Abstract
The study aimed to evaluate the relationship between immunonutritional indicators such as the systemic immune-inflammation index (SII), the Naples prognostic score (NPS), nutritional risk index (NRI), serum albumin (ALB), total cholesterol (TC) and all-cause mortality in adult stroke patients. Data were obtained from the National Health and Nutrition Examination Survey (NHANES) databases for 2005–2018. To determine mortality outcomes, participants were matched with National Death Index records until December 31, 2019. Spearman’s correlation analysis and the random survival forest (RSF) were employed to assess the relationships among NPS, NRI, SII, ALB, and TC, and to determine the most predictive indicator for all-cause mortality in stroke patients. For the selected prognostic indicator, Kaplan–Meier survival analysis and Cox proportional hazards regression models were subsequently utilized to evaluate their associations with all-cause mortality in stroke patients. The study included 1076 stroke patients, with a median (IQR) age of 67 (56, 77) years. During a median follow-up of 67 months, a total of 372 (weighted 31%) stroke participants died from all causes. Among the immunonutritional indicators evaluated, NPS had the strongest predictive power for all-cause mortality in stroke patients. The Kaplan–Meier curve and Log-rank test showed that all-cause mortality was higher in the higher NPS group (3–4) compared to the lower NPS group (0–2) (P < 0.001). After adjusting for multiple potential confounders, the Cox regression model indicated that the higher NPS (3–4) group remained an independent predictor for higher all-cause mortality risk (HR = 1.89, 95% CI 1.44–2.47, P < 0.001). As a comprehensive evaluation index of inflammation and nutrition, NPS is a powerful predictor of all-cause mortality in stroke patients.
Similar content being viewed by others
Introduction
Stroke is an acute neurological disorder characterized by focal dysfunction resulting from the cerebral artery blockage and insufficient blood supply to the brain. This condition initiates a cascade of pathophysiological events, including an inflammatory response, excitotoxicity, calcium overload, and oxidative stress1. Clinical symptoms include hemiplegia, aphasia, depression, dysphagia, and cognitive impairment2. Globally affecting approximately 17.95 million people and causing an estimated 630,000 stroke-related deaths annually, stroke ranks as the second leading cause of mortality and the third largest contributor to disability-adjusted life years (DALYs) lost worldwide3,4. Consequently, it presents a significant public health challenge that severely threatens human health.
In stroke prevention and treatment, prognostic indicators serve as crucial tools for enhancing risk stratification and predicting patient survival outcomes. The National Institutes of Health Stroke Scale (NIHSS), while widely used for neurological assessment5, demonstrates suboptimal prognostic accuracy in ischemic stroke (73.7% sensitivity, 74.1% specificity)6. The Glasgow Coma Scale (GCS) evaluates consciousness but proves inadequate for sedated/intubated patients7, whereas the Barthel Index quantifies daily living functionality while neglecting post-stroke cognitive impacts8. Similarly, the SF-36 quality-of-life tool suffers from significant floor/ceiling effects limiting clinical utility9 While conventional neurological assessment tools primarily evaluate consciousness and physical function, their efficacy remains operator-dependent and is compromised by patient cooperation, with a limited capacity for dynamic acute-phase monitoring. In contrast, inflammation- and nutrition-related biomarkers derived from routine laboratory data objectively quantify the severity of neural injury while providing critical insights into inflammatory responses, immune competence, and coagulation status, thus enabling a comprehensive characterization of disease progression.
Stroke is a multifactorial pathology characterized by inflammation as a central mediator, with elevated inflammatory markers correlating with increased incidence and mortality10,11,12. Stroke typically triggers inflammatory responses, including the release of cytokines and chemokines, which initiate pathological processes such as vascular endothelial injury and thrombosis, ultimately resulting in brain tissue damage. Chronic inflammation can induce insulin resistance through inflammatory factors like tumor necrosis factor and C-reactive protein (CRP)13,14 leading to decreased serum albumin levels and body weight12,15. Previous studies have established strong associations between albumin levels16,17,18 and body mass index (BMI)19 with stroke severity and functional outcomes. Inflammation directly influences stroke prognosis and does so indirectly through the modulation of albumin and body weight. The interdependence among nutritional, immune, and prognostic parameters underscores the necessity for multidimensional indicator panels to enable a comprehensive assessment.
