Abstract
Rice grain chalkiness occurs when grains fill under heat stress, greatly reducing grain quality. But the effects of heat stress during grain filling on the subsequent development and adaptive traits remain to be elucidated. Here, we evaluated the effects of heat stress during parental grain filling on the thermotolerance of subsequent plants grown under heat stress after anthesis. Subsequent plants were grown from control (25 °C) and heat-exposed (30 °C) parental seeds under natural conditions until anthesis. Then plants were divided into three treatment groups—control [parental plants] – control [subsequent plants] (CC, 25 °C), control–heat (CH, 30 °C), and heat–heat (HH, 30 °C). Plants grown from heat-stressed seeds had thicker and shorter flag leaves, which delayed leaf senescence and improved photosynthesis under heat stress. HH plants also had significantly less chalkiness than those of CH plants. DNA methylation analysis revealed that heat stress during grain filling significantly hypomethylated promoters of starch biosynthesis genes and hypermethylated those of α-amylase genes. Consequently, HH plants had significantly higher expression of starch biosynthesis genes and suppressed expression of starch-degrading α-amylase genes than CH plants under heat stress. We propose that heat stress during grain filling induces adaptive transgenerational memory that allows subsequent plants to better cope with heat stress.
Similar content being viewed by others
Introduction
Global temperatures due to climate change are compromising agricultural production and food security worldwide. Rice (Oryza sativa L.), one of the world’s most important staple crops, is highly affected by high-temperature stress; every 1°C increase in minimum temperature during the growing season reduces total yield by ~ 10%1. In japonica rice, air temperature above 26 °C during grain filling causes grain chalkiness, a major cause of quality2,3,4 . Heat stress during grain filling downregulates starch-biosynthesis–related genes such as OsAGPS2b (ADP-Glc pyrophosphorylase subunit 2b), OsGBSSI (granule-bound starch synthase), and OsSuSy2 (sucrose synthase 2), and upregulates the genes encoding a starch-degrading enzyme, α-amylase (OsAmy), resulting in loosely packed starch granules with an opaque and chalky appearance2,3,5. Although many studies have proposed mechanisms of the effects of heat stress during grain filling on developing rice grains, the effects on the growth and development of subsequent plants remain to be elucidated.
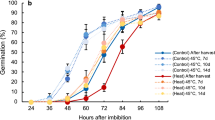
In many plant species, parental responses to environmental changes are passed on to the offspring6,7. In Arabidopsis thaliana, heat stress during parental seed development significantly alters the development of progeny: plants whose parents were heat-stressed acquired thermotolerance during reproductive stage6 and altered flowering time8. This so-called ‘transgenerational memory’ or ‘epigenetic memory’ occurs in a wide range of plant species, including rice, wheat, Arabidopsis and peanut, under various stress conditions, promoting adaptations in progeny7,9,10,11. Although many studies have demonstrated such effects, molecular analysis to identify key genes and underlying mechanisms are still limited. These transgenerational effects are known to be transferred through epigenetic markers, such as histone modifications and DNA methylation12,13,14. DNA methylation plays an important role in long-term memory of responses to developmental and environmental stresses via gene regulation15,16. In plants, it occurs in three contexts—CG, CHG, and CHH (where H stands for A, T, or C)—in which DNA methylation in the promoter region usually inhibits gene transcription17. In rice, the acquisition by successive generations of plants treated with repeated drought stress of non-random DNA methylation status for favorable phenotypic changes13 suggests a crucial role of DNA methylation in abiotic stress adaptations. We have reported that heat stress during grain filling significantly delays subsequent seed germination in rice and induces it in barley, through the epigenetic regulation of seed DNA methylation18,19. In rice, heat stress during grain filling induces hypermethylation of germination-related genes, including those encoding α-amylase, delaying germination by downregulating the expression of these genes after imbibition18, suggesting that heat-stress–induced changes in seed DNA methylation might lead to phenotypic changes during the subsequent development.
