Abstract
The emergence of neuropathic pain is significantly influenced by the impairment of mitochondrial processes. Ensuring the stability of mitochondrial activity requires a delicate equilibrium between the processes of mitochondrial fission and fusion. However, the specific alterations in mitochondrial activity across different models of neuropathic pain and the underlying mechanisms remain largely unclear. We developed a persistent compression injury (CCI) model targeting the sciatic nerve in mice. CCI induced pain like behaviors in mice, which were associated with increased levels of dynamin related protein 1 (Drp1) and decreased expression of the fusion protein OPA1 and an increase in the percentage of DRG nerve cell mitochondria in the fission form, and a decrease in percentage in the fusion form. Ultrastructural analysis showed that mitochondria in CCI mice were smaller in perimeter and area, adopting a more circular shape. Overexpression of OPA1 mediated by AAV attenuated pain hypersensitivity, lowered oxidative stress, and expanded mitochondrial circumference and area. Mdivi-1 treatment reduced pain, whereas blocking fusion with MYLS22 augmented pain and oxidative stress and further led to increased mitochondrial fragmentation. Our results illustrate that Mitochondria in DRG nerve cell are highly sensitive to neuropathic pain. Modulating mitochondrial fission and fusion through targeted gene overexpression and pharmacological inhibitors restores mitochondrial dynamics, reduces oxidative stress, and alleviates neuropathic pain in mice. These findings position mitochondrial dynamics as promising therapeutic targets for pain management.
Similar content being viewed by others
Introduction
Primary damage or dysfunction in the nervous system causes neuropathic pain1. Its hallmark clinical features include spontaneous pain and nociceptive hypersensitivity. Current pain relievers are classified according to their modes of operation into non-steroidal anti-inflammatory medications (NSAIDs), opioids, ion channel regulators and adjunctive pain therapies2. Among them, morphine and other opioid drugs are the most extensively utilized traditional analgesics in the clinical setting and exhibit a potent therapeutic efficacy in alleviating pain3. However, their application is constrained by major disadvantages, such as the potential for dependency, reduced efficacy over time, and adverse effects on breathing and blood circulation with extended use. These issues make opioids a “double-edged sword” in pain management. Addressing neuropathic pain remains a significant hurdle in clinical settings. Therefore, uncovering the underlying processes that contribute to this condition and formulating potent treatment options are critical focuses for both researchers and healthcare providers.
Mitochondria are maternally inherited, highly dynamic cytoplasmic organelles that primarily generate energy through oxidative phosphorylation4. Mitochondrial pathology including decreased energy production, deranged calcium imbalance, heightened reactive oxygen species (ROS) production and disrupted mitophagy lead to neuropathic pain5,6,7,8,9. Optimal mitochondrial performance depends on a balance between mitochondrial fission and fusion10. Excessive mitochondrial fission can result in fragmentation, impaired electron transport chain activity, excessive ROS accumulation, and the overactivation of pro-inflammatory pathways, ultimately leading to cellular damage11. For example, paclitaxel induces excessive mitochondrial fission in rodent dorsal root ganglion neuronal cells, causing swelling and vacuoles that can cause damage to the cells12. Similarly, Streptozotocin (STZ) induces overexpression of Drp1 in diabetic mice, which triggers endoplasmic reticulum stress and NLRP3 inflammatory vesicle activation, leading to the development of NP13. Despite these insights, the role of mitochondrial dynamics within DRG nerve cell under CCI conditions remains unclear. Specifically, the effects of upregulating or downregulating mitochondrial fission and fusion proteins on pain behaviors require further investigation.
Disruption of equilibrium of mitochondrial fission and fusion gives rise to intracellular oxidative stress and an increased generation of ROS14. ROS have been recently linked to chronic pain, especially neuropathic and inflammatory pain5. Based on a still increasing research, it is known that changes in mitochondrial dynamics and changes in their structure and morphology bring about the production of ROS15,16. The processes mitochondrial fission and fusion can be disrupted by ROS. Removal of excess ROS promotes the shift from a fission to fusion state of mitochondria, which is characterized by high levels of ROS and promotes mitochondrial fission and impairs mitochondrial function. Here, we show that p110, a new and specific peptide inhibitor of Drp1 enzyme activity, reduces mitochondrial hyperdivision and ROS levels, increases mitochondrial membrane potential, and enhances mitochondrial integrity17,18. Additionally, mutations in Opa1, which regulate mitochondrial morphology, result in elevated ROS generation and a greater vulnerability to oxidative stress19. Collectively, these research findings suggest that maintaining mitochondrial function through the equilibrium of mitochondrial fission and fusion could serve as an approach for the prevention and treatment of neuropathic pain.
Currently, MYLS22 is the only available pharmacological inhibitor of mitochondrial fusion. MYLS22 is a specific and non-toxic small-molecule blocker of OPA1. It suppresses mitochondrial fusion and exacerbates mitochondrial damage20. In contrast, Mdivi-1, identified through pharmacological analysis of mitochondrial fragmentation inhibitors, blocks Drp1 function by reducing its self-assembly. This particular small molecule inhibitor focuses on Drp1 to enhance mitochondrial performance and safeguard the intended cells21,22. Mdivi-1 has been previously shown to suppress mechanical nociceptive hypersensitivity induced by various pain promoting agents (e.g. tumor necrosis factor, nitric oxide (NO), glial-derived neurotrophic factor). Inflammatory and neuropathic pain conditions are frequently associated with these substances23. In this research, we employed MYLS22 and Mdivi-1 to modulate the proteins responsible for mitochondrial fission and fusion, examining their impact on neuropathic pain.
To achieve this, we utilized a mouse model of neuropathic pain, focusing on mitochondrial fusion-related proteins as our primary targets. By employing overexpression techniques, pharmacological inhibitors, and other interventions, we modulated mitochondrial fission and fusion in DRG nerve cell to assess their effects on pain behaviors in CCI mice.
Materials and methods
Animals
We utilized adult male C57BL/6 mice (8–10 weeks), which were sourced from Beijing SPF Biotechnology Co., Ltd (China). The rodents were kept in separate enclosures within a serene, properly aerated setting that featured advanced air purification technology. We prepared standard food and water for the mice and allowed them to have free access to them. The full experiment was conducted in a light and dark regulated environment at a constant 24 °C ± 0.5 °C room temperature for 12 h. The CCI mice were closely observed for recovery after surgery, and the surgical wounds were inspected daily to ensure that they were clean and free of infection, blood seepage and other abnormalities. The Animal Ethics Committee of the University of Zhengzhou approved all animal experiments (ethics number: ZZUIRB2022-48). Every experimental protocol was in strict compliance with the recognized guidelines for the ethical treatment and employment of laboratory animals. All methods were performed according to relevant guidelines and regulations and according to ARRIVE guidelines.
Surgical procedures of CCI model
We created a CCI model in C57BL/6 mice following well-documented protocols. Animals were sedated with isoflurane (flow rate of 0.3–0.5 lit/min, 3% for induction, 1.5–2% for maintenance, delivered at 4 ml/h). To expose the sciatic nerve, we made an incision and carefully retracted the surrounding tissues. The sciatic nerve was ligated at three points using 6–0 sutures, with a spacing of 0.5–1 mm between each ligature. The ligature tension was fine-tuned to cause mild spasms in the calf muscles or toes while being secured. In the sham surgery group, we performed identical procedures, including nerve exposure, but omitted the ligation step.