Immunonutrition is an emergent and interdisciplinary subject since it comprises various aspects related to nutrition, immunity, infections, inflammation, injury, or tissue damage20. The systemic immune-inflammation index (SII), nutritional risk index (NRI), and the Naples prognostic score (NPS) are biomarkers used to assess immunonutritional status in various disease conditions. Elevated SII correlates with increased mortality, recurrence risk, and functional impairment severity in stroke patients21. Lower NRI is associated with adverse outcomes in stroke patients, including higher mortality rates, prolonged hospitalization, and increased complications19. The NPS is a novel scoring system that combines inflammatory and nutritional assessments, initially developed for prognostic evaluation in colorectal cancer22. These indices are calculated using the following parameters: lymphocyte, neutrophil, and monocyte counts, serum albumin (ALB), total cholesterol (TC), height, and weight. These parameters offer advantages such as low cost, ease of acquisition, high accuracy, and good reproducibility, demonstrating significant potential for broad clinical application across various diseases.
Currently, evidence regarding the immunonutritional status and all-cause mortality in stroke patients is limited, and the prognostic significance of immunonutritional indicators remains unclear. This study aims to address these gaps by utilizing data from the National Health and Nutrition Examination Survey (NHANES) from 2005 to 2018, in conjunction with National Death Index (NDI) records up to December 31, 2019. The research investigates five immunonutritional indicators (NPS, NRI, SII, ALB, and TC) in stroke patients and their association with all-cause mortality. The objective is to identify prognostic assessment indicators for stroke with greater predictive value, thereby enhancing risk stratification and providing theoretical support for the development of prevention and treatment strategies.
Material and methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a nationwide study assessing the nutritional status of U.S. residents. Detailed data are available at https://www.cdc.gov/nchs/nhanes/index.htm. National Death Index (NDI) records were matched with NHANES data to obtain participant mortality information as of December 31, 2019. Participants without recorded deaths were considered alive throughout the follow-up period. Survival time was defined as the number of months between the NHANES interview date and the date of death or census. All-cause mortality encompasses death from any cause, including malignancy, chronic lower respiratory disease, influenza, pneumonia, and other diseases23.
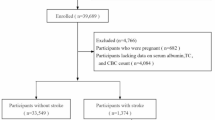
Stroke was determined based on the results of the self-administered standard questionnaire24. Specifically, when participants answered “yes” to the question “Ever told you had a stroke,” they were considered to have had a stroke. From the 70,190 participants in NHANES 2005–2018, this study excluded those under 20 years of age (N = 30,441), without a history of stroke (N = 38,157), and with missing stroke assessment data (N = 1). Additionally, participants lacking follow-up information (N = 84), hematological assessment data (N = 132), relevant covariates (N = 286), or with a Probability Sampling Unit (PSU) of 1 (N = 13) were excluded. The final sample comprised 1,076 stroke patients to evaluate the relationship between immunonutritional indicators and all-cause mortality (Fig. 1). The NHANES protocols were approved by the ethics review board and included the written informed consent of all participants, following the principles of the Declaration of Helsinki.
Assessment for Immunonutritional Indicators
The NPS is a comprehensive indicator reflecting both the nutritional and inflammatory status of patients, frequently used for prognostic assessment in oncology25. NPS was calculated from ALB, TC, the neutrophil–lymphocyte ratio (NLR), and lymphocyte–monocyte ratio (LMR). The scoring method is as follows: ALB ≥ 40 g/L, TC > 180 mg/dL, NLR < 2.96, or LMR > 4.44 was scored as 0, while ALB < 40 g/L, TC ≤ 180 mg/dL, NLR ≥ 2.96, or LMR ≤ 4.44 was scored as 126,27. NPS is the sum of the scores of each of the four factors26,27. NPS of 0 indicates normal nutritional status, whereas scores of 1–2 and 3–4 correspond to mild and moderate to severe malnutrition, respectively.
The Systemic Immune-inflammation Index (SII) integrates two components of the immune response: the innate immune response, primarily driven by neutrophils, and the adaptive immune response, supported by lymphocytes28. It serves as a prominent and stable index that reflects both local immune responses and systemic inflammatory markers. The SII was calculated as (platelet count × neutrophil count)/lymphocyte count.