Here, we focused on whether heat stress during parental grain filling causes phenotypic changes that confer thermotolerance of subsequent plants under heat stress during their grain filling. We hypothesized that epigenetic regulation induced by heat stress during grain filling has transgenerational effects on starch metabolism genes18,19. As heat stress during grain filling hypermethylates promoters of α-amylase genes in seeds, downregulating expression after imbibition18, we expected that it would alter seed DNA methylation levels specifically in promoters of starch-metabolism–related genes to cause transcriptional changes in subsequent plants that alter grain chalkiness. Our findings show that transgenerational effects in heat-exposed seeds can increase grain quality of subsequent plants that develop under heat stress, a promising way of improving rice production under global warming.
Results
Heat stress during parental grain filling altered flag leaf morphology and induced thermotolerance of subsequent plants grown under heat stress
At anthesis, flag leaves of subsequent plants grown from heat-exposed seeds were significantly shorter and thicker with increase in mesophyll cell layer than those of plants grown from control seeds, although leaf width was unchanged (Fig. 1A–F). These changes in leaf phenotype were not observed in penultimate leaves (Supplemental Fig. 1A–C). During grain filling under heat stress, SPAD values of CH plants (whose parents were not exposed to heat stress during grain filling) gradually decreased, indicating leaf senescence due to heat stress (Fig. 2A). However, those of HH plants (whose parents were exposed to heat stress) indicated significantly delayed leaf senescence (Fig. 2A), with less leaf oxidative damage, as the endogenous ROS content was significantly lower in HH than in CH (= CC) at 20 DAF (Supplemental Fig. 2). Heat stress significantly decreased photosynthetic rate, stomatal conductance, and transpiration rate in CH flag leaves relative to CC (control) (Fig. 2B–D), but significantly increased them in HH flag leaves relative to CH. These results suggest that HH plants had higher leaf thermotolerance under heat stress during grain filling (Fig. 2B–D). Combined with the leaf morphological changes, the results suggest that heat stress during parental grain filling induced thermotolerance in HH plants, delaying leaf senescence and promoting photosynthesis under heat stress.
Heat stress during grain filling caused flag leaf morphological changes of subsequent plants. A Flag leaf morphology and B longitudinal leaf section stained with 0.2% toluidine blue. C Flag leaf length (n = 20), D thickness (n = 10), E cell layer (n = 5), and F width (n = 10, N.S., not significant) of plants grown from control and heat-developed seeds (error bars represent SD). Significant differences are shown at **P < 0.01 according to Student’s t-test.Heat stress during grain filling caused flag leaf morphological changes of subsequent plants. A Flag leaf morphology and B longitudinal leaf section stained with 0.2% toluidine blue. C Flag leaf length (n = 20), D thickness (n = 10), E cell layer (n = 5), and F width (n = 10, N.S., not significant) of plants grown from control and heat-developed seeds (error bars represent SD). Significant differences are **p<0.01 according to Student’s t-test.
Flag leaf thermotolerance of subsequent plants subjected to heat stress during grain filling. A Flag leaf SPAD values from flowering to harvest of control [parent] – control [subsequent] (CC), control–heat (CH), and heat–heat (HH) plants (C = 25 °C, H = 30 °C) exposed during grain filling (error bars represent SE, n = 4). B Photosynthetic rate, C stomatal conductance, and D transpiration rate at 26 DAF (days after flowering, error bars represent SD, n = 3). Values with the same letter are not significantly different at P < 0.05 by Tukey’s test..
Heat stress during parental grain filling improved grain quality of subsequent plants grown under heat stress
Heat stress during grain filling significantly increased chalkiness in grains of CH plants relative to grains of CC plants (Fig. 3). However, HH plants developed significantly less chalkiness relative to grains of CH plants (Fig. 3). In addition, HH plants had fewer white-belly and white-backed grains than CH plants. These results suggest that parental exposure to heat stress during grain filling induces thermotolerance in subsequent plants, resulting in improved grain quality due to reduced chalkiness under heat stress.