Von Frey test
We used a von Frey filament (Stoelting Co., Wood Dale, IL, USA) to apply it vertically to the central plantar area to assess the mechanical sensitivity of hind paws by measuring the force needed to elicit a withdrawal reaction. The mice were accustomed to the experimental setting by being positioned on a wire grid surface for a minimum of 30 min before the test began. We responded the sole of the each rear foot with a 0.07 g von Frey filament that we made sure bend with each application. Each paw was activated 10 times, with a minimum gap of five minutes between the stimulation of the left and right rear paws. We repeated the stimulation procedure with a 0.4 g von Frey filament. For each trial a successful reaction was noted as foot retraction, trembling, or repeated paw licking. The number of positive reactions was calculated as a percentage of the total number of measurements to establish a paw withdrawal threshold.
Hargreaves test
Thermal hyperalgesia was assessed through the plantar thermal radiation technique. An infrared light was positioned at the center of the hind paw’s plantar surface to gauge the reaction to thermal stimulation. The typical mice showed initial withdrawal times ranging from 10 to 12 s. To prevent tissue damage in cases where the mice did not react, the exposure duration was limited to 20 s. Each rear foot was assessed on three separate occasions, with a minimum interval of 10 min between each test. We documented the response times, which included quick foot retraction, paw licking, or hopping. The mean delay from three tests was computed to establish the threshold for paw retraction.
Western-blot
We extracted protein samples from unilateral L3-5 DRGs of mice. To ensure sufficient protein mass, we pooled three DRGs from the same side of two mice. The samples were next chilled in a cytosolic extraction solution and centrifuged (10 min at 2000 rpm, 4 °C). The protein concentration was measured with a BCA protein quantification kit. A total of 15 μg of protein was subjected to electrophoresis on a 10% SDS–polyacrylamide gel. Subsequently, the proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with a 5% FBS solution for two hours at room temperature and then incubated with primary antibodies overnight at 4 °C. The following antibodies were employed: mouse anti-OPA1 (1:1000, BD Biosciences, RRID: 612606), rabbit anti-MFN2 (1:1000, Proteintech, RRID: 12186-1-AP), rabbit anti-DRP1 (1:1000, Abcam, RRID: AB184247), rabbit anti-β-actin (1:1000, Servicebio, RRID: GB-11001) and mouse anti-GAPDH (1:2000, Servicebio, RRID: GB11002). The membranes undergo three 10-min washes with TBST. Following this, they are incubated for two hours with a horseradish peroxidase-conjugated goat anti-rabbit IgG. (1:10,000, Abbkine, China). Using an enhanced chemiluminescent solution, the strips are developed in an imaging system. In the statistical analysis stage, the gray value of each lane was precisely measured with the help of Image J software. For statistics, β-actin or GAPDH was used as the internal reference, and the gray value of the target protein was divided by the gray value of the internal reference protein to obtain the preliminary ratio. Subsequently, the mean value of multiple sets of data in the control group was calculated, and finally each set of data was divided by this mean value to obtain the final normalized result.
Dorsal root ganglion microinjection
As previously described, DRG microinjections were performed. In summary, to reveal the unilateral L3 and L4 dorsal root ganglia, we performed a central incision in the lower back and removed the associated joint processes. Using a dissecting microscope, we administered 0.5 µL of AAV9 (1 × 1012 vg/ml) to each exposed DRG via a glass microcapillary connected to a Hamilton syringe. To ensure proper delivery, we left the injection pipette in place for 5 min before removal. All mice displayed normal locomotor activity following the procedure.
Electron microscopy
For the electron microscopy samples, we sedated the mice using isoflurane and then infused the L3-L5 dorsal root ganglia with a mixture of 4% paraformaldehyde and 4% glutaraldehyde in phosphate-buffered saline. The DRG tissues dissected were further fixed in a 2.5% glutaraldehyde solution. After fixation, tissues were washed 3 times with 0.1 M PBS, and stained with 1% osmium tetroxide for two hours. We dried the tissues at ambient temperature using a series of ethanol solutions (50%, 70%, 90%) followed by 100% acetone. We then embedded the samples in epoxy resin and placed them at ambient temperature to cure overnight. We utilized an ultramicrotome to generate sections with a thickness of 70 nm. These sections were then stained using 1% uranyl acetate for a duration of 15 min, followed by 1% lead citrate for 6 min. We captured the stained sections using a transmission electron microscope. The imaging technician was blinded to the experimental conditions to minimize bias. We analyzed the mitochondrial area and perimeter using Image-Pro Plus software to quantify morphological parameters.
Intrathecal injection
We performed intrathecal injections of Mdivi-1 (1.5 mg/kg, MCE, HY-15886, USA), MYLS22 (0.5 mg/kg, MCE, HY-136446, USA), or Mitotracker™ Deep Red probe (10 μl, 100 nM) into the L5-L6 spinal cord region using a Hamilton microsyringe. Successful injection was confirmed by an obvious tail flick response in the mice. After injection, we kept the needle in place for 1 min before gently rotating and withdrawing it to prevent leakage.
Mitotracker™ is a fluorescent dye that labels mitochondria in live cells. To assess mitochondrial distribution and subcellular morphology, we used the Mitotracker™ Deep Red probe (Mito-Red) in CCI mice. Imaging process was carried out using a confocal laser scanning microscope (Olympus FV 1000, Japan). To measure the quantity and size of individual mitochondria, ImageJ 3D Object Counter plug in was used.
DHE staining
To detect ROS, we sliced DRG tissues into 12 μm thick sections at − 20 °C. After the frozen sections were washed 3 times with 0.01 M PBS, diluted DHE solution (1.25 μ/ml) was added to the tissues and incubated for 30 min in a 37 °C incubator. The sections were then incubated for 30 s with DAPI (1:200, C1002, Beyotime Biotechnology, China). ROS were assessed with fluorescence microscopy to identify red fluorescence.
SOD activity detection
We measured superoxide dismutase (SOD) activity in the L3-L5 DRG of mice using the T-SOD assay kit (Elabscience, cat. E-BC-K020-M) as per the manufacturer’s directions. Values of OD were recorded and used them to calculate the SOD enzyme activity.
Statistical analysis
Carry out further statistical analysis by means of the GraphPad Prism 8.3.0 software. All measurements are given as means ± SD. When data followed a normal distribution and variance was homogeneous, comparisons between two groups were analyzed using an unpaired t-test. If the data had a normal distribution but non-homogenous variance, Welch’s correction unpaired t-test was used. For univariate comparisons involving two or more groups, we employed one-way ANOVA when the data followed a normal distributed with homogeneous variance. Tukey’s test was used for post-hoc comparisons. If the variance was not homogeneous, we applied the Brown-Forsythe ANOVA. The Kruskal–Wallis test was used for data that did not follow a normal distribution. For multivariate comparisons, we used two-way ANOVA when the data followed a normal distribution with homogeneous variance, followed by Sidak’s multiple comparison test. If the assumptions of normality and homogeneity were not met, we performed rank sum tests. Statistical significance was assumed in case the P value was less than 0.05.
Results
CCI induces upregulation of mitochondrial fission factor DRP1 and downregulation of mitochondrial fusion factors OPA1 and MFN2 in DRG
We assessed paw mechanical sensitivity (paw withdrawal threshold, PWT) and thermal sensitivity on the third and seventh days post-CCI with Von Frey test and hot plate nociceptometry respectively, and the control group was the sham surgery group. Withdrawal response to a Von Frey filament of 0.07 g and 0.4 g was markedly increased when stimulated on the surgical side.
On day 3, the CCI group exhibited higher withdrawal frequencies than the sham group (0.07 g, CCI: 50.833 ± 7.930%, sham: 7.500 ± 7.538%; 0.4 g, CCI: 71.667 ± 14.035%, sham: 32.500 ± 4.523%; P < 0.001). This difference persisted on day 7 (0.07 g, CCI: 60.833 ± 7.930%, sham: 9.167 ± 7.930%; 0.4 g, CCI: 78.333 ± 10.299%, sham: 30.000 ± 8.528%; P < 0.001) (Fig. 1A,B). The data show that CCI mice are more sensitive to 0.07 g stimulation than 0.4 g stimulation, and a strong pain response is elicited by mild stimulation, indicating that they are highly sensitive to mild stimulation. When the stimulus intensity was increased to 0.4 g, the response of CCI mice still increased, but the amplitude was limited, which may be close to the limit of the nociceptive response system.