The NRI is a widely used screening tool that objectively assesses nutritional status based on ideal weight, actual weight, and serum albumin level29. A higher NRI indicates a lower risk of malnutrition19. NRI = [(1.519 × serum albumin, g/dL) + (41.7 × weight (kg)/ideal body weight (IBW; kg))]30. Ideal body weight is determined via the Lorentz formula: for males, it is calculated as height (cm) − 100 − [(height (cm) − 150)/4]; for females, height (cm) − 100 − [(height (cm) − 150) / 2.5].
Baseline assessment
Baseline data on study participants were collected through questionnaires, physical examinations, and laboratory tests. The assessed variables included age (in years), sex (male or female), race (non-Hispanic white or other), ratio of family income to poverty (PIR), education level (categorized as lower than high school graduate, high school graduate or GED, some college and college graduate or above), body mass index (BMI, kg/m2), smoking status (non-smokers were defined as individuals who had smoked fewer than 100 cigarettes in their lifetime; others were classified as smokers), alcohol intake (non-drinkers were those who consumed fewer than 12 alcoholic drinks in the past year; others were considered drinkers), and physical activity levels (low, moderate, and high, with metabolic equivalent of task (MET) values of < 600, 600–3000, and > 3000, respectively). Additionally, the disease history was assessed using a standard self-administered questionnaire24. Specifically, participants were considered to have hypertension if they responded ‘yes’ to the question, 'Ever told you had high blood pressure?'. Similarly, individuals who answered ‘yes’ to ' Doctor told you have diabetes?' were classified as having diabetes.
Statistical analysis
In accordance with NHANES recommendations, the statistical analysis of this study considered sample weights, clustering, and stratified analysis. Whether the data were normally distributed was analyzed through visuals (histograms), and analytical methods (Kolmogorov–Smirnov Test). Since the continuous variables in the test results are basically skewed in distribution, the continuous variables are presented as median and interquartile range (median (IQR)), and between-group comparisons were conducted using the Wilcoxon rank sum test. Categorical variables are represented as frequencies (%) and compared using the chi-square (χ2) test.
Spearman’s correlation analysis was utilized to evaluate the correlation between NPS, NRI, SII, ALB, and TC. Variable screening was conducted using the random survival forests model (RSF) to identify the most significant predictors of all-cause mortality in stroke patients. A variable is deemed valuable for prognosis if its variable importance (VIMP) value exceeds 0; the larger the VIMP value, the greater its predictive power. Kaplan–Meier analysis, combined with the Log-rank test, was initially used to assess the relationship between NPS and all-cause mortality in this population. Subsequently, weighted multivariable Cox analysis was conducted to further investigate the reliability of this relationship after adjusting for multiple covariates. The Cox regression results are presented for three distinct models: the crude model, which is unadjusted; Model 1, which accounts for demographic factors such as age, gender, PIR, race, and education; and Model 2, which includes demographic factors along with BMI, smoking, alcohol consumption, physical activity, hypertension, and diabetes history. Additionally, the correlation between the components of NPS and all-cause mortality was explored based on Model 2 to further elucidate the association between NPS and all-cause mortality in stroke patients.
Subgroup and sensitivity analyses were conducted to investigate the association between NPS and all-cause mortality, thereby enhancing the robustness of this study. The subgroup analysis was stratified by the following characteristics: age (< 65 years or ≥ 65 years), sex (male or female), BMI (< 30 kg/m2 or ≥ 30 kg/m2), smoking status (non-smokers or smokers), alcohol intake (non-drinkers or drinkers), physical activity (low physical activity or moderate to high physical activity), hypertension (yes or no), and diabetes (yes or no).
Following the exclusion of cancer patients, further sensitivity analysis was performed. The variables analyzed included age (in years), sex (male or female), race (non-Hispanic white or other race), PIR (< 1 or ≥ 1), education level (categorized as lower than high school graduate, high school graduate or GED, some college and college graduate or above), BMI (kg/m2), smoking status (non-smoker or smoker), alcohol intake (non-drinker or drinker), and physical activity (low physical activity or moderate to high physical activity). Hypertension and diabetes were categorized as yes or no. All statistical analyses were conducted using R software (version 4.3.2). A P-value < 0.05 was considered for statistical significance.
