Abstract
Several chronic autoimmune diseases are characterized by elevated autoantibody Fab glycosylation. Whether Fab glycans link to disease state or development remains unclear, yet may serve as a marker thereof. Many autoimmune diseases are treated with B cell depletion therapies that particularly result in a decline of autoantibodies. The question arises whether B cell depletion therapy may have an impact on Fab glycosylation. Here, we investigated the longitudinal effects of B cell depletion therapy on Fab glycosylation of total IgG and IgG autoantibodies in rheumatoid arthritis (RA), pemphigus vulgaris (PV), ANCA-associated vasculitis (AAV), and multiple sclerosis (MS). Baseline Fab glycosylation was compared to 6–12 months into therapy by lectin affinity chromatography, determining Fab sialylation as an estimate of Fab glycosylation. We observed a modest decrease in Fab glycosylation of total IgG for RA (median 13.8%[IQR 11.7–16.3] – 9.1%[IQR8-11]) and PV (16.4%[IQR14.9–17.5] – 13.01%[IQR10.8–15.5]) after 6 months, whereas for AAV Fab glycosylation slightly increased (11.6%[IQR7.4–15] – 14.9%[IQR11.4–19.3]), and no changes were found for MS. Autoantibody titers (anti-CCP, anti-PR3, anti-Dsg3) had declined following B cell depletion therapy, yet their elevated Fab glycosylation levels were maintained. Taken together, Fab glycosylation levels of autoantibodies do not decrease upon B cell depletion therapy, thereby retaining their predictive potential as biomarker.
Similar content being viewed by others
Introduction
Autoimmunity occurs when a breach of tolerance leads to the recognition of self-antigens. B cells are key players in this process, as evidenced by success of B cell-targeted therapies in several human autoimmune diseases1. Several of disease-specific autoantibodies have been associated with increased Fab glycosylation. Fab glycans are posttranslational modifications of the antibody’s variable domain that are incorporated at N-glycosylation sites (N-X-S/T) which arise predominantly during somatic hypermutation (SHM)2,3,4. In the healthy population, 10–14% of serum IgG is estimated to be Fab glycosylated, whereas in for instance rheumatoid arthritis (RA), over 80% of anti-cyclic citrullinated peptide-2 (anti-CCP) autoantibodies are Fab glycosylated5. Fab glycans on anti-CCP antibodies were even linked to disease progression and proposed as potential biomarkers of disease development5,6. Mechanistically, Fab glycans diversify the antibody repertoire by potentially expanding specificity and function. The presence of Fab glycans might affect B cell responses by altering antigen binding, membrane transport, interactions with other proteins (such as lectins), or by enhancing B cell receptor (BCR) signaling7,8,9. Despite these findings, the direct link between elevated Fab glycosylation and autoimmunity remains unclear. Notably, increased Fab glycosylation of autoantibodies seems to occur particularly on autoantibodies present in chronic autoimmune diseases5,7,10. Nevertheless, chronic antigenic stimulation per se could not explain this phenomenon, as was shown by low Fab glycan levels on antigen specific antibodies in chronic viral infections such as herpesviruses and HIV5. Nevertheless, enhanced BCR signaling in the presence of Fab glycans may indicate a survival advantage and escape mechanism for B cell tolerance checkpoints and could explain their high prevalence in autoimmunity9.
Many chronic autoimmune diseases are treated with B cell depletion therapies. The central role of B cells in autoimmunity explains the efficacy of these therapies such as rituximab (RTX) and ocrelizumab (OCR)11. Both are therapeutic monoclonal antibodies targeting CD20, a transmembrane glycoprotein expressed on B cells from the late pre-B cell stage until plasma cell differentiation. Anti-CD20 therapy therefore specifically depletes naïve, activated, and memory B cells, while leaving antibody secreting plasma cells intact. In addition, replenishment of short-lived plasma cells is largely prevented by depletion of the naïve and memory B cell compartment12,13. In autoimmune diseases such as RA and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), B cell depletion therapy has been shown to induce a preferential decline in autoantibodies, respectively anti-Rheumatoid Factor/anti-CCP and anti-PR3, leaving serum IgG relatively stable. In most cases, total IgM falls more rapidly and drastically than IgG14,15,16,17,18,19,20,21, yet to which extent varies among literature, with some reporting only minor changes in IgM following B cell depletion therapy22,23,24,25. Most studies describe a small decrease in IgG, with some falling below the lower limit of normal particularly at long term16,17,18,19,21,22,23,26,27,28. However, the number of patients reaching hypogammaglobulinemia is consistently higher for IgM27,29,30,31,32.