Behavioral and mitochondrial fusion and fission protein expression changes at third and seventh days after CCI. A–F Behavioral assessments were conducted on mice at third and seventh days post-CCI. A The ipsilateral paw withdrawal threshold of 0.07 g was measured. B The ipsilateral paw withdrawal threshold of 0.4 g was measured. C The latency for thermal withdrawal on the ipsilateral side. D The contralateral paw withdrawal threshold of 0.07 g was measured. E The contralateral paw withdrawal threshold of 0.4 g was measured. F The latency for thermal withdrawal on the contralateral side. n = 12 per group. Two-way ANOVA succeeded by Sidak’s multiple comparisons test, ***P < 0.001 vs. sham group. G Changes in DRP1 protein expression in mouse DRG following CCI. H Changes in MFN2 protein expression in mouse DRG following CCI. I Changes in OPA1 protein expression in mouse DRG following CCI. n = 6 / 7 per group. One—way ANOVA and then Tukey’s multiple comparison procedure, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. sham group. Measurements are given as means ± SD.
Additionally, compared to sham controls, the thermal foot retraction latency was significantly shortened in the CCI group. On day 3, CCI mice had a shorter latency (6.417 ± 1.247 s) than sham mice (13.433 ± 1.072 s), and on day 7, CCI mice still had a shorter latency (7.092 ± 1.288 s vs. sham 12.817 ± 1.288 s) (Fig. 1C). There were no notable alterations in the mechanical withdrawal of the contralateral paw or the latency of thermal foot withdrawal (Fig. 1D-F). These results indicate that CCI mice are hypersensitive to both mechanical and thermal pain. We also assessed the levels of mitochondrial fission and fusion proteins in the DRG of CCI mice using Western blot techniques. The expression of DRP1, a crucial protein for mitochondrial fission, was markedly elevated in the DRG on the affected side on both the third and seventh days post-CCI (day 3: 5.941 ± 2.082, P < 0.001; day 7: 4.762 ± 2.090, P < 0.01) relative to the control group (Fig. 1G).
Mitochondrial fusion, regulated inversely to fission, was assessed by examining MFN2, a protein responsible for outer mitochondrial membrane fusion24,25. The expression of MFN2 was significantly downregulated in the DRG of CCI mice on both days 3 and 7 (day 3: 0.130 ± 0.063, P < 0.001; day 7: 0.517 ± 0.295, P < 0.05) compared to the sham group (Fig. 1H). The amounts of OPA1, a crucial protein implicated in the joining of the inner mitochondrial membrane and the upkeep of cristae structure26,27, were markedly reduced in the DRG of CCI mice (day 3: 0.428 ± 0.289, P < 0.05; day 7: 0.414 ± 0.209, P < 0.05) (Fig. 1I). These results indicate that CCI elicited augmentation of the mitochondrial fission protein DRP1 and a attenuation of the fusion proteins OPA1 and MFN2 in DRG.
Microscopic morphological changes in mitochondria after CCI with increased fragmentation, reduced volume, and network disruption in DRG nerve cell
Mitochondrial fission is characterized by a rise in the quantity of mitochondria and a decrease in their individual size28. In the Sham group, mitochondria in DRG nerve cell appeared as elongated tubules forming a highly interconnected network. In contrast, after CCI, mitochondria became spherical and shorter (Fig. 2A). The mitochondrial volume decreased significantly (CCI: 0.639 ± 0.135, P < 0.001) and the number of mitochondria increased (Sham: 14.95 ± 5.453, CCI: 25.30 ± 10.24, P < 0.001) in CCI DRG nerve cell compared to the Sham group (Fig. 2B,C).
CCI induces morphological changes and ultrastructural damage in mitochondria of DRG. A MitoTracker Red-stained representative confocal microscopy images of mitochondrial morphology after CCI. B Representative DRG nerve cell mitochondria electron microscopy images after CCI. Scale: 3 μm. C Mean Mitochondrial Volume Quantification. n = 6 per group. Unpaired t-test, ***P < 0.001 vs. sham group. D Quantification of mitochondrial number per cell. Line indicates median (n = 20 cells in each group). Unpaired t-test with Welch’s correction, ***P < 0.001 vs. sham group. E Measurement of mitochondrial area. F Measurement of mitochondrial perimeter. G Quantification of mitochondrial interconnectivity. Sham (n = 1,064 mitochondria), CCI (n = 1,146 mitochondria). Mann–Whitney U test, ***P < 0.001 vs. sham group. Measurements are given as means ± SD.
To delve deeper into the structural modifications, the fine architecture of the DRG mitochondria was analyzed using transmission electron microscopy (TEM) (Fig. 2D). We assessed three mitochondrial parameters: perimeter (μm), area (μm2), and interconnectivity (a lower score indicates more fragmentation). Neuronal mitochondria in CCI mice were significantly shorter in perimeter (Sham: 1.532 ± 0.651 μm, CCI: 0.910 ± 0.388 μm, P < 0.001), smaller in area (Sham: 0.145 ± 0.092 μm2, CCI: 0.061 ± 0.047 μm2, P < 0.001), and exhibited reduced interconnectivity (Sham: 0.089 ± 0.022, CCI: 0.060 ± 0.018, P < 0.001) on day 7 after CCI (Fig. 2E-G). These indicate that after CCI, the expression of mitochondrial fission proteins is high and that of fusion proteins is low, causing alterations in the quantity and structure of mitochondria.
AAV-mediated upregulation of OPA1 in DRG reduces pain sensitivity in CCI mice
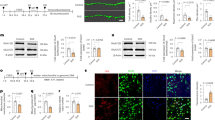
To explore the function of OPA1 in pain hypersensitivity caused by CCI, we employed AAVs to adjust the levels of mitochondrial fusion proteins. Specifically, we overexpressed OPA1 by injecting AAV-OPA1 into the operated-side L3-L4 DRG three weeks prior to CCI surgery. One week after CCI, we isolated the operated-side L3-L4 DRG for further analysis (Fig. 3A). Microscopic examination confirmed successful delivery of AAV-OPA1 to the DRG of CCI mice (Fig. 3B). Western blotting also confirmed that OPA1 overexpression (OE) led to an increase in OPA1 protein levels (CCI + AAV-NC: 0.470 ± 0.223, CCI + AAV-OPA1: 0.957 ± 0.376; P < 0.05) (Fig. 3C).
DRG microinjection of AAV-OPA1 for the exploration of its effects on neuropathic pain. A Experimental design for microinjecting AAV-OPA1 or control virus into the DRG. B Fluorescence images of the overexpression of OPA1 by viral injection into the DRG. Scale: 50 μm. C Levels of OPA1 protein in CCI mice post-microinjection of an adeno-associated virus construct designed for overexpression were analyzed. n = 7 per group. One—way ANOVA and then Tukey’s multiple comparison procedure, **P < 0.01 vs. sham group, #P < 0.05 vs. CCI + AAV-NC group. D–F DRG microinjection of AAV-OPA1 in DRG attenuates CCI mice pain behaviors. D The paw withdrawal threshold of 0.07 g on the same side was measured. E The paw withdrawal threshold of 0.4 g on the same side was measured. F The latency of thermal withdrawal on the same side. n = 12 per group. Two-way ANOVA succeeded by Sidak’s post-hoc test for multiple comparisons test, ***P < 0.001 vs. sham group, ###P < 0.001 vs. CCI + AAV-NC group. Measurements are given as means ± SD.