Results
Baseline characteristics
The study encompassed 1,076 stroke patients with a median age (IQR) of 67 (56, 77) years. The cohort was predominantly non-Hispanic white (72%) with 44% male representation. Median hematological parameters were as follows: lymphocyte count 1.90 × 103 cells/µL, neutrophil count 4.30 × 103 cells/µL, and monocyte count 0.60 × 103 cells/µL. Median (IQR) values for ALB, TC, NRI, and SII were 41.00 (39.00, 44.00) g/L, 181.00 (154.00, 213.00) mg/dL, 61.34 (53.72, 70.37), and 520.80 (360.00, 720.14), respectively. The most prevalent NPS was 2, representing 34% of the cohort (Supplementary Table S1).
In this study, there were 372 people in the all-cause death group and 704 people in the survival group. The frequencies of smoking status, alcohol intake, and diabetes in the survival group and all-cause death group were similar (P > 0.05). Compared with the survival group, the all-cause death group was more likely to be non-Hispanic whites who were older, had lower BMI, were less physically active, had a higher prevalence of hypertension, and had higher NPS (P < 0.001). Detailed baseline characteristics are shown in Supplementary Table S1.
Prognostic value of immunonutritional indicators
Spearman’s correlation analysis elucidated the relationships among NPS, NRI, SII, ALB, TC, and all-cause mortality. Results demonstrated a significant positive correlation between NPS and SII (r: 0.41), and significant negative correlations between NPS and both ALB (r: − 0.47) and TC (r: − 0.55) (Fig. 2A).
Prognostic value of NPS in adult stroke patients. (A) Spearman’s correlation analysis was used to calculate the correlation coefficients among immunonutritional indicators. (B) Randomized survival forest (RSF) was used to assess the prognostic importance of immunonutritional indicators in predicting all-cause mortality in stroke patients. NRI nutritional risk index, NPS Naples prognostic score, SII systemic immune-inflammation index, ALB serum albumin, TC total cholesterol.
RSF analysis was employed to assess the variable importance (VIMP) of immunonutritional indicators in predicting all-cause mortality. As illustrated in Fig. 2B, NPS exhibited markedly higher variable importance for all-cause mortality compared to other indicators, underscoring its superior predictive potential.
Relationship between NPS and mortality
Table 1 delineates the baseline characteristics of participants stratified by the higher NPS group (3–4) and the lower NPS group (0–2). Significant inter-group disparities were observed across multiple parameters, including age, race, smoking status, diabetes, hypertension, lymphocyte, neutrophil, and monocyte counts, ALB, TC, SII, and mortality status (all P < 0.05).
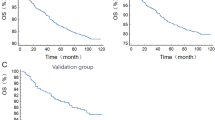
During the study period, 372 participants succumbed to various causes, with a median survival time of 69 months. Kaplan–Meier analysis, in conjunction with the log-rank test, was utilized to preliminarily assess the association between NPS and all-cause mortality in stroke patients. Figure 3 demonstrates a significant positive correlation between elevated NPS and increased risk of all-cause mortality (P < 0.001).
Table 2 presents the outcomes of three multivariate Cox regression models. The crude model, which did not account for any covariates, revealed that the risk of all-cause mortality in the higher NPS group was 2.56 times greater than that in the lower NPS group (95% CI 1.94–3.38, P < 0.001). Model 1, adjusted for age, sex, race, PIR, and education level, indicated that the risk of all-cause mortality in the higher NPS group was 1.90 times that of the lower NPS group (95% CI 1.43–2.51, P < 0.001). Model 2 further controlled for additional factors based on Model 1. The results from Model 2 indicated that the risk of all-cause mortality in the higher NPS group remained elevated, at 1.89 times that of the lower NPS group (95% CI 1.44–2.47, P < 0.001). All cox regression results of Model 2 are shown in Supplementary Table S2.