Since autoimmune diseases with heavily Fab glycosylated autoantibodies are often treated with B cell depletion therapy, the question arises whether B cell depletion therapy could have an effect on Fab glycosylation. It has been reported that rituximab treatment may alter the glycosylation profiles of the glycans attached to the conserved N-glycosylation site in the IgG fragment crystallizable (Fc) region, possibly affecting their inflammatory potential33,34. Whether B cell depletion therapy has a similar effect on autoantibody Fab glycosylation in chronic autoimmune diseases has not been investigated yet. Here, we investigated the longitudinal effects of B cell depletion therapy on Fab glycosylation of serum IgG and autoantibodies in three autoantibody mediated autoimmune diseases: RA, pemphigus vulgaris (PV), and AAV plus a neurological autoimmune disorder likely to be indirectly driven by B cells: Multiple Sclerosis (MS)35.
Results
IgM and IgG titers fall in all diseases but MS following B cell depletion therapy
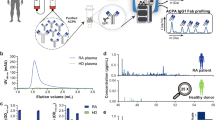
Patients diagnosed with RA (n = 15), PV (n = 10), AAV (n = 11), and MS (n = 15) that received B cell depletion therapy were included in the study. Characteristics of the patients are summarized in Table 1. First, we investigated the effect of B cell depletion therapy on antibody titers in patient sera of our RA, PV, AAV, and MS cohorts (Fig. 1 A,B). As IgM titers are often reported to be more sensitive to B cell depletion therapy than IgG, we firstly measured IgM in all groups to obtain a complete picture. IgM levels significantly dropped upon B cell depletion therapy for RA, PV, and AAV (for PV only significant at t = 12), whereas no significant changes were observed for the MS group in response to B cell depletion therapy. Next, we studied total IgG titers following B cell depletion therapy. Interestingly, the patterns observed for IgG closely matched those for IgM in all groups; if significant decreases in IgM had been found, so were for IgG. For PV IgM levels fluctuated, mostly within the normal range (IgM: 0.4–2.3 g/L), yet with significant decrease (50.4%) after 12 months of treatment. Similarly, IgG levels of PV patients had significantly decreased at 12 months of treatment, with 80% of patients dropping below the lower limit of normal (LLN; IgG < 7 g/L) (6.1 g/L [IQR 4.6–7.0]). Corresponding to the pattern of IgM, no significant differences were found in IgG titers in MS patients following B cell depletion therapy (median at baseline: 8.6 g/L [IQR 6.4–9.5], t = 6: 8.6 g/L [IQR 6.0–9.8], t = 12: 7.8 g/L [IQR 6.5–10.1]), both staying within the normal range. RA and PV patients had baseline IgM and IgG within or above the normal range (IgG: 7–16 g/L), whereas we noticed that 63.6% of AAV patients had baseline levels below the LLN for IgM (0.4 g/L [IQR 0.2–0.5]) and 18.2% below the LLN for IgG (median 8.7 g/L [IQR 7.8–11.5]).
Total IgM and IgG titers following B cell depletion therapy. Longitudinal antibody titers are shown for RA (red), PV (yellow), AAV (green), and MS (pink). A Total IgM levels (g/L) at 0, 6, and 12 months after initiation of B cell depletion therapy. Connected dots represent a patient. Grey area indicates normal range: 0.4–2.3 g/L IgM. B. Total IgG (g/L) as measured at 0, 6, and 12 months after start B cell depletion therapy. Grey area indicates normal range: 7–16 g/L. Statistical differences were determined using a Wilcoxon matched-pairs signed rank test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
B cell depletion therapy-induced decrease in autoantibodies
Next we studied the effect of B cell depletion therapy on antigen specific antibody levels in patients with autoantibody mediated autoimmune diseases in our cohorts, namely RA (anti-CCP), PV (anti-Dsg3), and AAV (anti-PR3) (Fig. 2). All three autoimmune diseases showed a significant decline in autoantibodies in the first 6 months of treatment (RA: 53.2%; PV: 18.4%; AAV: 94.8%). Anti-Dsg3 levels showed a trend of further decline in the next 6 months, whereas anti-PR3 levels showed a trend towards stabilization.
Auto-antibody titers following B cell depletion therapy. Longitudinal auto-antibody IgG titers are shown for RA; anti-CCP (red), PV; anti-Dsg3 (yellow), and AAV; anti-PR3 (green) in arbitrary units (AU)/mL. Connected dots represent a single patient. Statistical differences were determined using a Wilcoxon matched-pairs signed rank test. *p < 0.05, **p < 0.01, ***p < 0.001.