We then assessed the impact of OPA1 OE in the DRG on pain behaviors in CCI mice. Our results showed: OPA1 OE reduced the frequency of mechanical foot retraction in response to a 0.07 g stimulus (D7: Sham: 11.667 ± 5.774%, CCI + AAV-NC: 57.500 ± 9.653%, CCI + AAV-OPA1: 20.000 ± 7.385%; P < 0.01) and a 0.4 g stimulus (D7: Sham: 30.000 ± 8.528%, CCI + AAV-NC: 68.333 ± 13.371%, CCI + AAV-OPA1: 34.167 ± 5.149%; P < 0.01). Additionally, OPA1 OE increased the latency for thermal foot retraction (D7: Sham: 12.658 ± 1.174 s, CCI + AAV-NC: 8.100 ± 1.904 s, CCI + AAV-OPA1: 12.458 ± 1.456 s; P < 0.01) compared to the CCI group (Fig. 3D-F). These findings show that selective enhancement of OPA1 expression in dorsal root ganglia could reduce pain in mice with chronic constriction injury.
Morphological restoration of mitochondria following OPA1 overexpression in CCI mice
Mito-Red analysis revealed significant increased mitochondrial volume following OPA1 OE (Fig. 4A) (CCI + AAV-NC: 0.675 ± 0.106, CCI + AAV-OPA1: 1.178 ± 0.271; P < 0.01). Additionally, the number of mitochondria in DRG nerve cell decreased significantly (Sham: 16.17 ± 7.420, CCI + AAV-NC: 27.19 ± 11.65, CCI + AAV-OPA1: 16.91 ± 7.025; P < 0.001) compared to the CCI group (Fig. 4B-C). Electron microscopy showed that OPA1 overexpression in CCI mice resulted in larger mitochondrial area (Fig. 4D) (Sham: 0.145 ± 0.092 μm2, CCI + AAV-NC: 0.060 ± 0.044 μm2, CCI + AAV-OPA1: 0.137 ± 0.105 μm2; P < 0.001), longer mitochondrial circumference (Sham: 1.532 ± 0.651 μm, CCI + AAV-NC: 0.934 ± 0.394 μm, CCI + AAV-OPA1: 1.495 ± 0.690 μm; P < 0.001), and increased mitochondrial interconnectivity (Sham: 0.089 ± 0.022, CCI + AAV-NC: 0.059 ± 0.016, CCI + AAV-OPA1: 0.084 ± 0.025; P < 0.001) (Fig. 4E-G). These results suggest that raising levels of OPA1 facilitates the fusion of mitochondria in the sensory nerve cells of mice with chronic constriction injury.
Microscopic morphological changes in mitochondria after overexpression of OPA1. A Confocal microscopy images showcasing mitochondrial structure stained with MitoTracker Red. Scale: 20 μm. B Representative DRG nerve cell mitochondria electron microscopy images after overexpression of OPA1. C Mean Mitochondrial Volume Quantification. n = 6 per group. Unpaired t-test, ***P < 0.001 vs. CCI + AAV-NC group. D Quantification of mitochondrial number per cell. Line indicates the median. CCI + AAV-NC (n = 36 cells), CCI + AAV-OPA1 (n = 23 cells). E Measurement of mitochondrial area. F Measurement of mitochondrial perimeter. G Quantification of mitochondrial interconnectivity. CCI + AAV-NC (n = 1118 mitochondria), CCI + AAV-OPA1 (n = 1007 mitochondria). Mann–Whitney U test, ***P < 0.001 vs. CCI + AAV-NC group. Measurements are given as means ± SD.
Mitochondrial fusion inhibitor MYLS22 induces pain and mitochondrial fragmentation in mice
Further evaluate OPA1’s role in neuropathic pain, we used the mitochondrial fusion inhibitor MYLS22 to modulate mitochondrial fusion proteins. Mice were treated with continuous intrathecal injections of MYLS22 (0.5 mg/kg) for 3 days in a physiological state (Fig. 5A). The reduction of OPA1 protein expression by MYLS22 treatment was confirmed through Western blot analysis (MYLS22: 0.566 ± 0.140, P < 0.05) (Fig. 5B). Behavioral tests demonstrated that MYLS22 significantly increased the frequency of mechanical paw withdrawal (0.07 g, Left, D3, MYLS22: 47.500 ± 6.216%, P < 0.001; Right, D3, MYLS22: 49.167 ± 9.003%, P < 0.001) and reduced the latency of thermal paw withdrawal (Left, D3, MYLS22: 8.233 ± 1.574 s, P < 0.001; Right, D3, MYLS22: 8.700 ± 1.053 s, P < 0.001) compared to the control group (Fig. 5C-H). These findings suggest that MYLS22 causes increased mechanical and thermal pain sensitizations in mice.
Mitochondrial fusion inhibitor MYLS22 causes pain in mice. A Experimental design for the intrathecal injection of MYLS22. B The levels of OPA1 protein in CCI mice following the intrathecal administration of MYLS22. n = 6 per group. Unpaired t-test, *P < 0.05. C The left paw withdrawal threshold of 0.07 g was measured. D The right paw withdrawal threshold of 0.07 g was measured. E The left paw withdrawal threshold of 0.4 g was measured. F The right paw withdrawal threshold of 0.4 g was measured. G The latency for thermal withdrawal of the left paw. H The latency for thermal withdrawal of the right paw. n = 12 per group. Two-way ANOVA in combination with Sidak’s multiple comparisons test, ***P < 0.001 vs. Vehicle group. I MitoTracker Red-stained representative confocal microscopy images of mitochondrial morphology. Scale: 20 μm. J Representative DRG nerve cell mitochondria electron microscopy images following the intrathecal administration of MYLS22. Scale: 3 μm. K Mean Mitochondrial Volume Quantification. n = 6 per group. Unpaired t-test, ***P < 0.001 vs. Vehicle group. L Quantification of mitochondrial number per cell. Line indicates the median. Vehicle group: n = 42 cells, MYLS22: n = 42 cells. M Measurement of mitochondrial area. N Measurement of mitochondrial perimeter. O Quantification of mitochondrial interconnectivity. Control (n = 1064 mitochondria), MYLS22 (n = 1026 mitochondria). Mann–Whitney U test, ***P < 0.001 vs. Vehicle group. Measurements are given as means ± SD.
Mito-Red analysis revealed that after intrathecal injection of MYLS22, mitochondria became spherical and shortened (Fig. 5I). Mitochondria exhibited a decrease in size (MYLS22: 0.579 ± 0.063, P < 0.001) and an increase in number (Vehicle: 17.79 ± 8.356, MYLS22: 26.48 ± 8.755) (Fig. 5J-K). Electron microscopy further showed that mitochondria in the MYLS22-treated group were smaller in circumference (Fig. 5L) (Vehicle: 1.532 ± 0.651 μm, MYLS22: 1.349 ± 0.739 μm), reduced in area (Vehicle: 0.145 ± 0.092 μm2, MYLS22: 0.104 ± 0.096 μm2), and exhibited decreased interconnectivity (Vehicle: 0.089 ± 0.022, MYLS22: 0.068 ± 0.024) compared to the control group (Fig. 5M-O). These findings indicate that MYLS22-induced mitochondrial fusion inhibition resulted in fragmentation of DRG mitochondria.
