed for both cases: with a single antenna radiator and with the antenna enabled with PRS. The SAR value for the single antenna is 1.21 W/kg, while with PRS, it increases to 1.59 W/kg, though it remains within safe limits shown in Fig. 7a and b. To ensure compliance with SAR regulations, an input power of 50 milliwatts was used for both scenarios. Despite the notable increase in directive gain with PRS, the SAR value remains only slightly higher and still within safe thresholds. Moreover, the lossy nature of breast tissues affects the antenna’s frequency response due to power absorption across various layers. The penetration of EM waves into tissue layers is essential for identifying dielectric contrasts. The penetration depth determines how far the waves can effectively travel within each tissue layer, aiding in assessing the sensor’s sensitivity to detecting tumors. The penetration depth depends on the skin depth factor, which varies with the dielectric properties of each layer, leading to differing levels of absorption and scattering. Based on loss tangent the material can be classified as good conductor, good dielectric and lossy dielectric. The loss tangent and penetration depth can be evaluated by using the following Eqs. (2) and (3)34,35
if (\(\frac{\sigma }{\omega \varepsilon }\gg 1\)) represents the good conductor or \((\frac{\sigma }{\omega \varepsilon }\ll 1)\) exhibits the good dielectric medium. The corresponding values of loss tangent have been displayed in Table. 4, that confirm the different tissues are lossy dielectrics. The penetration depth in lossy dielectrics is determined by the attenuation constant (1\α) from the relation34
where
Experimental results
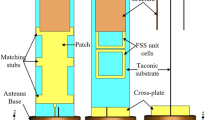
To validate the experimental outcomes, the proposed antenna-sensor is fabricated on an RT Duroid 5880 substrate, featuring a dielectric constant of 2.2, a loss tangent of 0.009 and a thickness of 0.787 mm by using conventional PCB process. Additionally, the PRS is constructed on a Rogers TMM 13i laminate of thickness 3.8 mm of dielectric constant of 12.2 and a loss tangent of 0.0019. The PRS is placed towards the ground plane of antenna with the help of foam material of dielectric constant 1.1. The fabricated prototype’s top and bottom views and antenna under test are presented in Fig. 8. The top- and bottom-plane of monopole radiator is shown in Fig. 8a, while Fig. 8b shows the planes of PRS. The proposed PRS enabled antenna sensor is tested in an anechoic chamber to evaluate the gain and radiation patterns as shown in Fig. 8c and d respectively.
A realistic heterogeneous breast phantom equivalent to female breast tissues is developed using natural materials, such as distilled water, agar powder, propylene glycol, safflower oil, ensuring preservation of the dielectric properties of skin, fat, fibro-glandular tissues, and tumor. The detailed methodology and composition of material in preparing these heterogeneous breast phantoms with and without tumor are comprehensively discussed in33,34. Later, the dielectric constant is measured for each homogeneous layer the help of MS46122B shock line Vector Network Analyzer (VNA) using dielectric resonator method35. The radius of overall phantom is considered 50 mm with dimensions are considered same of simulation studies. The corresponding images of these realistic phantoms can be seen in Fig. 8e and 8f respectively. To ensure the performance of the fabricated antenna-sensor, it is tested for S-parameters along with PRS at two different distances 2 mm and 6 mm. Figure 9a depicts the S11/S22/S12 vs frequency response when antenna-sensors are placed at 2 mm away from the breast phantom. The S11 is observed of − 38 dB while S22 observed of − 48 dB at 2.015 GHz. When antenna sensors moved away further and placed at a distance of 6 mm from the breast, the S11 is observed of − 28.8 dB and S22 is observed of − 52 dB at 2.02 GHz shown in Fig. 9b. Thus, it is quite evident from the measurements results that a significant difference in the magnitude of S11 and S22 are observed in the presence of tumor. Figure 9c, illustrates the antenna-senor setup used for identifying the tumor presence.