Heat stress during grain filling alters DNA methylation levels of starch-metabolism–related gene promoters in seeds
To elucidate the key factors behind the inhibition of grain chalkiness in subsequent plants of heat-exposed parents, we focused on starch biosynthesis genes, which are downregulated, and starch-degrading (α-amylase) genes, which are upregulated by heat stress during grain filling, promoting chalkiness in rice2,3,5. We expected that heat stress during grain filling would alter seed DNA methylation levels specifically in promoters of starch-metabolism–related genes to cause transcriptional changes in subsequent plants that alter grain chalkiness. The promoter regions of starch biosynthesis genes OsAGPS2b, OsGBSSI, and OsSuSy2 were significantly hypomethylated in heat-developed seeds relative to the control (Fig. 4A). Conversely, those of α-amylase genes OsAmy1A and OsAmy3D were significantly hypermethylated relative to the control (Fig. 4B). Thus, heat stress during parental grain filling significantly altered seed DNA methylation levels of starch-metabolism–related gene promoters, which was considered to contribute to transcriptional changes of subsequent plants.
Relative DNA methylation levels of starch biosynthesis and starch-degrading gene promoters in control seeds and heat-developed seeds. Relative methylation levels at promoters of A starch biosynthesis and B starch-degrading genes in control and heat-developed parental seeds. Plots show data from 4 biological replicates. Significant differences were calculated according to Student’s t-test—P < 0.05*.
Transgenerational heat stress memory induces transcriptional changes of starch metabolism genes in developing grains of subsequent plants under heat stress
We analyzed the transcriptional levels of starch biosynthesis genes and α-amylase genes in the grains of subsequent plants during grain filling under heat stress by qRT-PCR. In developing seeds at 15 DAF, the expression of starch biosynthesis genes was significantly downregulated in grains of CH plants (grown at 30 °C) relative to those of CC (control) plants, especially that of OsGBSSI, which was reduced to 31.5% (Fig. 5A). The expression of α-amylase genes was significantly upregulated in grains of CH plants relative to those of CC plants (OsAmy1A to 94.2×, OsAmy3D to 4.89×) (Fig. 5B). The significant downregulation of starch biosynthesis genes and upregulation of α-amylase genes in CH confirm the effects of heat stress during grain filling on developing grains. Conversely, the expression of starch biosynthesis genes was significantly upregulated in grains of HH plants (whose parents were exposed to heat stress) relative to those of CH plants (OsAGPS2b to 2.2×, OsGBSSI to 2.5×, and OsSuSy2 to 2.5×) under the same heat stress condition (Fig. 5A). And the expression of α-amylase genes was significantly downregulated in grains of HH plants relative to those of CH grains (OsAmy1A to 49%, OsAmy3D to 29.4%) (Fig. 5B). However, heat stress did not enhance the expression of OsAmy3D relative to CC. These results show that starch biosynthesis was increased and starch degradation was decreased in grains of HH plants relative to those of CH plants under the same heat stress conditions, in negative correlation with seed DNA methylation levels of the gene promoters. Thus, heat stress during grain filling altered seed DNA methylation levels in the promoters of starch-metabolism–related genes, which regulated transcriptional changes in developing seeds under heat stress and improved grain quality.
Relative expression of starch-metabolism–related genes during grain filling of subsequent plants at 15 DAF. A Starch biosynthesis genes OsAGPS2b, OsGBSSI, OsSuSy2; B α-amylase genes OsAmy1A, OsAmy3D. Error bars show SD of total chalky grain percentage (n = 3). Bars with the same letter are not significantly different at P < 0.05 by Tukey’s test..