Mdivi-1 inhibitor alleviates neuropathic pain and promotes mitochondrial fusion in CCI model mice
We utilized the mitochondrial fission blocker Mdivi-1 to perturb mitochondrial dynamics to explore the function of DRP1 in pain sensitization produced by CCI. The study was conducted after 3 days of continuous intrathecal injection of Mdivi-1 (1.5 mg/kg) starting on day 8 post-CCI (Fig. 6A). Western blot analysis confirmed that Mdivi-1 treatment reduced DRP1 protein expression (CCI: 2.151 ± 1.045, CCI + Mdivi-1: 0.967 ± 0.384; P < 0.05) (Fig. 6B). We subsequently assessed the impact of Mdivi-1 on pain indicators in mice with CCI. Behavioral assays demonstrated that intrathecal injection of Mdivi-1 significantly reduced the frequency of mechanical foot retraction (0.07 g, D10, Sham: 10.000 ± 8.528%; CCI: 56.364 ± 9.244%; CCI + Mdivi-1: 18.333 ± 7.177%; P < 0.01; 0.4 g, D10, Sham: 30.833 ± 5.149%; CCI: 66.364 ± 6.742%; CCI + Mdivi-1: 33.333 ± 7.785%; P < 0.001) and increased the latency of thermal foot retraction (Sham: 11.825 ± 0.952 s, CCI: 7.164 ± 1.142 s, CCI + Mdivi-1: 11.258 ± 0.737 s; P < 0.001) compared to the CCI group (Fig. 6C-E). These findings indicate that the application of Mdivi-1 decreased both mechanical and heat-induced pain sensitivity in mice subjected to CCI.
Intrathecal injection of Mdivi-1 for the exploration of its effects on neuropathic pain. A Experimental design for intrathecal injection of Mdivi-1. B The amounts of DRP1 protein in CCI mice after the intrathecal administration of Mdivi-1. n = 6 per group. One—way ANOVA and then Tukey’s multiple comparison procedure, *P < 0.05 vs. sham group, #P < 0.05 vs. CCI group. C The ipsilateral paw withdrawal threshold of 0.07 g was measured. D The ipsilateral paw withdrawal threshold of 0.4 g was measured. E The latency for thermal withdrawal of ipsilateral side. n = 11 / 12 per group. Two-way ANOVA succeeded by Sidak’s multiple comparison test, ***P < 0.001 vs. sham group, ###P < 0.001 vs. CCI group. F Confocal microscopy images showcasing mitochondrial structure stained with MitoTracker Red. Scale: 20 μm. G Representative DRG nerve cell mitochondria electron microscopy images after the intrathecal administration of Mdivi-1. Scale: 3 μm. H Mean Mitochondrial Volume Quantification. n = 6 per group. Unpaired t-test; **P < 0.01 vs. CCI group. I Quantification of mitochondrial number per cell. Line indicates the median. CCI (n = 39 cells), CCI + Mdivi-1 (n = 33 cells). J Measurement of mitochondrial area. K Measurement of mitochondrial perimeter. L Quantification of the mitochondrial interconnectivity. CCI (n = 1146 mitochondria), CCI + Mdivi-1 (n = 962 mitochondria). Mann–Whitney U test, ***P < 0.001 vs. CCI group. Measurements are given as means ± SD.
The Mito-Red analysis showed a marked rise in mitochondrial size following the intrathecal administration of Mdivi-1 (Fig. 6F) (CCI: 0.682 ± 0.137, CCI + Mdivi-1: 1.030 ± 0.185). However, after Mdivi-1 treatment, the quantity of mitochondria in DRG nerve cell saw a notable reduction (Sham: 16.17 ± 7.420, CCI: 25.77 ± 9.158, CCI + Mdivi-1: 17.76 ± 6.805) when compared to the CCI group (Fig. 6G-H). Electron microscopy further demonstrated that mitochondrial circumference (Fig. 6I) (Sham: 1.532 ± 0.651 μm, CCI: 0.910 ± 0.388 μm, CCI + Mdivi-1: 1.357 ± 0.690 μm), area (Sham: 0.145 ± 0.092 μm2, CCI: 0.061 ± 0.047 μm2, CCI + Mdivi-1: 0.126 ± 0.109 μm2), and interconnectivity (Sham: 0.089 ± 0.022, CCI: 0.060 ± 0.018, CCI + Mdivi-1: 0.081 ± 0.029) were all significantly improved in DRG nerve cell of CCI mice after Mdivi-1 administration (Fig. 6J-L). These findings suggest that Mdivi-1 promotes mitochondrial fusion in DRG nerve cell of CCI mice.
OPA1 upregulation and Mdivi-1 injection alleviate CCI-induced ROS elevation, while MYLS22 exacerbates oxidative damage
Growing research connects modifications in mitochondrial architecture and form, influenced by shifts in mitochondrial dynamics, to the generation of reactive oxygen species29,30. To test whether this was the case for DRG nerve cell, we measured reactive ROS levels with DHE staining (Fig. 7A). DHE serves as an exceptionally precise and sensitive indicator for identifying and measuring the accumulation of ROS31. Our findings indicated a substantial elevation in ROS levels within DRG nerve cell following CCI, as opposed to the sham group. Furthermore, overexpression of OPA1 and intrathecal injection of Mdivi-1 both reduced ROS accumulation in DRG nerve cell of CCI mice (CCI: 2.602 ± 0.152, CCI + AAV-NC: 2.143 ± 0.229, CCI + AAV-OPA1: 1.324 ± 0.218, CCI + Mdivi-1: 1.295 ± 0.261) (Fig. 7B). In contrast, ROS accumulation was significantly higher in DRG nerve cell after MYLS22 treatment compared to the control group (Vehicle: 1.079 ± 0.063, MYLS22: 1.654 ± 0.440, P < 0.05) (Fig. 7C).
Targeted upregulation of OPA1 and intrathecal injection of Mdivi-1 alleviates CCI-induced ROS elevation, whereas intrathecal injection of MYLS22 exacerbates oxidative damage. A Images that are representative of DRG ROS signals detected by means of DHE staining. B Mean fluorescence intensity of DHE after targeted OPA1 upregulation and intrathecal injection of Mdivi-1. One—way ANOVA and then Tukey’s multiple comparison procedure, ***P < 0.001 vs. sham group, ###P < 0.001 vs. CCI or CCI + NC group. C Fluorescence intensity of DHE after intrathecal administration of MYLS22 is shown. n = 6 per group. Unpaired t-test with Welch’s correction, *P < 0.05 vs. sham group. D Detection of SOD activity after DRG microinjection of OPA1 overexpressing virus. E Detection of SOD activity after intrathecal injection of Mdivi-1. One—way ANOVA and then Tukey’s multiple comparison procedure, *P < 0.05, **P < 0.01 vs. sham group, ##P < 0.01, ###P < 0.001 vs. CCI group. F Assessment of SOD levels following the intrathecal administration of MYLS22. Unpaired t-test, *P < 0.05 vs. Vehicle group. n = 6 per group. Measurements are given as means ± SD.
The cellular redox state is strictly modulated by the balance between oxidative and antioxidant systems, which involve various enzymes. Within the nervous system, SOD, catalase, glutathione peroxidase, and heme oxygenase collaborate with NAD(P)H to regulate the balance of reactive oxygen species32. SOD is a major antioxidant enzyme whose activity increases significantly to stop oxidative reactions when the body is subjected to oxidative stress33. To evaluate the impact of treatments on SOD activity in DRG nerve cell, we assessed its activity across experimental groups. Our data revealed a significant drop in the SOD activity after CCI when contrasted with the control group. However, overexpression of OPA1 (Sham: 42.24 ± 3.474, CCI + AAV-NC: 36.26 ± 4.527, CCI + AAV-OPA1: 46.56 ± 3.285) and intrathecal injection of Mdivi-1 (Sham: 71.00 ± 7.247, CCI: 45.77 ± 11.11, CCI + Mdivi-1: 68.09 ± 10.67) increased SOD activity in DRG nerve cell of CCI mice (Fig. 7D-E). In contrast, SOD activity was reduced following MYLS22 treatment compared to the control group (Vehicle: 66.20 ± 2.372, MYLS22: 60.49 ± 5.250) (Fig. 7F). These findings suggest that targeted upregulation of OPA1 and intrathecal injection of Mdivi-1 alleviate CCI-induced oxidative stress, while MYLS22 exacerbates oxidative damage.