Further exploration of the associations between individual NPS components and all-cause mortality in stroke patients, utilizing the fully adjusted Model 2 (Supplementary Table S3). The analysis revealed that lymphocyte count (HR = 1.05, 95% CI 1.03–1.08), neutrophil count (HR = 1.16, 95% CI 1.08–1.25), and monocyte count (HR = 1.74, 95% CI 1.24–2.43) were independent risk factors for increased all-cause mortality in stroke patients (all P < 0.05). Moreover, participants with ALB ≤ 40 g/dL had 1.48 times higher all-cause mortality than those with ALB > 40 g/dL (95% CI 1.19–1.84, P < 0.01). Interestingly, The TC did not demonstrate a significant association with all-cause mortality in this cohort of stroke patients (P = 0.439).
In the subgroup analysis of all-cause mortality among stroke patients (Fig. 4), we found no significant interaction between subgroup variables, including age, gender, BMI, smoking status, alcohol intake, physical activity, hypertension, and diabetes with NPS (all P for interaction > 0.05). Based on the analysis of a single subgroup variable, in the low exercise intensity subgroup, stroke patients in the higher NPS group exhibited a significantly higher mortality risk compared to those in the lower NPS group (HR = 2.46, 95%CI: 1.86–3.26, P < 0.001). In the Moderate/high exercise intensity subgroup, no significant difference in mortality risk was observed among stroke patients across different NPS groups (P = 0.579). Additionally, a sensitivity analysis, performed after excluding cancer patients from the stroke population, yielded results consistent with our primary findings (Supplementary Table S4). The concordance observed in the subgroup and sensitivity analyses substantiate the stability and clinical applicability of our results, reinforcing the potential utility of NPS as a prognostic tool in stroke patients.
Discussion
This study, leveraging data from the NHANES 2005–2018 cohort in conjunction with National Death Index (NDI) records, elucidated the independent association between immunonutritional indicators and all-cause mortality among stroke patients. Our findings demonstrate that the NPS serves as a robust prognostic indicator for predicting all-cause mortality of stroke patients. Notably, a higher NPS (3–4) emerged as a significant risk factor for increased all-cause mortality. Furthermore, we identified that individual components of the NPS—lymphocyte, neutrophil, and monocyte counts, as well as ALB—function as independent predictors of all-cause mortality in stroke patients. These results provide compelling evidence supporting the clinical application of NPS in stroke patients, potentially facilitating more effective risk stratification and the development of targeted therapeutic interventions.
Stroke ranked as the second leading cause of mortality following ischemic heart disease, remains a pressing public health concern that demands urgent attention4. A growing body of evidence underscores the intricate relationship between inflammation and stroke pathogenesis31. This interconnection manifests not only through the local inflammatory response triggered by neuronal death and the subsequent release of damage-associated molecular patterns (DAMPs) at the site of injury10 but also through the association between elevated systemic inflammation and poor clinical outcomes in stroke patients32. Our investigation reveals that the three inflammatory cell types incorporated in the NPS—lymphocytes, neutrophils, and monocytes—exhibit independent associations with all-cause mortality in stroke patients. Lymphocytes are postulated to exert neuroprotective effects, contributing to improved neurological function33. Conversely, neutrophils have been implicated in the induction of oxygen free radicals, potentially exacerbating brain damage34,35, with elevated neutrophil levels correlating with poor prognosis in stroke patients32. Moreover, both monocytes and neutrophils serve as sources of matrix metalloproteinase-9 (MMP-9), which may precipitate hemorrhagic transformation (HT) and symptom exacerbation36,37. These findings underscore the value of incorporating lymphocyte, neutrophil, and monocyte counts into prognostic indicators such as the NPS, enabling a more comprehensive and dynamic assessment of patients’ inflammatory status.
In addition to the three cell types involved in inflammatory regulation, the NPS incorporates serum albumin and total cholesterol as indicators of nutritional status. Our results demonstrate a significant inverse relationship between elevated albumin levels and all-cause mortality in stroke patients. While stroke is recognized as an inflammatory condition capable of inducing hepatic production of acute-phase proteins, serum albumin levels may decrease due to increased tissue fluid volume and systemic consumption38. Consequently, reduced serum albumin levels not only indicate malnutrition but also reflect a persistent systemic inflammatory response10. A cohort study has established low serum albumin as an independent determinant of poor outcomes in ischemic stroke39. Wang et al.40 posited that higher serum albumin levels may confer improved prognosis in ischemic stroke patients. Interestingly, our study found no significant relationship between total cholesterol levels and all-cause mortality in stroke patients. While hypercholesterolemia is a well-established risk factor for ischemic stroke41, some studies have reported an association between higher total cholesterol levels and improved stroke outcomes42. The relationship between total cholesterol levels and stroke outcomes remains controversial and warrants further investigation.