Discussion
Heat stress during grain filling of parental plants improved grain quality and increased the thermotolerance of subsequent plants under heat stress. Plants grown from heat-developed seeds had shorter but thicker flag leaves. In perennial ryegrass (Lolium perenne L.) under moderate heat stress, leaf thickness and leaf H2O2 content had a significant negative correlation, and leaf thickness and a photosynthetic parameter (Fv/Fm) had a positive correlation, indicating the importance of leaf thickness in oxidative damage due to H2O2 generation and photosynthetic activity under heat stress20. It was suggested that shorter and thicker flag leaves of HH plants was one of the factors that might contribute to delayed leaf senescence on account of lower endogenous H2O2 levels, with a higher photosynthetic rate, stomatal conductance, and transpiration rate under heat stress than in CH plants. As the flag leaf photosynthetic rate during grain filling and chlorophyll content are important factors that counter grain chalkiness in rice21, we assume that the flag leaf morphological and physiological changes conferred thermotolerance in HH plants. To be noted, the morphological changes of penultimate leaves were not observed, suggesting that this phenomenon might be specific to flag leaves. To the best of our knowledge, no key gene that determines flag leaf length and thickness without affecting leaf width in cereals has yet been identified, so how heat stress during grain filling affected subsequent leaf morphological change at the molecular level remains to be investigated.
HH plants (whose parents had been exposed to heat stress during grain filling) was less affected by heat stress and showed better grain quality than CH (whose parents had not been exposed) plants under the same heat stress. At high temperatures, rice plants develop chalky grains owing to transcriptional changes in starch-metabolism–related genes2,3,5. Heat stress during grain filling significantly upregulates α-amylase gene expression in developing grains5,22. However, it also causes hypermethylation of α-amylase promoter regions in fully developed seeds, which suppresses α-amylase gene expression during seed germination18. In barley, it causes hypomethylation of genes for gibberellic acid biosynthesis (HvGA3ox) and abscisic acid catabolism (HvABA8’OHs) in seeds, enhancing their transcription after imbibition to promote seed germination19. These findings indicate roles of seed DNA methylation status in transcriptional changes during the later phase of plant development to determine phenotypic changes in subsequent plants.
Heat stress during grain filling significantly hypomethylated starch biosynthesis gene promoters and hypermethylated α-amylase gene promoters in fully developed seeds. As hypermethylation plays an important role in transcriptional suppression (and hypomethylation in gene activation)17, the negative relationship between transcriptional level and seed DNA methylation of the promoters of starch-metabolism–related genes was revealed. Under high temperatures, starch biosynthesis genes are usually suppressed, leading to insufficient starch accumulation as one of the main factors causing chalkiness2,3. However, parental exposure to heat stress during grain filling led to hypomethylation of starch biosynthesis gene promoters, resulting in significantly higher gene expression in HH plants than in CH plants. A previous study reported the critical role of α-amylase in chalkiness induction under heat stress: RNAi-mediated suppression of α-amylase gene expression in developing grains resulted in fewer chalky grains under high-temperature stress5. Here, OsAmy1A and OsAmy3D expression was highly induced by heat stress in CH, as reported previously2,5. However, transgenerational memory induced by heat stress during grain filling of parental plants significantly inhibited this α-amylase gene expression in HH plants, especially that of OsAmy3D, whose expression was suppressed to the same level as in CC plants. Since OsAmy3D is expressed in the ventral side of grains under heat stress23, we suspect that strong suppression due to hypermethylation of its promoter might play an important role in reducing belly-white chalky grains of HH plants.
Recent studies have reported stress-induced transgenerational effects of the parental environment on the adaptive traits of progeny. In common wheat (Triticum aestivum L.), heat stress priming during grain filling also leads to higher grain yield, photosynthetic rate, and antioxidant activity in progeny grown under heat stress7. Progeny of durum wheat (Triticum turgidum L.) parents subjected to drought stress during the reproductive stage show acquired stress tolerance in terms of yield performance and grain quality traits24. Besides cereals, peanut (Arachis hypogaea L.) showed transgenerational memory under water deficit stress, with better seedling emergence and greater yield than progeny of unstressed parents under field conditions10. These findings, together with our results, suggest that changes in parental environments could play an important role in determining agronomic traits of progeny in a wide range of crop plants. Our study also suggests that transgenerational memory induced by seed DNA methylation level is one of the factors involved in subsequent acquired thermotolerance. Besides DNA methylation, other epigenetic markers such as histone modification14 and post-transcriptional regulation of miRNA24, which are also reported to play important roles in subsequent memory, need to be elucidated for the further explanation of the underlying mechanisms.