Discussion
Increasingly neuropathic pain, a crippling long-term condition, is associated with mitochondrial dynamic disruptions, in particular mechanisms of mitochondrial fission and fusion34. There is no doubt that mitochondria are important in neuronal operations, producing ATP, controlling calcium levels, preventing ROS and effects on cell death35. These mechanisms require stability of mitochondrial fission and fusion, and misregulation of this equilibrium is presumed to be a major contributing factor to the pathogenesis of neuropathic pain. In this research, we explored the impact of mitochondrial dynamics on neuropathic pain using a CCI model. This model was first developed by Bennett and Xie in rats to mimic human neuropathic pain36. This model is characterized by pain sensitivity to mechanical and thermal stimuli and spontaneous pain 1 d after surgery, and the pain caused by nerve ligation can last 15–30 days and has the clinical characteristics of neuropathic pain37. Generally, at about 7 days after surgery, the inflammatory response and nerve remodeling caused by nerve injury gradually stabilize, and pain-related behaviors also tend to stabilize38,39. Our findings indicate that pain behaviors triggered by CCI have a connection with substantial changes in mitochondrial dynamics within the DRG. These changes include an increase in expression of the mitochondrial fission protein DRP1, decrease in levels of the fusion protein OPA1 and an increase in ROS levels. The ultrastructural examination showed that these molecular modifications were linked to significant changes in the structure of mitochondria, including a decrease in area, perimeter, and interconnectivity, as well as a shift to a more spherical shape.
Here we show that changes in DRG mitochondrial dynamics are closely correlated to pain behaviors induced by CCI. We observed that stable overexpression of OPA1, and treatment with the mitochondrial division blocker Mdivi-1, attenuated CCI-induced pain behaviors. Consistent with these results, ultrastructural analysis showed that OPA1 overexpression and DRP1 inhibition significantly improved mitochondrial morphology, including enhanced area, perimeter, and interconnectivity. Furthermore, treatment with the mitochondrial fusion inhibitor MYLS22 exacerbated nociceptive sensitization and induced mitochondrial fragmentation. Collectively, our data suggest vulnerability of mitochondria to neuropathic pain and the rational use of mitochondrial fission and fusion processes as a novel approach for pain therapy.
Neurons rely heavily on mitochondria to sustain their intricate architecture and operational capabilities. They are involved in ATP generation, Ca2+ buffering, neurotransmitter synthesis and degradation, ROS generation and elimination, apoptosis, and metabolism40,41. These functions are regulated by mitochondrial dynamics42. Disruptions in mitochondrial dynamics contribute significantly to functional impairments in sensory neurons and the degeneration of neuronal terminals. The processes of mitochondrial fission and fusion are crucial for the creation of synapses and the growth of dendritic spines. Alterations in these processes hinder the transport of organelles to synapses, leading to the loss of dendritic spine mitochondria. This, in turn, impairs synapse formation and disrupts neurotransmission43. Our results underscore the critical role of maintaining a harmonious state of mitochondrial fission and fusion in nerve cells. We propose that mitochondria in the DRG participate importantly in pain regulation and that their morphology is changed in the neuropathic pain condition.
Modifications in mitochondrial dynamics profoundly affect a wide range of mitochondrial activities, such as calcium balance, cell death processes, ROS generation, energy conversion, and the proper transport and distribution of mitochondria within neurons44. Mitochondrial fission is initiated by DRP1, which binds to Fis1 on the mitochondrial surface, with its activity regulated by phosphorylation at serine 616 and 63745. These changes in the levels of proteins involved in mitochondrial fusion (Mfn1 and OPA1) and fission (DRP1) in both spinal cord and DRG are associated with inflammatory pain caused by complete freund’s adjuvant (CFA) and neuropathic pain caused by CCI in rats46,47. Inhibition of DRP1 alleviated mechanical nociceptive hypersensitivity in these pain models. We confirmed these results in a murine CCI model and further explored the function of DRP1 by employing the mitochondrial fission blocker Mdivi-1. Our results confirm that inhibiting DRP1 reduces mitochondrial fragmentation and alleviates CCI-induced neuropathic pain by restoring mitochondrial morphology.
The fusion of the mitochondrial inner membrane is crucially dependent on OPA1 that maintains the architecture of cristae and that of the respiratory chain protein complexes48,49. Studies have shown that knocking down or mutating OPA1 leads to mitochondrial fragmentation, while high OPA1 expression inhibits mitochondrial fission and enhances fusion capacity. In mice, overexpression of OPA1 can rescue mitochondrial dysfunction related to respiratory chain complex deficiencies50.Moreover, the upregulation of OPA1 significantly decreases neuronal cell death and mitigates issues related to mitochondrial impairment51. However, the potential of OPA1 in alleviating neuropathic pain has not been explored before. In this research, we observed that downregulating OPA1 with the mitochondrial fusion inhibitor MYLS22 induced mitochondrial fragmentation in DRG nerve cell, transforming mitochondria into spherical shapes and resulting in nociceptive hypersensitivity in mice. It is worth noting that the effect of intrathecal MYLS22 injection on pain for 3 consecutive days was significant, and given the possible adaptive changes in the organism, future studies could explore the effect at a broader time scale such as 7 or 10 days. Conversely, upregulation of OPA1 using AAV-OPA1 viral vectors inhibited mitochondrial fragmentation, promoted fusion, and alleviated CCI-induced neuropathic pain. Therefore, we speculate that the previous research by Gabriel Civileto et al. may be that OPA1 causes changes in respiratory complexes by changing the morphology of mitochondria, thereby alleviating mitochondrial diseases. Our work extends and builds upon these findings by highlighting OPA1’s role in neuropathic pain.
Mitochondria are key producers of ROS, and the mitochondrial dynamics disorder observed in our study might be one of the causes for the increased ROS production in CCI-induced neuropathic pain52. Early factors leading to overproduction of ROS and mitochondrial dysfunction have been reported to be imbalances in mitochondrial fusion and fission53. For instance, hyperglycemic conditions lead to mitochondrial fragmentation and subsequent ROS overproduction through Drp1 activation, while promoting mitochondrial fusion can prevent this overproduction54. Blocking fission of mitochondria in alveolar type II cells of mice has been found to abate paraquat induced production of ROS and subsequent cell death55. Other experiments revealed that the compound, Mdivi-1, reduced oxidative stress and limited cell death in cultured primary hippocampal cells derived from rats. Moreover, inhibiting MFN1 and MFN2 revealed that mitochondrial fission correlates with increased ROS generation56. Mutations in OPA1 that disrupt mitochondrial morphology lead to elevated ROS production and heightened sensitivity to oxidative stress19. Furthermore, decrease of OPA1 levels and increase of Drp1 levels in rat models of neuropathic pain were correlated with oxidative stress, and was counteracted in both cases with mitochondria targeted antioxidants.