Subgroup analyses revealed no significant interaction effects across variables, reinforcing the robustness of our findings. Notably, within the low exercise intensity subgroup, the higher NPS group exhibited a greater risk of mortality compared to the moderate/high exercise intensity subgroup. This observation suggests that moderate to high physical activity may partially mitigate the increased mortality risk associated with elevated NPS in stroke patients. The WHO 2020 Guidelines advocate for 150–300 min of moderate or 75–150 min of vigorous weekly aerobic activity for adults, including stroke patients43. The American Stroke Association (ASA) emphasizes that physical activity reduces blood pressure, enhances endothelial function, decreases insulin resistance, improves lipid metabolism, and may assist in weight reduction44. Enhancing physical activity can improve the prognosis of stroke patients by regulating coagulation mechanisms, including the enhancement of platelet aggregation and the reduction of fibrinogen levels45,46. Overall, although the interaction effects in this study were not significant and the intensity of physical activity did not notably alter the association between NPS and the risk of mortality, the positive impact of physical activity on stroke remains important. Stroke patients should be encouraged to engage in moderate to high-intensity physical activities when their physical conditions allow, as this can enhance bodily functions and reduce mortality risk47. Furthermore, NPS should be regarded as an independent risk factor, warranting attention regardless of the level of physical activity.
In conclusion, our findings underscore the intricate interplay between immunonutritional status and stroke prognosis. The NPS, by comprehensively integrating these aspects, provides a dynamic prediction of stroke patient outcomes with high predictive value and clinical applicability. Our results indicate that an elevated NPS score is associated with increased all-cause mortality in stroke patients. This suggests that interventions targeting nutrition and immune-inflammatory processes may have the potential to mitigate the risk of all-cause mortality in stroke patients. The strengths of this study include its prospective design and the utilization of a nationally representative sample of adult stroke patients in the United States, enhancing the generalizability of our findings. Additionally, we employed a comprehensive array of statistical analysis methods, including multivariable-adjusted Cox analysis, subgroup analysis, and sensitivity analysis, to minimize the impact of confounding factors on our results. Furthermore, the NPS, as a composite indicator of inflammation and nutrition, offers the advantages of ease of derivation, calculation, and economic feasibility for clinical application. However, due to the inherent limitations of the NHANES dataset, we were unable to conduct a comprehensive comparison of the significance of NPS and NHISS scores in predicting the prognosis of stroke patients, which constitutes a major limitation of this study. NPS relies on routine laboratory testing data and has specific requirements for basic medical experimental equipment, thereby limiting the global accessibility of NPS. Furthermore, our analysis did not account for several potential confounding factors that may influence the study outcomes, such as pharmacological interventions.
In summary, this study demonstrates a significant positive correlation between NPS and all-cause mortality in a representative cohort of stroke patients in the United States. Our findings confirm the important predictive role of the NPS as a comprehensive indicator of immunonutrition, validating its potential clinical applicability as a tool for risk stratification and timely intervention in stroke patients. These results provide a robust foundation for developing evidence-based strategies for stroke prevention and treatment, potentially leading to improved patient outcomes and reduced mortality rates. Additionally, we look forward to conducting a multi-center study with a larger sample size to verify the applicability of NPS to the population and further compare the predictive value of NPS with other new biomarkers.
Data availability
The datasets analyzed during the current study are publicly available in the National Health Nutrition Survey (NHANES), https://www.cdc.gov/nchs/nhanes/index.htm. Association of National Center for Health Statistics survey data with the National Death Index—Public Use Related Death Records 2019. From: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm.
References
Shah, H., Paul, G. & Yadav, A. K. Surface-tailored nanoplatform for the diagnosis and management of stroke: Current strategies and future outlook. Mol. Neurobiol. 61, 1383–1403 (2024).
Jiang, Y. et al. The interplay between cytokines and stroke: a bi-directional Mendelian randomization study. Sci. Rep. 14, 17657 (2024).