Increased Fab glycosylation of autoantibodies maintained during B cell depletion therapy
Subsequently, serum samples were submitted to SNA lectin chromatography to estimate the Fab sialylation level of total IgG for the different cohorts (Fig. 3A). The method enriches for Fab glycosylated IgG, as more than 90% of Fab glycans contain at least two sialic acid residues which are required for SNA binding, whereas for Fc glycans this is less than 1%, of which most are even shielded from SNA binding in between the two CH2domains36,37,38,39,40. Both for RA and PV a drop was observed after 6 months of treatment (RA: median 13,8% [IQR 11.7–16.3] – 9.1% [IQR 8–11], PV: 16.4% [IQR 14.9–17.5] – 13.01% [IQR 10.8–15.5]), which further declined between 6 and 12 months for PV (median 13.0% [IQR 10.8–15.5] – 11,3% [IQR 9.7–12.3]). For AAV an increase was seen 6 months after treatment initiation (median 11.6% [IQR 7.4–15] – 14.9% [IQR 11.4–19.3]). Overall, the Fab glycosylation levels remained essentially within the normal range, and for MS no significant differences were found (median T = 0: 8.7% [IQR 7.4–12.1], T = 6: 9.8% [IQR 8.9–11.2], T = 12: 9% [IQR 7.3–10.5]).
Fab sialylation level of total IgG and auto-antibodies following B cell depletion therapy. A Percentage of total IgG that is Fab sialylated at 0, 6, and 12 months after start of B cell depletion therapy. In RA (red), PV (yellow), AAV (green), and MS (pink). Connected dots represent a patient. B Percentage of auto-antibodies that is Fab sialylated at 0, 6, and 12 months following B cell depletion therapy. For RA; anti-CCP (red), PV; anti-Dsg3 (yellow), and AAV; anti-PR3 (green). Statistical differences were determined using a Wilcoxon matched-pairs signed rank test. *p < 0.05, **p < 0.01, ***p < 0.001.
Subsequently, we measured Fab sialylation levels within the autoantibody compartment (Fig. 3B). At baseline, Fab sialylation levels were elevated for all three autoantibodies (anti-CCP: 86.1% [IQR 70.9–89.9], anti-Dsg3: 58.3% [43.6–59.5], anti-PR3: 37.5% [IQR 29.7–66.9%]) compared to 13.8%, 16.4%, and 11.6% of total IgG for RA, PV, and AAV respectively. No significant differences were observed for anti-CCP and anti-Dsg3 fab sialylation in response to B cell depletion therapy. Fab sialylation of anti-PR3 was increased in most AAV patients 12 months after treatment initiation.
Discussion
In the human autoimmune diseases RA, PV, and AAV (marked by increased Fab glycosylation of autoantibodies), we report changes in total serum IgG Fab glycosylation following B cell depletion therapy. In MS, a neurological autoimmune disease where B cells likely play a role, yet lacking the (known) presence of autoantibodies, no change in IgG Fab glycosylation was found. Antibody titers followed a similar trend. Changes in total serum IgM and IgG were only observed in autoantibody-mediated autoimmune diseases, not in MS. We report a reduction in antibody titers following B cell depletion therapy with strongest impact on autoantibody levels and smaller changes in total IgM and IgG. The elevated levels of Fab glycosylation of most autoantibodies were maintained during B cell depletion therapy as hardly any differences were observed after therapy. However, anti-PR2 Fab glycosylation levels in AAV were increased 12 months into treatment.
Autoantibodies are often heavily Fab glycosylated, at least in several B cell mediated autoimmune diseases. We have observed a trend in which B cell depletion therapy only affects Fab glycosylation of total serum IgG in autoantibody mediated diseases (RA, PV, AAV) leaving IgG Fab glycosylation in neurological autoimmune disorder MS unchanged. The reduction in Fab glycosylation of total IgG could be a reflection of the stronger effect of B cell depletion therapy on autoantibody levels relative to non-autoimmune/total IgG, and may indicate that autoantibodies comprise a substantial portion of Fab-glycosylated antibodies in the total IgG pool. Additionally, anti-CCP/anti-PR3/anti-Dsg3 are often of the IgG4 isotype, an IgG subclass carrying enhanced levels of Fab glycans, often produced by short-lived plasma cells, which could contribute to the quick loss of a heavily Fab glycosylated part of the IgG pool. In line with this reasoning, it could merely be the result of the loss of short-lived plasma cell pool and therefore be an indication that the route of short-lived plasma cell development has increased susceptibility of N-glycosylation site incorporation in the Fab arm. However, the lack of changes observed in MS IgG antibodies argues against this analogy. Overall, the changes in Fab glycosylation of total IgG are small and stay within the same range as the healthy population.