Persistent pain, and particularly neuropathic and inflammatory conditions are substantially influenced by ROS5. Peripheral nerve injury leads to increased ROS levels in DRG cells. In animal models, intrathecal injection of the ROS donor TBHP induces mechanical nociceptive hypersensitivity, with heightened pain behavior linked to the inactivation of antioxidant enzymes SOD 1 and 257. In the case of animals developing neuropathic pain induced by vincristine (VCR), cisplatin and oxaliplatin, increased levels of lipid peroxidation and protein oxidation have been detected in their spinal cords. It is well established that ROS are crucial mediators of the onset and progression of neuropathic pain57,58. Our study corroborates these findings, showing increased ROS levels and decreased SOD activity in the DRG following CCI. Administration of systemic or intrathecal antioxidants, including phenyl-N-tert-butyl nitrone (PBN) or 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), nearly fully eliminates mechanical hypersensitivity in rodent models of neuropathic pain produced by spinal nerve ligation (SNL) within a few hours. Countless clinical trials have manifested that free radical scavengers, including N-acetylcysteine (NAC), dimethyl sulfoxide, vitamin C, and phenyl-N-tert-butyl nitrone can alleviate the symptoms and signs of neuropathic pain59,60. Our observations are consistent with these observations, and our research reveals that decreasing levels of ROS in the DRG relieves CCI induced both mechanical and thermal hyperalgesia.
In animal models of neuropathic pain induced by bortezomib, oxaliplatin, cisplatin, or VCR, excessive mitochondrial fusion in both peripheral nerves and DRG nerve cell leads to mitochondrial swelling and vacuolization61. These impaired mitochondria instead activate the mitochondrial permeability transition pore (mPTP), which causes Ca2+ discharge, disturbs membrane potential, and heightens neuronal excitability leading to the development of neuropathic pain62. Mdivi-1, a mitochondrial fission inhibitor, alleviates mechanical hyperalgesia in mice induced by TNFα, glial-derived neurotrophic factors, and NO63. Additionally, mitochondria-targeted antioxidant (Mito-TEMPO) enhances OPA1 expression and promotes mitochondrial fusion, thereby mitigating CCI-induced hyperalgesia64. Our results align with these findings. Moreover, our prior research has demonstrated that SIRT3, a deacetylase, is involved in modulating the processes of mitochondrial fission and fusion (data not yet published). Future studies will examine how regulating its upstream and downstream pathways affects neuropathic pain. This work builds on and extends our previous research. Despite its significant contributions, this study has some limitations. For instance, employing genetically modified mice to delve deeper into the functions of mitochondrial fission and fusion proteins in neuropathic pain could offer more conclusive findings. Future studies ought to delve into the regulatory processes both preceding and following changes in mitochondrial dynamics as a reaction to CCI.
In short, our findings indicate that critical regulators of mitochondrial fission and fusion, including Drp1 and Opa1, are disrupted and serve as the leading cause of mitochondrial dysfunction in neuropathic pain. By increasing Opa1 expression or inhibiting Drp1 activity, we successfully restored mitochondrial homeostasis and reduced oxidative stress in DRG. This restoration of mitochondrial homeostasis effectively alleviated neuropathic pain symptoms in mice and demonstrates the potential of modulating mitochondrial dynamics as a novel treatment for neuropathic pain. Future studies could target mitochondrial fission fusion for the development of novel analgesic drugs, thereby applying the fundamental findings regarding the regulation of mitochondrial dynamics to clinical practice.
Data availability
Data are available upon request from the corresponding author to support the results of this study.
References
Finnerup, N. B., Kuner, R. & Jensen, T. S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 101(1), 259–301 (2021).
Gurba, K. N., Chaudhry, R. & Haroutounian, S. Central neuropathic pain syndromes: Current and emerging pharmacological strategies. CNS Drugs 36(5), 483–516 (2022).
Mücke, M., Phillips, T., Radbruch, L. et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Db Syst Rev 3(3), CD012182 https://doi.org/10.1002/14651858.CD012182.pub2 (2018).
Machado, N. D. et al. Targeting mitochondrial oxidative phosphorylation: Lessons, advantages, and opportunities. Br. J. Cancer 129(6), 897–899 (2023).
Kallenborn-Gerhardt, W., Schroder, K. & Schmidtko, A. NADPH oxidases in pain processing. Antioxidants (Basel), 11(6) (2022).
Doyle, T. M. & Salvemini, D. Mini-review: Mitochondrial dysfunction and chemotherapy-induced neuropathic pain. Neurosci. Lett. 760, 136087 (2021).
Dai, C. Q., Guo, Y. & Chu, X. Y. NEUROPATHIC pain: The dysfunction of Drp1, mitochondria, and ROS homeostasis. Neurotox Res. 38(3), 553–563 (2020).
Zeng, Y. et al. Melatonin improves mitochondrial dysfunction and attenuates neuropathic pain by regulating SIRT1 in dorsal root ganglions. Neuroscience 534, 29–40 (2023).
Li, J. et al. Combination of autophagy and NFE2L2/NRF2 activation as a treatment approach for neuropathic pain. Autophagy 17(12), 4062–4082 (2021).
Dong, F. F. et al. Mitochondrial fusion and fission are required for proper mitochondrial function and cell proliferation in fission yeast. FEBS J. 289(1), 262–278 (2022).
Jezek, J., Cooper, K.F. & Strich, R. The impact of mitochondrial fission-stimulated ROS production on pro-apoptotic chemotherapy. Biology (Basel). 10(1) (2021).
Xu, Y., Jiang, Z. & Chen, X. Mechanisms underlying paclitaxel-induced neuropathic pain: Channels, inflammation and immune regulations. Eur. J. Pharmacol. 933, 175288 (2022).
Jheng, H. F. et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell Biol. 32(2), 309–319 (2012).
Craige, S. M. et al. Interplay of ROS, mitochondrial quality, and exercise in aging: Potential role of spatially discrete signaling. Redox Biol. 77, 103371 (2024).
Jiang, Y. et al. Caveolin-1 controls mitochondrial damage and ROS production by regulating fission - fusion dynamics and mitophagy. Redox Biol. 52, 102304 (2022).
Kim, B. & Song, Y. S. Mitochondrial dynamics altered by oxidative stress in cancer. Free Radic. Res. 50(10), 1065–1070 (2016).
Song, Z. et al. Inhibition of Drp1- Fis1 interaction alleviates aberrant mitochondrial fragmentation and acute kidney injury. Cell Mol. Biol. Lett. 29(1), 31 (2024).
Srivastava, A., et al., Therapeutic potential of P110 peptide: New insights into treatment of Alzheimer’s disease. Life (Basel), 13(11) (2023).
Quintana-Cabrera, R. et al. Opa1 relies on cristae preservation and ATP synthase to curtail reactive oxygen species accumulation in mitochondria. Redox Biol. 41, 101944 (2021).
Herkenne, S., Ek, O., Zamberlan, M. et al. Developmental and Tumor Angiogenesis Requires the Mitochondria-Shaping Protein Opa1. Cell Metab 31(5), 987-1003 e8 (2020).
Chuang, Y. C. et al. Peroxisome proliferator-activated receptor-gamma dependent pathway reduces the phosphorylation of dynamin-related protein 1 and ameliorates hippocampal injury induced by global ischemia in rats. J. Biomed. Sci. 23(1), 44 (2016).
Wu, Q. R. et al. High glucose induces Drp1-mediated mitochondrial fission via the Orai1 calcium channel to participate in diabetic cardiomyocyte hypertrophy. Cell. Death Dis. 12(2), 216 (2021).
Ferrari, L. F. et al. Role of Drp1, a key mitochondrial fission protein, neuropathic pain. J. Neurosci. 31(31), 11404–11410 (2011).
Gao, S. & Hu, J. Mitochondrial fusion: The machineries in and out. Trends Cell Biol. 31(1), 62–74 (2021).
Ding, M. et al. Mfn2-mediated mitochondrial fusion alleviates doxorubicin-induced cardiotoxicity with enhancing its anticancer activity through metabolic switch. Redox Biol. 52, 102311 (2022).
Noone, J., O’Gorman, D. J. & Kenny, H. C. OPA1 regulation of mitochondrial dynamics in skeletal and cardiac muscle. Trends Endocrinol. Metab. 33(10), 710–721 (2022).
Nyenhuis, S. B. et al. OPA1 helical structures give perspective to mitochondrial dysfunction. Nature 620(7976), 1109–1116 (2023).