Figure 10 illustrates the simulated and measured 2D radiation patterns of proposed PRS enabled monopole antenna at a frequency of 2.02 GHz at two principal cut-planes (ϕ = 0°) and (ϕ = 90°). These plots reveal that the proposed antenna exhibits a unidirectional radiation pattern, attributed to the reflective surface is positioned on the backside of the monopole antenna. The cross-polarization level is measured 20 dB below the co-polarization level in both principal cut planes in the broadside direction, and a front-to-back ratio (FTBR) of 14 dB is observed in the same direction. Table 5 provides a comparative analysis of the proposed antenna-sensor parameters with those of existing designs in the literature. The proposed antenna stands out for its simplicity in sensors is capable to detect the presence of malignant tissues based on its dielectric contrast sensing ability, making it easily functional and effective for tumor detection. The uniqueness of the proposed antenna-sensor lies in its simplicity and high detection ability that can detect the tumor’s presence near the fibro glandular layer due to its low operating frequency 2 GHz and high directive gain.
Unlike many previous studies, this work includes experimental validation using a realistic heterogeneous breast equivalent phantom, closely mimicking the properties of actual breast tissues. The results demonstrate a clear distinction in return loss between the normal breast tissue and the presence of a tumor. Additionally, a consistent agreement is observed between the experimental findings and the simulated results, further confirming the reliability of the proposed design. Moreover, the proposed one offers advantages of miniaturized design that allows for ease of use in clinical settings, a critical advantage over bulkier sensors. Also, it offers a high efficiency of 95–99% across the 1.9–2.1 GHz frequency range, thus it minimizes power loss, ensuring that a high proportion of the input power is directed toward tissue interaction. The proposed sensor setup utilizes two compact antenna-sensors for breast tumor detection, offering a simple yet effective design that enhances portability. Despite its straightforward construction, the system provides a clear distinction between normal and malignant tissues with the help of S-parameters. Optimal matching is maintained without the need for any matching medium or lumped loads when the sensor is in proximity of the tissue.
Conclusion
In this study, we present a novel near-field microwave monopole antenna-sensor incorporating a partially reflective surface (PRS). The PRS-enhanced sensor has a compact 50 × 50 × 18.8 mm3 size, operates at 2 GHz. By integrating the PRS, the sensor achieves higher directive gain, significantly improving its ability to detect deep-seated tumors within breast tissue. The sensor’s effectiveness is demonstrated through numerical simulations, which detect variations in a breast phantom. Validation is performed through measurements on an artificially created heterogeneous phantom, designed to replicate breast tissue properties. A strong correlation is observed between simulated and measured S-parameters within the 1.9–2.1 GHz frequency range. This antenna-sensor offers a viable, non-invasive solution for breast tissue characterization and accurate tumor detection, demonstrating its potential for early-stage breast cancer diagnosis. While the study provides promising results, certain parameters require further exploration. Future research will focus on developing a low-cost, portable breast cancer detecting system using proposed antenna sensor. A detailed investigation will be conducted to analyze power dissipation and energy distribution across different layers of the breast phantom. Understanding microwave energy absorption at varying depths will help optimize the sensor design for improved sensitivity and signal penetration in real-world clinical applications. Furthermore, specific absorption rate (SAR) analysis will be expanded to evaluate the impact of tumor positioning within the breast phantom. Once the system is fully developed and verified for SAR compliance, clinical trials will be conducted in an authorized hospital following all ethical approvals and safety regulations.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Srinivasan, D. & Gopalakrishnan, M. Breast cancer detection using adaptable textile antenna design. J. Med. Syst. 43, 1–10. https://doi.org/10.1007/s10916-019-1314-5 (2019).
De Jesus Aragao, A., Sanches, B., De Carvalho, D. & Van Noije, W. A. M. Low-cost portable system prototype for breast cancer detection using UWB signals. In Proc.—2020 13th Int. Congr. Image Signal Process. Biomed. Eng. Informatics, CISP-BMEI 2020 715–720, https://doi.org/10.1109/CISP-BMEI51763.2020.9263688 (2020).