Johnson, C. O. et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 439–458 (2019).
Feigin, V. L. et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 17, 18–29 (2022).
Mistry, E. A. et al. Predicting 90-day outcome after thrombectomy: Baseline-adjusted 24-hour NIHSS is more powerful than NIHSS score change. Stroke 52, 2547–2553 (2021).
Ramachandran, K., Radha, D., Gaur, A., Kaliappan, A. & Sakthivadivel, V. Is the National Institute of Health Stroke Scale a valid prognosticator of the aftermath in patients with ischemic stroke?. J Family Med Prim Care 11, 7185–7190 (2022).
Huang, P. et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res. Ther. 10, 300 (2019).
Pellicciari, L. et al. Trunk control test as a main predictor of the modified barthel index score at discharge from intensive post-acute stroke rehabilitation: Results from a multicenter Italian Study. Arch. Phys. Med. Rehabil. 105, 326–334 (2024).
Xu, Q. et al. Factors influencing disabled stroke survivors’ quality of life in rural China: Based on the structural characteristics and psychometric properties of the SF-36 assessment. J. Clin. Med. 12, 3012 (2023).
Wang, A. et al. Association of serum albumin to globulin ratio with outcomes in acute ischemic stroke. CNS Neurosci. Ther. 29, 1357–1367 (2023).
Shi, K. et al. Global brain inflammation in stroke. Lancet Neurol. 18, 1058–1066 (2019).
Chen, X., Hong, C., Guo, Z., Huang, H. & Ye, L. Association between advanced lung cancer inflammation index and all-cause and cardiovascular mortality among stroke patients: NHANES, 1999–2018. Front. Public Health 12, 1370322 (2024).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Donath, M. Y., Dinarello, C. A. & Mandrup-Poulsen, T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 19, 734–746 (2019).
Eckart, A. et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: A prospective study. Am. J. Med. 133, 713-722.e7 (2020).
Bucci, T. et al. Albumin levels and risk of early cardiovascular complications after ischemic stroke: A propensity-matched analysis of a global federated health network. Stroke 55, 604–612 (2024).
Zhou, H. et al. Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc. Neurol. 6, 458–466 (2021).
Zhang, Q. et al. Serum albumin level is associated with the recurrence of acute ischemic stroke. Am. J. Emerg. Med. 34, 1812–1816 (2016).
Misirlioglu, N. F. et al. The relationship between neutrophil-lymphocyte ratios with nutritional status, risk of nutritional indices, prognostic nutritional indices and morbidity in patients with ischemic stroke. Nutrients 16, 1225 (2024).
Venter, C. Immunonutrition: diet diversity, gut microbiome and prevention of allergic diseases. Allergy Asthma Immunol. Res. 15, 545–561 (2023).
Geraghty, J. R. et al. Systemic immune-inflammation index predicts delayed cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery 89, 1071–1079 (2021).
Zhu, N. et al. Naples prognostic score as a novel prognostic prediction indicator in adult asthma patients: A population-based study. World Allergy Organ. J. 16, 100825 (2023).
Li, B. et al. Association of serum uric acid with all-cause and cardiovascular mortality in diabetes. Diabetes Care 46, 425–433 (2023).
Yang, T. et al. Associations of dietary fats with all-cause mortality and cardiovascular disease mortality among patients with cardiometabolic disease. Nutrients 14, 3608 (2022).
Guo, H. & Wang, T. Predictive role of Naples prognostic score for survival in esophageal cancer: A meta-analysis. Medicine 103, e38160. https://doi.org/10.1097/MD.0000000000038160 (2024).
Xu, B. et al. Clinical implications of Naples prognostic score for patients with resected cholangiocarcinoma: A real-world experience. J. Inflamm. Res. 17, 655–667 (2024).
Li, S. et al. Naples Prognostic Score as a novel prognostic prediction tool in video-assisted thoracoscopic surgery for early-stage lung cancer: A propensity score matching study. Surg. Endosc. 35, 3679–3697 (2021).
Yang, C. et al. Prognostic value of systemic immune-inflammation index (SII) in patients with glioblastoma: A comprehensive study based on meta-analysis and retrospective single-center analysis. J. Clin. Med. 11, 7514 (2022).