In conclusion, our study shows that heat stress during parental grain filling can induce thermotolerance of subsequent plants through morphological and transcriptional changes, offering a promising and sustainable way to improve agricultural production under global warming.
Materials and methods
Plant materials and cultivation methods
Ten 3-week-old seedlings of rice (Oryza sativa L. ‘Nipponbare’), which developed from fully germinated seeds, were transplanted into a 1/5000-a Wagner pot with 8.75 g of basal fertilizer (4% N, 4% P2O-equivalent, 4% K2O-equivalent) and 0.85 g of sigmoid-type coated urea (N: 41%) in mid June 2020. Plants were grown under natural conditions at the Kyushu University Ito campus farm (33°37′N, 130°25′E), Fukuoka, Japan. An additional 0.5 g of ammonium sulfate (21% N) was applied twice, at the maximum tillering stage and the panicle booting stage. At the maximum tillering stage, tillers were removed, leaving only the main stem to develop. Anthesis, defined as the day when more than 50% of the spikelets in the panicle were fertilized in 50% of panicles, was set as 0 days after flowering (0 DAF). At 0 DAF, plants were transferred to two constant-temperature treatments, control (25 °C) and heat (30 °C), in growth chambers in a phytotron (Biotron Center, Kyushu University) under natural light with 70% humidity. Seeds from each treatment were harvested at 49 DAF, dried at room temperature for 1 week, and stored at − 30 °C to maintain vigor before sowing in the next season (Fig. 6A).
In the next season, seeds were sown and grown as above. At anthesis, plants grown from seeds of each treatment were divided into three treatment groups—control–control (CC, 25 °C), control–heat (CH, 30 °C), and heat–heat (HH, 30 °C)—with names representing the parent and subsequent plant temperatures during grain filling until harvest at 49 DAF (Fig. 6B).
Evaluation of flag leaf phenotype
After the flag leaves had fully emerged, their lengths were measured. The centralof each was collected and stored in formalin aceto-alcohol (FAA; 5:5:50:40 formaldehyde: acetic acid: ethanol: distilled water) before analysis. On a cryostat (CM 1950, Leica Microsystems K.K.), 15-µm-thick samples were cut in FSC 22 blue compound (Leica Microsystems K.K.), stained with 0.2% toluidine blue and viewed under a microscope (BZ-X710, Keyence); leaf thickness (n = 20), width (n = 10), and number of mesophyll cell layers adjacent to vascular bundles (n = 5) were measured in ImageJ v. 12.5a software. The average thickness and cell layer of both sides of vascular bundles at four distinct places on the same leaf center gave the average leaf thickness.
Photosynthetic rate, stomatal conductance and transpiration rate, and SPAD measurements
At 26 DAF, a sunny day, between 08:00 and 14:00, leaf photosynthetic rate, stomatal conductance, and transpiration rate were measured in the middle of flag leaves (n = 3) with an LCi Portable Photosynthesis System (LI-COR Biosciences) at a chamber temperature of 25 °C (CC) or 30 °C (CH and HH), under photosynthetically active radiation of 1500 µmol photons m− 2 s− 1 in atmospheric CO2. SPAD values were measured every 5 days with a SPAD-502 Plus meter (Konica Minolta). Three flag leaves from each pot were measured at the top, middle, and bottom to generate an average SPAD value (n = 4). The same flag leaves were measured at the same three spots every time.
Endogenous ROS content of flag leaves
At 20 DAF, flag leaf samples (pooled from three leaves per one biological replicate; total n = 3) were weighed and were then homogenized in 2 mL of 0.2 M perchloric acid on ice. The homogenates were centrifuged at 13 000 rpm at 4 °C for 15 min. We added 0.2 mL of 4 M KOH to 0.2 mL of sample supernatant and centrifuged it at 1000× g at 4 °C for 3 min. The endogenous H2O2 content was measured with a peroxidase-based assay with 3-dimethylaminobenzoic acid and 3-methyl-2-benzothiazolinone hydrazone as previously described25,26.