Kaur, G. & Kaur, A. A low cost and efficient breast cancer detection method with a staircase-shaped ultrawide band dielectric resonator antenna using monostatic radar-based microwave imaging technique. Sadhana Acad. Proc. Eng. Sci. https://doi.org/10.1007/s12046-022-01901-7 (2022).
Devika Menon, M. & Rodrigues, J. Efficient ultra-wideband radar based non-invasive early breast cancer detection. IEEE Access 11, 84214–84227. https://doi.org/10.1109/ACCESS.2023.3303333 (2023).
Porter, E., Coates, M. & Popovic, M. An early clinical study of time-domain microwave radar for breast health monitoring. IEEE Trans. Biomed. Eng. 63, 530–539. https://doi.org/10.1109/TBME.2015.2465867 (2016).
Das, C., Chowdhury, M. Z. & Jang, Y. M. A novel miniaturized mmWave antenna sensor for breast tumor detection and 5G communication. IEEE Access 10, 114856–114868. https://doi.org/10.1109/ACCESS.2022.3216858 (2022).
Elsherif, D. N. & Makkey, M. Y. Early detection of breast cancer using microstrip patch antenna. ICEEM 2021—2nd IEEE International Conference on Electronic Engineering https://doi.org/10.1109/ICEEM52022.2021.9480646 (2021).
Islam, M. T., Mahmud, Md. Z., Misran, N., Takada, J.-I. & Cho, M. Microwave breast phantom measurement system with compact side slotted directional antenna. IEEE Access 5, 5321–5330. https://doi.org/10.1109/ACCESS.2017.2690671 (2017).
Abbak, M., Çayören, M. & Akduman, I. Microwave breast phantom measurements with a cavity-backed Vivaldi antenna. IET Microwaves Antennas Propag. 8, 1127–1133. https://doi.org/10.1049/iet-map.2013 (2014).
El Atlas, N., El Aroussi, M. & Wahbi, M. Computer-aided breast cancer detection using mammograms: A review. 2014 2nd World Conf. Complex Syst. WCCS 2014 6, 626–631, https://doi.org/10.1109/RBME.2012.2232289 (2014).
Islam, M. S., Kaabouch, N. & Hu, W. C. A survey of medical imaging techniques used for breast cancer detection. IEEE Int. Conf. Electro Inf. Technol. 1–5 https://doi.org/10.1109/EIT.2013.6632694 (2013).
Lu, M., Xiao, X., Pang, Y., Liu, G. & Lu, H. Detection and localization of breast cancer using UWB microwave technology and CNN-LSTM framework. IEEE Trans. Microw. Theory Tech. 70, 5085–5094. https://doi.org/10.1109/TMTT.2022.3209679 (2022).
Chaudhary, S. S., Mishra, R. K., Swarup, A. & Thomas, J. M. Dielectric properties of normal & malignant human breast tissues at radiowave & microwave frequencies. PubMed 21, 76–79 (1984).
Islam, M. T., Mahmud, M. Z., Islam, M. T., Kibria, S. & Samsuzzaman, M. A low cost and portable microwave imaging system for breast tumor detection using UWB directional antenna array. Sci. Rep. 9, 1–13. https://doi.org/10.1038/s41598-019-51620-z (2019).
Aldhaeebi, M. A. & Almoneef, T. S. Dipole array sensor for microwave breast cancer detection. IEEE Access 11, 91375–91384. https://doi.org/10.1109/ACCESS.2023.3304694 (2023).
Aldhaeebi, M. A., Almoneef, T. S., Attia, H. & Ramahi, O. M. Near-field microwave loop array sensor for breast tumor detection. IEEE Sens. J. 19, 11867–11872. https://doi.org/10.1109/ACCESS.2023.3304694 (2019).
Afyf, A. et al. Novel antenna structure for early breast cancer detection. Procedia Eng. 168, 1334–1337. https://doi.org/10.1016/j.proeng.2016.11.365 (2016).
Gabriel, C. Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies. https://doi.org/10.21236/ada303903, (1996).