Wang, P. et al. Application of four nutritional risk indexes in perioperative management for esophageal cancer patients. J. Cancer Res. Clin. Oncol. 147, 3099–3111 (2021).
Chen, L. et al. Nutritional risk index predicts survival in patients with breast cancer treated with neoadjuvant chemotherapy. Front. Nutr. 8, 786742 (2022).
Jenny, N. S. et al. Inflammatory cytokines and ischemic stroke risk: The REGARDS cohort. Neurology 92, e2375–e2384 (2019).
Sandvig, H. V. et al. Plasma inflammatory biomarkers are associated with poststroke cognitive impairment: The Nor-COAST study. Stroke 54, 1303–1311 (2023).
Macrez, R. et al. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 10, 471–480 (2011).
Gong, P. et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J. Neuroinflamm. 18, 51 (2021).
Ceulemans, A.-G. et al. The dual role of the neuroinflammatory response after ischemic stroke: Modulatory effects of hypothermia. J. Neuroinflamm. 7, 74 (2010).
Xu, X. R. et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 53, 409–430 (2016).
Gong, P. et al. Neutrophil-lymphocyte ratio predicts post-thrombolysis early neurological deterioration in acute ischemic stroke patients. Brain Behav. 9, e01426 (2019).
Li, Q. et al. Naples prognostic score is an independent prognostic factor in patients with operable endometrial cancer: Results from a retrospective cohort study. Gynecol. Oncol. 160, 91–98 (2021).
Hansen, C. K., Christensen, A., Havsteen, I., Ovesen, C. & Christensen, H. Prevalence of early neurological deterioration after IV—thrombolysis in acute ischaemic stroke patients—A hospital-based cohort study. Clin. Neurol. Neurosurg. 171, 58–62 (2018).
Wang, C. et al. Serum albumin is negatively associated with hemorrhagic transformation in acute ischemic stroke patients. Cerebrovasc. Dis. 47, 88–94 (2019).
De Carvalho, L. S. F. Total cholesterol and the risk of stroke: A double-edged sword or a blunt knife?. Atherosclerosis 270, 191–192 (2018).
Markaki, I., Nilsson, U., Kostulas, K. & Sjöstrand, C. High cholesterol levels are associated with improved long-term survival after acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 23, e47–e53 (2014).
Bull, F. C. et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54, 1451–1462 (2020).
Furie, K. L. et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke https://doi.org/10.1161/STR.0b013e3181f7d043 (2011).
Muscella, A., Stefàno, E. & Marsigliante, S. The effects of exercise training on lipid metabolism and coronary heart disease. Am. J. Physiol. Heart Circ. Physiol. 319, H76–H88 (2020).
Thyfault, J. P. & Bergouignan, A. Exercise and metabolic health: beyond skeletal muscle. Diabetologia 63, 1464–1474 (2020).
Reimers, C. D., Knapp, G. & Reimers, A. K. Exercise as stroke prophylaxis. Dtsch. Ärztebl. Int. 106, 715–721 (2009).
Acknowledgements
The authors would like to express their sincere gratitude to all of the researchers whose work contributed to and informed this research.
Funding
This work was financially supported by the Weifang Science and Technology Development Plan (2022RKX121). The funder had no role in study design, data collection, interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
K.D.: Methodology, Software, Formal analysis, Writing—Original Draft; S.F.: Investigation, Visualization; X.Z., Y.L., F.C., J.J.: Investigation; Z.W.: Funding acquisition; C.W., P.W.:Conceptualization, Resources, Writing—Review & Editing, Supervision, Project administration. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Data analyzed in this study were obtained from the NHANES. The protocols involved were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB), and conducted in accordance with the Declaration of Helsinki, and informed consent from all participants was documented.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Deng, K., Feng, S., Cheng, F. et al. Association of immunonutritional indicators with all-cause mortality in adult stroke patients. Sci Rep 15, 14762 (2025). https://doi.org/10.1038/s41598-025-99158-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99158-7
Keywords
This article is cited by
-
The Ratio of Red Blood Cell Distribution Width To Serum Albumin and its Association with Cardiovascular and All-Cause Mortality Risk in Diabetic Patients: A Cohort Study Using NHANES Data from 1999 to 2018
High Blood Pressure & Cardiovascular Prevention (2025)