Analysis of degree of grain chalkiness
Grains were harvested from each pot (= 1 replicate; total n = 5) in each treatment group and were dried at room temperature for 1 week. Hulled grains were filtered through a 1.7-mm sieve and analyzed to determine the degree of grain chalkiness as previously described7.
Gene expression analysis via quantitative real-time PCR
At 15 DAF, we sampled developing grains to collect one biological replicate from each pot and stored them at − 80 °C (n = 3). Total RNA was extracted from 0.5 g of frozen grains by using the SDS/phenol/LiCl method27. cDNA was synthesized from the extracted RNA using ReverTra Ace reverse transcriptase (Toyobo) as in the manufacturer’s protocol. qRT-PCR was performed in a CFX Connect Optics Module real-time PCR detector (Bio-Rad) with SYBR green (Toyobo) according to the manufacturers’ instructions, using primers synthesized by Sigma-Aldrich (Supplemental Table 1) with PCR thermal cycling conditions as described in our previous study (Suriyasak et al. 2020). The results were normalized by the ddCt method28. to the expression level of the OsActin housekeeping gene.
DNA methylation analysis using methylated DNA immunoprecipitation–qPCR
We extracted genomic DNA from 40 seeds, giving one biological replicate (total n = 4), of control and heat-developed seeds with a DNeasy Plant Mini Kit (Qiagen). The DNA was sheared to about 300–500 bp by sonication (Picoruptor2, Diagenode). We immunoprecipitated sheared DNA (1 µg) with a Methylated DNA Immunoprecipitation Kit (Active Motif) according to the manufacturer’s protocol. We then performed methylated DNA immunoprecipitation–qPCR (MeDIP-qPCR) according to the manufacturer’s method to identify the locus-specific relative DNA methylation level, using the primers listed in Supplemental Table 2.
Statistical analysis and visualization software
Statistical analyses were performed in SPSS software v. 28.0.0.0 (IBM). Differences among treatments were analyzed by one-tailed Student’s t-test and Tukey’s test. Graphs were generated and visualized in OriginPro 2021b (OriginLab) and Excel v. 16.90.2 software.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary information files.
References
Peng, S. et al. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA. 101, 9971–9975 (2004).
Yamakawa, H., Hirose, T., Kuroda, M. & Yamaguchi, T. Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant. Physiol.144, 258–277 (2007).
Tanamachi, K. et al. Differential responses to high temperature during maturation in heat-stress-tolerant cultivars of Japonica rice. Plant. Prod. Sci.19, 300–308 (2016).
Miyahara, K. et al. Identification of chromosome regions for high-temperature tolerance in the Japonica rice cultivar ‘genkitsukushi’ (Oryza sativa L). Plant. Prod. Sci.26, 88–99 (2022).
Hakata, M. et al. Suppression of α-amylase genes improve quality of rice grain ripened under high temperature. Plant. Biotechnol. J.10, 1110–1117 (2012).
Whittle, C. A., Otto, S. P. & Johnston, M. O. Krochko J. E. Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany87, 650–657 (2009).
Wang, X. et al. Heat priming induces transgenerational tolerance to high temperature stress in wheat. Front. Plant. Sci.7, 1–12 (2016).
Groot, M. A. et al. Transgenerational effects of mild heat in Arabidopsis thaliana show strong genotyping specificity that explained by climate at origin. New. Phytol. 215, 1221–1234 (2017).
He, Y. & Li, Z. Epigenetic environmental memories in plants: establishment, maintenance, reprogramming. Trends Genet.34, 856–866 (2018).
Racette, K., Zurweller, B., Tillman, B. & Rowland, D. Transgenerational stress memory of water deficit in peanut production. Field Crops Res.248, 107712 (2020).