Chaturvedi, D., Ganesh, T. & Kumar, A. Design and Analysis of Wearable Monopole Antenna Sensor. In 2023 Photonics Electromagn. Res. Symp. PIERS 2023—Proc. 2003–2007, https://doi.org/10.1109/PIERS59004.2023.10221430 (2023).
Lee, N. Y. et al. Microwave tomography technology test-bed system for study breast cancer detection technology based on electromagnetic field. 2006 IEEE Antennas and Propagation Society International Symposium 1–4, (2008).https://doi.org/10.1109/aps.2008.4619672
Sugitani, T., Kubota, S., Toya, A., Xiao, X. & Kikkawa, T. A Compact 4 ×: 4 planar uwb antenna array for 3-D breast cancer detection. iEEE Antennas Wirel. Propag. Lett. 12, 733–736. https://doi.org/10.1109/LAWP.2013.2270933 (2013).
Yun, X., Fear, E. C. & Johnston, R. H. Compact antenna for radar-based breast cancer detection. IEEE Trans. Antennas Propag. 53, 2374–2380. https://doi.org/10.1109/TAP.2005.852308 (2005).
Subramanian, S., Sundarambal, B. & Nirmal, D. Investigation on simulation-based specific absorption rate in ultra-wideband antenna for breast cancer detection. IEEE Sens. J. 18, 10002–10009. https://doi.org/10.1109/JSEN.2018.2875621 (2018).
Huang, W. & Kishk, A. A. Compact dielectric resonator antenna for microwave breast cancer detection. IET Microwaves Antennas Propag. 3, 638–644. https://doi.org/10.1049/iet-map.2008.0170 (2009).
Gibbins, D. et al. A comparison of a wide-slot and a stacked patch antenna for the purpose of breast cancer detection. IEEE Trans. Antennas Propag. 58, 665–674. https://doi.org/10.1109/TAP.2009.2039296 (2010).
Meriche, M. A., Attia, H., Messai, A., Mitu, S. S. I. & Dennis, T. A. Directive wideband cavity antenna with single-layer meta-superstrate. IEEE Antennas Wirel. Propag. Lett. 18, 1771–1774. https://doi.org/10.1109/LAWP.2019.2929579 (2019).
Von Trentini, G. Partially reflecting sheet arrays. IRE Trans. Antennas Propag. 4, 666–671. https://doi.org/10.1109/TAP.1956.1144455 (1956).
Attia, H. V-Band High-Gain slot antenna using single layer partially reflective surface. In 2022 16th European Conference on Antennas and Propagation (EuCAP) 877 (4 pp), https://doi.org/10.1049/cp.2018.1236 (2018).
Samantaray, D. & Bhattacharyya, S. A Gain-enhanced slotted patch antenna using metasurface as superstrate configuration. IEEE Trans. Antennas Propag. 68, 6548–6556. https://doi.org/10.1109/TAP.2020.2990280 (2020).
Attia, H., Abdelghani, M. L. & Denidni, T. A. Wideband and high-gain millimeter-wave antenna based on FSS fabry-perot cavity. IEEE Trans. Antennas Propag. 65, 5589–5594. https://doi.org/10.1109/TAP.2017.2742550 (2017).
Konstantinidis, K., Feresidis, A. P. & Hall, P. S. Broadband sub-wavelength profile high-gain antennas based on multi-layer metasurfaces. IEEE Trans. Antennas Propag. 63, 423–427. https://doi.org/10.1109/TAP.2014.2365825 (2015).
Bailey, W. H. et al. Synopsis of IEEE Std C95.1TM-2019 ‘IEEE standard for safety levels with respect to human exposure to electric, magnetic, and electromagnetic fields, 0 Hz to 300 GHz’. IEEE Access 7, 171346–171356. https://doi.org/10.1109/ACCESS.2019.2954823 (2019).
Islam, M. T., Samsuzzaman, M., Kibria, S. & Islam, M. T. Experimental breast phantoms for estimation of breast tumor using microwave imaging systems. IEEE Access 6, 78587–78597. https://doi.org/10.1109/ACCESS.2018.2885087 (2018).