Increased Fab glycosylation was observed for autoantibodies of all groups at baseline. During B cell depletion therapy, the high level of Fab glycosylation was maintained or in case of AAV even increased at 12 months into treatment. Given that anti-PR3 titers fell upon B cell depletion therapy, the highly Fab glycosylated autoantibodies may point to a selective advantage of PR3-directed B cells that recur after B cell depletion. In any case, based on our data, B cell depletion therapy may not alter the potential of Fab glycans on autoantibodies to serve as biomarker for disease as their levels remains intact. Fab glycans on anti-CCPs antibodies were for instance suggested to have predictive value on disease development6. Aside from the presence of these Fab glycans it may be worthwhile to investigate their composition by mass spectrometry based glycopeptide profiling. Generally, Fab glycans are complex-type N-linked glycans that contain high amounts of galactose, sialic acid, and bisecting N-acetylglucosamine yet little core fucosylation3. For Fc glycans the composition has been linked to an antibody’s inflammatory potential and state of disease. Low galactose has for instance been associated with an inflammatory profile and afucosylated anti-SARS-CoV-2 antibodies were linked to a more severe disease COVID-19 disease course41,42. Whether the composition of Fab glycans of autoantibodies may change over time, or due to B cell depletion therapy, and how this impacts disease state is not known yet and requires further investigation.
A potential risk of B cell depletion therapy is the loss of protective humoral immunity, either by loss of existing protective antibody production or by the prevention of development of memory or plasma B cells to new antigens. Often a substantial decline in IgM is seen, while IgG remains relatively more stable. The short half-life of IgM and the short-lived character of most IgM producing plasma cells as opposed to B cell depletion therapy -resistant long-lived plasma cells that account for most serum IgG, could explain the stronger decline in IgM versus IgG. AAV stood out for low titers at the start of treatment, especially for IgM, potentially explained by the use of cyclophosphamide as pre- or co-medication. The combination of CYC and RTX has been described to increase the chance of developing hypogammaglobulinemia and a stronger decline of IgM22,30. Several studies showed lower baseline IgG after CYC treatment prior to RTX30,43, and even severe hypogammaglobulinemia was reported after combinational therapy of CYC and RTX in lymphoma44,45.
Next to known B cell mediated autoimmune diseases with disease-specific autoantibodies, we tested the effects of B cell depletion therapy in MS, a neurological autoimmune diseases which was thought to be T cell driven until the success of B cell depletion therapy led to the notion that B cells must play a central role in MS as well. Apart from fluctuations in IgM, no drastic changes were observed in antibody titers. As expected, the success of B cell depletion therapy in MS can probably not be attributed to changes in humoral immunity as compared to the other diseases discusses.
Overall we report modest changes in Fab glycosylation on the total IgG level in autoantibody mediated chronic autoimmune diseases, whereas levels of Fab glycosylation of autoantibodies remain elevated, during B cell depletion therapy.
Methods
Study participants
This study is part of the Target-2-B! (T2B!) multicenter consortium across the Netherlands that follows up on the study by Koers et al. 2023. Fab glycosylation levels were found to be increased on IgG autoantibodies of several chronic autoimmune diseases, among which RA, PV and AAV. In order to study the effect of B cell depletion therapy of such elevated Fab glycosylation, we included patients with RA, PV, and AAV that received RTX B cell depletion therapy. As an example of a non-autoantibody mediated autoimmune disease we additionally included a group of MS patients that received OCR treatment. Serum samples were drawn prior to the start of treatment, at 6 months of treatment, and for all but RA at 12 months of treatment. Experiments were performed in accordance with relevant guidelines/regulations were approved by local ethical committees, and all patients provided informed consent.
RA
For the RA group, 15 patients (female 80.0% [12/15]) were included with a median age of 53 (between 34 and 68) years at sampling baseline at Leiden University Medical Center (UMC) (Table 1). All patients had active disease (median baseline DAS 5.04) at the start of B cell depletion therapy. They were treated with repeated infusions of 1000 mg rituximab (RTX) with 100 mg methylprednisolone pre-medication at first 2–3 infusions. The study protocol was approved by the Ethics Committee of the Leiden University Medical Center.