Tang, Q. et al. Dynamin-related protein 1-mediated mitochondrial fission contributes to IR-783-induced apoptosis in human breast cancer cells. J. Cell Mol. Med. 22(9), 4474–4485 (2018).
Koren, S. A. et al. All-optical spatiotemporal mapping of ROS dynamics across mitochondrial microdomains in situ. Nat. Commun. 14(1), 6036 (2023).
Brillo, V., et al., Mitochondrial dynamics, ROS, and cell signaling: A blended overview. Life (Basel) 11(4) (2021).
Hervera, A. et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 20(3), 307–319 (2018).
Obeme-Nmom, J. I. et al. Regulation of redox enzymes by nutraceuticals: A review of the roles of antioxidant polyphenols and peptides. Food Funct. 15(22), 10956–10980 (2024).
Kishore, L., Kaur, N. & Singh, R. Effect of Kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in Streptozotocin-induced diabetic neuropathy. Inflammopharmacology 26(4), 993–1003 (2018).
Willemen, H. et al. Inflammation-induced mitochondrial and metabolic disturbances in sensory neurons control the switch from acute to chronic pain. Cell Rep. Med. 4(11), 101265 (2023).
Monzel, A. S., Enriquez, J. A. & Picard, M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat. Metab. 5(4), 546–562 (2023).
Bennett, G. J. & Xie, Y. K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33(1), 87–107 (1988).
Zhi, M. J. et al. Application of the chronic constriction injury of the partial sciatic nerve model to assess acupuncture analgesia. J. Pain Res. 10, 2271–2280 (2017).
Wan, K. et al. Electroacupuncture alleviates neuropathic pain by suppressing ferroptosis in dorsal root ganglion via SAT1/ALOX15 signaling. Mol. Neurobiol. 60(10), 6121–6132 (2023).
Shao, S. et al. Divanillyl sulfone suppresses NLRP3 inflammasome activation via inducing mitophagy to ameliorate chronic neuropathic pain in mice. J. Neuroinflamm. 18(1), 142 (2021).
Guan, S., Zhao, L. & Peng, R. Mitochondrial respiratory chain supercomplexes: from structure to function. Int. J. Mol. Sci.. 23(22) (2022).
Song, J., Herrmann, J. M. & Becker, T. Quality control of the mitochondrial proteome. Nat. Rev. Mol. Cell Biol. 22(1), 54–70 (2021).
Ketenci, M., Zablocki, D. & Sadoshima, J. Mitochondrial quality control mechanisms during diabetic cardiomyopathy. JMA J. 5(4), 407–415 (2022).
Cerqueira, F. M. et al. Diluted serum from calorie-restricted animals promotes mitochondrial beta-cell adaptations and protect against glucolipotoxicity. FEBS J. 283(5), 822–833 (2016).
Adebayo, M. et al. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 35(6), e21620 (2021).
Li, X. et al. Ischemia-induced cleavage of OPA1 at S1 site aggravates mitochondrial fragmentation and reperfusion injury in neurons. Cell. Death Dis. 13(4), 321 (2022).
Xie, M. et al. 2-Bromopalmitate attenuates inflammatory pain by maintaining mitochondrial fission/fusion balance and function. Acta Biochim. Biophys. Sin. (Shanghai) 53(1), 72–84 (2021).
Kun, L. et al. Hyperbaric oxygen promotes mitophagy by activating CaMKKbeta/AMPK signal pathway in rats of neuropathic pain. Mol. Pain 15, 1744806919871381 (2019).
Sessions, D. T. et al. Opa1 and Drp1 reciprocally regulate cristae morphology, ETC function, and NAD(+) regeneration in KRas-mutant lung adenocarcinoma. Cell Rep. 41(11), 111818 (2022).
Cartes-Saavedra, B. et al. OPA1 disease-causing mutants have domain-specific effects on mitochondrial ultrastructure and fusion. Proc. Natl. Acad. Sci. USA 120(12), e2207471120 (2023).
Civiletto, G. et al. Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metab. 21(6), 845–854 (2015).
Dong, T. et al. Opa1 prevents apoptosis and cisplatin-induced ototoxicity in murine cochleae. Front. Cell Dev. Biol. 9, 744838 (2021).
Dai, C. Q., Guo, Y. & Chu, X. Y. Neuropathic pain: The dysfunction of Drp1, mitochondria, and ROS homeostasis. Neurotox. Res. 38(3), 553–563 (2020).
Fao, L. & Rego, A. C. Mitochondrial and redox-based therapeutic strategies in Huntington’s disease. Antioxid Redox Signal 34(8), 650–673 (2021).
Rovira-Llopis, S. et al. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 11, 637–645 (2017).
Zhao, G. et al. Crosstalk between mitochondrial fission and oxidative stress in paraquat-induced apoptosis in mouse alveolar type II cells. Int. J. Biol. Sci. 13(7), 888–900 (2017).
Dhapola, R. et al. Recent advances in molecular pathways and therapeutic implications targeting mitochondrial dysfunction for Alzheimer’s disease. Mol. Neurobiol. 59(1), 535–555 (2022).
Shi, Y., Yuan, S. & Tang, S. J. Reactive oxygen species (ROS) are critical for morphine exacerbation of HIV-1 gp120-induced pain. J. Neuroimmune Pharmacol. 16(3), 581–591 (2021).
Zhou, Y. Q. et al. The therapeutic potential of Nrf2 inducers in chronic pain: Evidence from preclinical studies. Pharmacol. Ther. 225, 107846 (2021).
Simasingha, N. et al. Efficacy of dexamethasone and N-acetylcysteine combination in preventing post-embolization syndrome after transarterial chemoembolization in hepatocellular carcinoma. World J. Gastroenterol. 29(5), 890–903 (2023).
Paez-Hurtado, A. M., Calderon-Ospina, C. A. & Nava-Mesa, M. O. Mechanisms of action of vitamin B1 (thiamine), B6 (pyridoxine), and B12 (cobalamin) in pain: A narrative review. Nutr. Neurosci. 26(3), 235–253 (2023).
Waseem, M. et al. Role of mitochondrial mechanism in chemotherapy-induced peripheral neuropathy. Curr. Drug Metab. 19(1), 47–54 (2018).
Ma, J. et al. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer 124(11), 2289–2298 (2018).
Ferrari, L. F. et al. Role of Drp1, a key mitochondrial fission protein, in neuropathic pain. J. Neurosci. 31(31), 11404–11410 (2011).
Zhan, L. et al. Effect of mito-TEMPO, a mitochondria-targeted antioxidant, in rats with neuropathic pain. NeuroReport 29(15), 1275–1281 (2018).
Acknowledgements
Not applicable.
Funding
Natural Science Foundation of China (No. 81901335 assigned to Y.Y.S. and No. 82203969 assigned to Y.R.X.) sponsored this work.
Author information
Authors and Affiliations
Contributions
Yanyan Sun and Jing Cao conceptualized and designed the project; Liu Xie and Wanting Chang performed the behavioral tests, western blotting examination, immunofluorescence experiments, Mitochondrial morphology examination; Liu Xie, Wanting Chang, Linna Song analyzed the data; Liu Xie and Qingqing Yang carried out CCI model and assisted in immunofluorescence experiments; Liu Xie wrote the manuscript. Yanyan Sun and Yiran Xu revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not relevant.
Consent for publication
All authors are in agreement to take on the responsibility for their contributions and have sanctioned the submitted version of the paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, L., Cao, J., Xu, Y. et al. Mechanistic study of modulating mitochondrial fission and fusion to ameliorate neuropathic pain in mice. Sci Rep 15, 15571 (2025). https://doi.org/10.1038/s41598-025-99300-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99300-5