Bhavani, M. V. L., Chaturvedi, D., Lanka, T. & Kumar, A. Development of a QMSIW antenna sensor for tumor detection utilizing a hemispherical multilayered dielectric breast-shaped phantom. IEEE Sens. J. 24, 32080–32089. https://doi.org/10.1109/JSEN.2024.3450990 (2024).
Lazebnik, M. et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 52, 6093–6115. https://doi.org/10.1088/0031-9155/52/20/002 (2007).
Bah, M. H., Hong, J. S. & Jamro, D. A. Study of breast tissues dielectric properties in UWB range for microwave breast cancer imaging. Adv. Comput. Sci. Res. https://doi.org/10.2991/cisia-15.2015.129 (2015).
Moussa, M. N., Madi, M. A. & Kabalan, K. Y. Breast tumor detection, sizing and localization using a 24-element antenna array. IEEE J. Biomed. Heal. Informatics 26, 5109–5121. https://doi.org/10.1109/JBHI.2022.3189640 (2022).
Bhargava, D. & Rattanadecho, P. Microwave imaging of breast cancer: Simulation analysis of SAR and temperature in tumors for different age and type. Case Stud. Therm. Eng. 31, 101843. https://doi.org/10.1016/j.csite.2022.101843 (2022).
Kumar, H. & Kumar, G. Compact planar Yagi-Uda antenna with improved characteristics. In 2017 11th Eur. Conf. Antennas Propagation, EUCAP 2017 2008–2012, (2017).https://doi.org/10.23919/EuCAP.2017.7928412
Hamza, M. N., Koziel, S. & Pietrenko-Dabrowska, A. Design and experimental validation of a metamaterial-based sensor for microwave imaging in breast, lung, and brain cancer detection. Sci. Rep. 14, 1–17 (2024).
Hamza, M. N., Islam, M. T. & Koziel, S. Advanced sensor for non-invasive breast cancer and brain cancer diagnosis using antenna array with metamaterial-based AMC. Eng. Sci. Technol. an Int. J. 56, 101779 (2024).
Imran, A. I. & Elwi, T. A. A cylindrical wideband slotted patch antenna loaded with frequency selective surface for MRI applications. Eng. Sci. Technol. Int. J. 20, 990–996 (2017).
Bashar, B. S. et al. Reconfigurable antenna based CLRH inclusions for 5G wireless networks. In 2024 4th International Conference on Artificial Intelligence and Signal Processing (AISP) 1–5 (IEEE, 2024).
Bashar, B. S. et al. Design of Metasurface Antenna for 5G Applications. In 2024 4th International Conference on Artificial Intelligence and Signal Processing (AISP) 1–4 (IEEE, 2024).
Bashar, B. S. et al. Miniaturized metamaterial antenna for 5.7 GHz services. In 2024 4th International Conference on Artificial Intelligence and Signal Processing (AISP) 1–5 (IEEE, 2024).
Acknowledgements
This work is supported by DST-SERB Power Grant project No. “SPG/2021/002829”.
Author information
Authors and Affiliations
Contributions
Conceptualization: Divya Chaturvedi, Tiruganesh Lanka; Formal analysis: Divya Chaturvedi, Tiruganesh Lanka, Arvind Kumar; Visualization: MVL Bhavani, Tiruganesh Lanka; Writing original draft: Divya Chaturvedi, Tiruganesh Lanka; Writing—review and editing: Arvind Kumar, MVL Bhavani,Tiruganesh Lanka; Software and Resources: Tiruganesh Lanka, Arvind Kumar, Divya Chaturvedi.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lanka, T., Chaturvedi, D., Bhavani, M.V.L. et al. Design of PRS enabled monopole slotted-antenna sensor for breast tumor detection. Sci Rep 15, 19332 (2025). https://doi.org/10.1038/s41598-025-99102-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99102-9
Keywords
This article is cited by
-
An accurate analytical modeling method for microwave-based breast tumor detection and phantom manufacturing
Biomedical Engineering Letters (2025)