PV
At the Medical Center of Groningen 10 patients (female 70.0% [7/10])diagnosed with PV were included in the study of which three were diagnosed with mucocutaneous PV (mcPV), four with mucosal PV (mPV), and three with pemphigus foliaceus (PF). The patients had a median age of 56 (ranging between 31 and 67) at the start of B cell depletion therapy (Table 1). All but one received two infusions of 1000 mg two weeks apart, the other patient received two doses of 500 mg instead. Nine patients received a dose of 500 mg six months later, and eight received a fourth dose of 500 mg after another six months. Six patients were treated with prednisone, and three with azathioprine during B cell depletion therapy. The sere were obtained from the diagnostic biobank of the Center for Blistering diseases in the University Medical Center of Groningen, a tertiary referral center in the Netherlands. The Medical Research Involving Human Subjects Act does not apply to this type of non-interventional study with leftover materials for diagnostic purposes.
AAV
At the Medical Center of Groningen 11 patients (female 36.4% [4/11]) diagnosed with AAV were included. They had a median age of 53 years (between 21 and 77) at sampling baseline and received RTX monotherapy, either two doses of 1000 mg two weeks apart, or four doses of 375 mg/m2 (Table 1). The study was approved by the Medical Ethical Committee (METc) of the UMC Groningen (UMCG).
MS
For the MS cohort, 15 patients (female 40% 6/15]) were included at Amsterdam UMC. One patient was diagnosed with relapsing–remitting MS (RRMS), all others were diagnosed with primary progressive MS (PPMS). The median age was 57 (between 46 and 62) at the start of B cell depletion therapy (Table 1). They received 4 infusions of ocrelizumab, of which the first two with a dose of 300 mg and the second two with 600 mg. Patients were treated with 100 mg methylprednisolone prior to each ocrelizumab infusion. No other disease modifying therapy was administered before B cell depletion therapy. The study was approved by the Amsterdam UMC Medical Ethical Committee.
Lectin affinity chromatography
Sialylated proteins were purified from patient samples by Sambucus nigra agglutinin (SNA) affinity chromatography as described previously40. Serum samples were dialyzed in 1 × Tris–HCl buffered saline (TBS, 10 mM Tris, 140 mM NaCl, pH 7.4) prior to dilution of 30 uL sample in 230 uL TBS. The total 260 uL diluted sample was subsequently loaded on a SNA agarose column (1 mL, Vector Laboratories) and fractionated in 0.5 mL fractions with the ÄKTAprime plus system (GE Healthcare) at 0.2 mL/minute. The sialylated proteins were eluted with 0.5M lactose, 0.2M acetic acid elution buffer at 0.8 mL/minute. Fractions were pooled to reach a sialylated protein enriched (SNA +) fraction and a sialylated protein depleted (SNA-) fraction. The SNA + was dialyzed against phosphate buffered saline (PBS) at 4 °C. Samples were stored at 4 °C until further testing. To determine Fab sialylation level of antibodies in patient sera, total IgG and antigen-specific IgG concentrations were measured in both the SNA + and SNA- fractions as described below. By dividing the amount of (antigen specific) antibody in the SNA + fraction over the amount of antibodies in (SNA + + SNA-) fraction, the percentage of Fab sialylated antibodies could be determined.
IgM and IgG enzyme-linked immunosorbent assay (ELISA)
Serum samples were tested for IgM and both the sialylated protein enriched and depleted fractions for IgG concentration by ELISA. 96-well flat bottom plates (Nunc MaxiSorp, Denmark) were coated with 2 ug/mL coating antibody (monoclonal mouse anti-human IgM (MH15-1, Sanquin) or IgG (MH16-1, Sanquin) in PBS. The next day plates were washed with 0.02% PBS/Tween 20 after which 100 ul sample diluted in HPE buffer (Sanquin) was incubated for 1 h at room temperature. Pooled serum with a known IgM and IgG concentration was used as a calibrator. After a washing step with 0.02% PBS/Tween 20, 1 ug/mL detection antibody (MH15-1, HRP-labeled for IgM, MH16-1, HRP-labeled for IgG, Sanquin) was added and incubated for 1 h. Finaly, plates were developed using TMB substrate solution (1-Step Ultra TMB ELISA, ThermoScientific) diluted in H2O and stopped with 0.2M H2SO4. Absorbance was measured at 450 and 540 nm.
Antigen specific immunoassays
Anti-CCP
IgG antibodies to CCP2 were measured by ELISA as described previously46. Biotinylated CCP-2 citrullin peptide (1µg/mL) was diluted in PBS supplemented with 0.1% bovine serum albumin (BSA) to coat Microcoat streptavidin microplates-high capacity plates for 1 h at room temperature. Samples were diluted 1:50 in PBS/1% BSA/0.05% Tween 20 and incubated at 37 °C for 1 h. Rabbit anti-human HRP labelled IgG (diluted 1:5000, P0214; Dako, Glostrup, Denmark) was used to detect anti-CCP2 IgG. Subsequently, samples were developed using 2.2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) mixed with 0.05% H2O2. Absorbance was measured at 415 nm.