Perrella, G., Baurle, I. & Zanten, M. V. Epigenetic regulation of thermomorphogenesis and heat stress tolerance. New. Phytol. 234, 1144–1160 (2022).
Kou, H. P. et al. Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance by progenies to the stress in rice. J. Plant. Physiol.168, 1685–1693 (2011).
Zheng, X. et al. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci. Rep.7, 39843 (2017).
Liu, J. et al. An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell. Res.29, 379–390 (2019).
Crisp, P. A., Ganguly, D., Eichten, S. R., Borevitz, J. O. & Pogson, B. J. Reconsidering plant memory: intersections between stress Recovedry, RNA turnover, and epigenetics. Sci. Adv.2, e1501340 (2016).
Lang, Z. et al. Critical roles of DNA demethylation in the activation of ripening-induced genes and Inhibition of ripening-repressed genes of tomato fruit. Proc. Natl. Acad. Sci. U S A. 114, E4511–E4519 (2017).
He, L. et al. DNA methylation-free Arabidopsis reveals crucial roles of DNA methylation in regulating gene expression and development. Nat. Commun.13, 1335 (2022).
Suriyasak, C. et al. Mechanism of delayed seed germination caused by high temperature during grain filling in rice (Oryza sativa L). Sci. Rep.10, 17378 (2020).
Sakai, Y., Suriyasak, C., Inoue, M., Hamaoka, N. & Ishibashi, Y. Heat stress during grain filling regulates seed germination through alterations of DNA methylation in barley (Hordeum vulgare L). Plant. Mol. Biol.110, 325–332 (2022).
Soliman, W. S., Fujimori, M., Tase, K. & Sugiyama, S. Heat tolerance and suppression of oxidative stress: comparative analysis of 25 cultivars of the C3 grass Lolium perenne. Environ. Exp. Bot.78, 10–17 (2015).
Yang, Y. et al. PGL, encoding chlorophyllide a Oxygenase 1, impacts leaf senescence and indirectly affects grain yield and quality in rice. J. Exp. Bot.67, 1297–1310 (2016).
Suriyasak, C. et al. Reactive oxygen species induced by heat stress during grain filling of rice (Oryza sativa L.) are involved in occurrence of grain chalkiness. J. Plant. Physiol.216, 52–57 (2017).
Nakata, M. et al. High temperature-induced expression of rice α-amylases in developing endosperm produces Chalky grains. Front. Plant. Sci.8https://doi.org/10.3389/fpls.2017.02089 (2017).
Liu, H., Able, A. J. & Able, J. A. Small RNAs and their targets are associated with transgenerational effects of water-deficit stress in durum wheat. Sci. Rep.11, 3613 (2021).
Ishibashi, Y. et al. Reactive oxygen species are involved in Gibberellin/abscisic acid signaling in barley aleurone cells. Plant. Physiol.158, 1705–1714 (2012).
O’Kane, D., Gill, V. & Burdon, R. Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta198, 371–377 (1996).
Chirgwin, J. M., Przybyla, E., MacDonald, R. J. & Rutter, W. J. Isolation of biological active ribonucleic acid from sources enriched in ribonuclease. Biochemistry198, 371–377 (1979).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucl. Acids Res.29, 2002–2007 (2001).
Acknowledgements
We express our gratitude to the staffs of Kyushu University, Center for Advanced Instrumental and Educational Supports, particularly Ishihara D., Kawamura T. and Matsuishi T. for crop cultivation assistance.
Author information
Authors and Affiliations
Contributions
S.C., H.N., and I.Y. designed the experiments, S.C., K.R., M.R., S.Y., N.TH. and H.N. performed the experiments, S.C., and Y.I. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Suriyasak, C., Kawaguchi, R., Matsumoto, R. et al. Adaptive memory induced by heat stress during grain filling enhances subsequent thermotolerance in rice (Oryza sativa L.). Sci Rep 15, 14135 (2025). https://doi.org/10.1038/s41598-025-99146-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99146-x