Anti-Dsg3
To measure anti-Dsg3 IgG antibodies, the MESACUP™−2 TEST Desmoglein 3 ELISA kit (MBL, Tokyo, Japan) was used. Samples were measured within the range of detection, generally diluted a 100-fold. Detection was performed according to manufacturer’s instructions with an anti-IgG conjugate included in the kit.
Anti-PR3
Measurement of anti-PR3 IgG antibodies was performed by a slightly adjusted in-house ELISA as previously described47,48. In short, 1.3 µg/mL goat anti-mouse IgG-Fc (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) was coated on 96-well plates (Greiner Bio-One) in 0.1M carbonate buffer pH 9.6 and incubated for a minimum of 24 h at 4 °C. Plates were washed and mouse anti-human PR3 (0.624µg/mL, Hycult Biotech) in 0.1M TRIS/HCL pH 8.0 containing 0.05% Tween-20, 0.3M NaC1, 2% BSA and 1% normal goat serum (incubation buffer) was added and incubated for 2 h at room temperature. Plates were washed and an extract of azurophilic granules of normal human neutrophils in incubation buffer was added to the plates and incubated overnight at 4 °C. Samples were diluted in PBT and after washing, added to the wells and incubated for 2 h at room temperature. A positive patient sera pool was used as calibrator. Samples were blocked with 0.5% normal mouse serum in incubation buffer and incubated for 30 min at room temperature. Goat anti-human IgG Fc-AP (1:1000) (Southern Biotec) was used to detect IgG. Plates were developed with p-nitrophenyl-phosphate disodium and the reaction was stopped with 5M NaOH. Absorbance was measured at 405 nm.
Statistical analysis
Statistical differences either between baseline and 6 months or between baseline and 12 months were analyzed using the non-parametric rank test: Wilcoxon matched-pairs signed rank test. The test was performed for groups of which data was available for both timepoints. The statistical analysis were carried out using GraphPad Prism 9.1.1.
Data availability
Data for this publication is available upon reasonable request from the corresponding author.
Abbreviations
- AAV:
-
ANCA-associated vasculitis
- ANCA:
-
Antineutrophil cytoplasmic antibody
- BCR:
-
B cell receptor
- CCP:
-
Cyclic citrullinated peptide
- COVID-19:
-
Corona virus disease 2019
- CYC:
-
Cyclophosphamide
- Dsg3:
-
Desmoglein 3
- ELISA:
-
Enzyme-linked immunosorbent assay
- Fc:
-
Fragment crystallizable
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IQR:
-
Interquartile range
- LLN:
-
Lower limit of normal
- MS:
-
Multiple Sclerosis
- OCR:
-
Ocrelizumab
- OD:
-
Optical density
- PPMS:
-
Primary progressive multiple sclerosis
- PR3:
-
Proteinase 3
- PV:
-
Pemphigus vulgaris
- RA:
-
Rheumatoid arthritis
- RTX:
-
Rituximab
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SHM:
-
Somatic Hypermutation
- SNA:
-
Sambucus nigra agglutinin
References
Pisetsky, D. S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 19, 509–524 (2023).
Dunn-Walters, D., Boursier, L. & Spencer, J. Effect of somatic hypermutation on potential N-glycosylation sites in human immunoglobulin heavy chain variable regions. Mol. Immunol. 37, 107–113 (2000).
van de Bovenkamp, F. S., Hafkenscheid, L., Rispens, T. & Rombouts, Y. The Emerging Importance of IgG Fab Glycosylation in Immunity. J. Immunol. 196, 1435–1441 (2016).
Vergroesen, R. D. et al. B-cell receptor sequencing of anti-citrullinated protein antibody (ACPA) IgG-expressing B cells indicates a selective advantage for the introduction of N -glycosylation sites during somatic hypermutation. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2017-212052 (2017).
Koers, J., Sciarrillo, R., Derksen, N. I. L. & Vletter, E. M. Differences in IgG autoantibody Fab glycosylation across autoimmune diseases. J. Allergy Clin. Immunol. 151, 1646–1654 (2023).
Hafkenscheid, L. et al. N -Linked Glycans in the Variable Domain of IgG Anti-Citrullinated Protein Antibodies Predict the Development of Rheumatoid Arthritis. Arthr. Rheumatol. 71, 1626–1633 (2019).
Rombouts, Y. et al. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann. Rheum. Dis. 75, 578–585 (2016).
Volkov, M. et al. IgG Fab Glycans Hinder FcRn-Mediated Placental Transport. J. Immunol. 210, 158–167 (2023).
Kissel, T. et al. Surface Ig variable domain glycosylation affects autoantigen binding and acts as threshold for human autoreactive B cell activation. Sci. Adv. 8, 1–22 (2022).
Zhou, X. et al. Antibody glycosylation in autoimmune diseases. Autoimmun. Rev. 20, 102804 (2021).
Lee, D. S. W., Rojas, O. L. & Gommerman, J. L. B cell depletion therapies in autoimmune disease: Advances and mechanistic insights. Nat. Rev. Drug Discov. 20, 179–199 (2021).
Reff, M. E. et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83, 435–445 (1994).
Leandro, M. J., Cambridge, G., Ehrenstein, M. R. & Edwards, J. C. W. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arth. Rheum. 54, 613–620 (2006).
Cambridge, G. et al. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthr. Rheum. 48, 2146–2154 (2003).
De La Torre, I. et al. Total serum immunoglobulin levels in patients with RA after multiple B-cell depletion cycles based on rituximab: Relationship with B-cell kinetics. Rheumatology 51, 833–840 (2012).
Popa, C., Leandro, M. J., Cambridge, G. & Edwards, J. C. W. Repeated B lymphocyte depletion with rituximab in rheumatoid arthritis over 7 yrs. Rheumatology 46, 626–630 (2006).
Cortazar, F. B. et al. Effect of Continuous B Cell Depletion With Rituximab on Pathogenic Autoantibodies and Total IgG Levels in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthr. Rheumatol. 69, 1045–1053 (2017).
Jones, R. B. et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthr. Rheum. 60, 2156–2168 (2009).
Jonsdottir, T. et al. Treatment of refractory SLE with rituximab plus cyclophosphamide: Clinical effects, serological changes, and predictors of response. Ann. Rheum. Dis. 67, 330–334 (2007).
Vallerskog, T. et al. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin. Immunol. 122, 62–74 (2007).
Bennett, C. M. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood 107, 2639–2642 (2006).
Venhoff, N. et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS ONE 7, e37626 (2012).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti–tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthr. Rheum. 54, 2793–2806 (2006).
Pijpe, J. et al. Rituximab treatment in patients with primary Sjögren’s syndrome: An open-label phase II study. Arthr. Rheum. 52, 2740–2750 (2005).
Moser, T. et al. Long-term immunological consequences of anti-CD20 therapies on humoral responses to COVID-19 vaccines in multiple sclerosis: An observational study. Ther. Adv. Neurol. Disord. https://doi.org/10.1177/17562864221092092 (2022).
Kormelink, T. G. et al. Decrease in immunoglobulin free light chains in patients with rheumatoid arthritis upon rituximab (anti-CD20) treatment correlates with decrease in disease activity. Ann. Rheum. Dis. 69, 2137–2144 (2010).
van Vollenhoven, R. F. et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann. Rheum. Dis. 72, 1496–1502 (2013).
Hauser, S. L. et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 376, 221–234 (2017).
Kridin, K. & Ahmed, A. R. Post-rituximab immunoglobulin M (IgM) hypogammaglobulinemia. Autoimmun. Rev. 19, 102466 (2020).
Marco, H. et al. The effect of rituximab therapy on immunoglobulin levels in patients with multisystem autoimmune disease. BMC Musculoskelet Disord. 15, 178 (2014).
Keystone, E. et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: An open-label extension analysis. Arthr. Rheum. 56, 3896–3908 (2007).
Reddy, V., Martinez, L., Isenberg, D. A., Leandro, M. J. & Cambridge, G. Pragmatic Treatment of Patients With Systemic Lupus Erythematosus With Rituximab: Long-Term Effects on Serum Immunoglobulins. Arthr. Care Res. (Hoboken) 69, 857–866 (2017).
Schmidt, D. E., De Haan, N., Sonneveld, M. E. & Porceli, L. IgG-Fc glycosylation before and after rituximab treatment in immune thrombocytopenia. Sci. Rep. https://doi.org/10.1038/s41598-020-59651-7 (2020).
Font, G. et al. IgG N-glycosylation from Patients with Pemphigus Treated with Rituximab. Biomedicines 10, 1774 (2022).
Cencioni, M. T., Mattoscio, M., Magliozzi, R., Bar-Or, A. & Muraro, P. A. B cells in multiple sclerosis - from targeted depletion to immune reconstitution therapies. Nat. Rev. Neurol. 17, 399–414 (2021).
Guhr, T. et al. Enrichment of Sialylated IgG by Lectin Fractionation Does Not Enhance the Efficacy of Immunoglobulin G in a Murine Model of Immune Thrombocytopenia. PLoS ONE 6, e21246 (2011).
Käsermann, F. et al. Analysis and Functional Consequences of Increased Fab-Sialylation of Intravenous Immunoglobulin (IVIG) after Lectin Fractionation. PLoS ONE 7, e37243 (2012).
Stadlmann, J. et al. A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics 9, 4143–4153 (2009).
Culver, E. L. et al. Unique patterns of glycosylation in immunoglobulin subclass G4-related disease and primary sclerosing cholangitis. J. Gastroenterol. Hepatol. 34, 1878–1886 (2019).
van de Bovenkamp, F. S. et al. Adaptive antibody diversification through N-linked glycosylation of the immunoglobulin variable region. Proc. Natl. Acad. Sci. U S A 115, 1901–1906 (2018).
Kemna, M. J. et al. Galactosylation and Sialylation Levels of IgG Predict Relapse in Patients With PR3-ANCA Associated Vasculitis. EBioMedicine 17, 108–118 (2017).
Van Coillie, J. et al. The BNT162b2 mRNA SARS-CoV-2 vaccine induces transient afucosylated IgG1 in naive but not in antigen-experienced vaccinees. EBioMedicine 87, 1–19 (2023).
Roberts, D. M. et al. Rituximab-associated hypogammaglobulinemia: Incidence, predictors and outcomes in patients with multi-system autoimmune disease. J. Autoimmun. 57, 60–65 (2015).
Irie, E. et al. Severe hypogammaglobulinemia persisting for 6 years after treatment with rituximab combined chemotherapy due to arrest of B lymphocyte differentiation together with alteration of T lymphocyte homeostasis. Int. J. Hematol. 91, 501–508 (2010).
Walker, A. R. et al. Profound hypogammaglobulinemia 7 years after treatment for indolent lymphoma. Cancer Invest. 26, 431–433 (2008).
Verpoort, K. N. et al. Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthr. Rheum. 56, 3949–3952 (2007).
Vanderlocht, J., van Beers, J. J. B. C., Limburg, P. C., Damoiseaux, J. & Roozendaal, C. Antigen-specific detection of autoantibodies against myeloperoxidase (MPO) and proteinase 3 (PR3). Methods Mol. Biol. 1901, 153–176 (2019).
Gueirard, P. et al. Anti-myeloperoxidase antibodies: Immunological characteristics and clinical associations. J. Autoimmun. 4, 517–527 (1991).
Acknowledgements
We acknowledge the support of patient partners, private partners and active colleagues of the T2B consortium; see website: www.target-to-b.nl.
Funding
This collaboration project is financed by the PPP Allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen (SGF) under projectnumber LSHM18055-SGF to stimulate public–private partnerships and co-financing by health foundations that are part of the SGF. This study was supported by ReumaNederland. This study was supported by a research grant from the Landsteiner Foundation for Blood Transfusion (Grant1626) to TR. This study was supported by a research grant from the Vasculitis Foundation to PH (2016) (http://www.vasculitisfoundation.org/research/research-program/).
Author information
Authors and Affiliations
Consortia
Contributions
AMV: conceptualization, data curation, investigation, writing original draft. JK: conceptualization, data curation, investigation. NILD: investigation. LH, ZK, JK, AR, PH, BH, DW, RT, NAB: curated clinical data. TWK, SMH, AB and all other authors reviewed and edited the manuscript. TR: conceptualization, methodology, supervision, writing – original draft.
Corresponding author
Ethics declarations
Competing interests
JK received research grants for multicentre investigator-initiated trials DOT-MS (NCT04260711, ZonMW), Supernext (NCT04225312, Treatmeds) and BLOOMS (NCT05296161, ZonMW and Treatmeds); received consulting fees from F Hoffmann-La Roche, Biogen, Teva, Merck, Novartis, and Sanofi/Genzyme (all payments to institution); reports speaker relationships with F Hoffmann-La Roche, Biogen, Immunic, Teva, Merck, Novartis, and Sanofi/Genzyme (all payments to institution); and is on the adjudication committee of MS clinical trials of Immunic (payments to institution only). All other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Valk, A.M., Koers, J., Derksen, N.I.L. et al. Elevated Fab glycosylation of autoantibodies maintained during B cell depletion therapy. Sci Rep 15, 14770 (2025). https://doi.org/10.1038/s41598-025-99226-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99226-y
Keywords
This article is cited by
-
Antibody glycosylation in neuroimmune diseases
Journal of Translational Medicine (2025)