Abstract
To explore the mechanisms by which nitrogen alleviates drought stress in Phoebe bournei, this study integrated drought treatment with exogenous nitrogen application to assess physiological characteristics and employed transcriptome sequencing to decipher transcriptional responses. The results indicated that nitrogen fertilizer mitigated leaf wilting in P. bournei under drought stress and significantly enhanced leaf dry weight, fresh weight, thickness, and chlorophyll content. Furthermore, nitrogen improved photosynthesis by inhibiting stomatal closure, enhancing light energy absorption, and accelerating electron transport in PSII. 11 photosynthesis-related genes, including PFP, TRY, LQY, FTSH, FRO, CURT, PETF, ATPF, PETA, CRRSP, and MEN and 17 carbohydrate metabolism-associated genes, such as PWD, GBE1, GAPA, PFKA, RFS, ISA, GLGC, PGK, ALDO, GUX, RX9, MIOX, HCT, BAM, MPFP, and ERNI exhibited differential expression in response to nitrogen. Moreover, nitrogen treatment significantly modulated plant hormone metabolism, with 44 upregulated and 14 downregulated differentially expressed genes (DEGs) primarily associated with jasmonic acid (JA) synthesis and signaling. These findings provide new insights into enhancing the drought tolerance of P. bournei in the context of global climate change.
Similar content being viewed by others
Introduction
Drought is one of the most significant environmental factors limiting plant growth, affecting both agriculture and forestry, and is a major abiotic stressor for plants. Drought leads to decreased plant biomass, stomatal closure, and photoinhibition, while also affecting physiological processes such as water transport and photosynthetic assimilation in plants1,2. Under drought stress, the photosynthesis, leaf water potential, and water content of plants are significantly reduced3. Currently, numerous studies have reported on the impacts of drought stress on forest trees. For instance, drought treatment has resulted in wilting, curling, and even shedding of poplar leaves, with long-term drought causing greater damage than short-term drought4. Drought has led to a reduction in leaf area and suppressed branch growth in black poplar; it has also decreased the number of leaves and lowered biomass5. Phoebe bournei, belonging to the Lauraceae family and the Phoebe genus, is an evergreen large tree6,7. Its wood boasts a strong fragrance, high durability, and dense and tough texture, making it a premium material for furniture and possessing significant economic value. However, due to the persistent drought caused by global warming, the production of P. bournei has been declining year by year. Consequently, the development of corresponding strategies to address the challenges posed by climate change has become particularly necessary.
Photosynthesis is the primary means of biological carbon sequestration and sustaining normal growth and development in plants. Under drought stress, the physiological metabolism and mechanisms of photosynthesis in plants are affected, leading to a reduction in photosynthetic efficiency8,9. In the early stages of drought, plants close their stomata to reduce transpiration, thereby preventing water loss and enhancing water use efficiency10. Under persistent and severe drought conditions, the intercellular CO2 concentration in leaves remains unchanged or even increases, suggesting that the reduction in photosynthesis in this context is not due to stomatal limitation. At this stage, the photosystem II (PSII) reaction center is damaged, disrupting the electron transport chain and inhibiting electron transport. Consequently, excess light energy accumulates, chlorophyll content declines, the photosynthetic apparatus is damaged, and reactive oxygen species (ROS) levels increase, potentially causing irreversible damage or plant death10,11,12. The accumulation of ROS resulting from this process further accelerates the peroxidation radical chain reaction of membrane lipids and the peroxidation and de-esterification reactions of membrane lipids, thereby impairing the normal function of the cell membrane13,14. Malondialdehyde (MDA), as the end product of membrane lipid peroxidation, can reflect the degree of stress experienced by plants15.
Plant hormones are essential regulators of plant growth and development. Under drought stress, plant hormones regulate the synthesis, transport, and signaling of endogenous hormones, triggering physiological responses and becoming the most sensitive active substances in plants to drought stress16. JA is a pivotal signal in activating plant responses to biotic stress, and concurrently, it plays a role similar to abscisic acid (ABA) in abiotic stress. JA exerts significant functions in regulating root water absorption and transport, aiding plants in absorbing water from soil under limited water conditions. These mechanisms include stomatal closure and antioxidant enzyme activation, enabling plants to adapt to water-limited conditions17,18. JA can also protect PSII reaction centers from salt stress-induced damage, maintain normal carbon assimilation, and reduce peroxidation caused by salt stress.
Carbohydrates, the primary products of photosynthesis, are essential energy substrates for plant growth and development. The synthesis and metabolism of carbohydrates play critical roles in regulating plant growth and stress resistance. Under drought stress, plants regulate sugar accumulation and distribution to mitigate damage and maintain growth19. Drought stress significantly affects gene expression related to carbohydrate regulation in plant cells20,21. Changes in soluble sugar content act as signaling molecules, regulating the expression of key genes involved in plant defense responses and metabolic processes, thereby controlling plant resistance and growth22.
In this study, exogenous nitrogen was applied to P. bournei seedlings under drought stress to examine physiological responses, including leaf parameters, photosynthetic characteristics, and oxidative stress.
Transcriptome analysis was then performed to identify differentially expressed genes in seedling leaves. This study aimed to explore how nitrogen influences the adaptive responses of P. bournei to drought stress.
Materials and method
Plant materials
On June 1, 2024, healthy 1-year-old P. bournei seedlings of uniform size were selected and planted in plastic pots (15.5 cm diameter × 14 cm height). Local forest yellow soil was used, with 1.5 kg of air-dried soil per pot. One seedling was planted in each pot for an artificial drought simulation experiment. Ammonium nitrogen was applied at two rates: 0.8 g/plant NH4+ (NT), and the control treatment (CK) did not accept any ammonium nitrogen. Fertilization was based on the actual nitrogen content of the compound used, with the amount of the compound calculated according to the designed nitrogen level. Fertilizer was applied in three equal doses at 30-day intervals, with solid fertilizer placed around the root zone. Phosphorus and potassium fertilizers were applied at a ratio of N: P2O5: K2O = 1:1:1, based on the moderate nitrogen rate, using the same application method.
After 30 days of treatment, morphological, photosynthetic, and chlorophyll fluorescence parameters were measured. Central leaves were collected for carbon and nitrogen tests, physiological and biochemical analyses, and metabolomic and transcriptomic studies.
Severe drought stress was applied by maintaining soil moisture at 30–35% (control, CK), with nitrogen added (NT). Uniform management practices were adopted. During the stress period, soil moisture was maintained within the target range using the weighing method at fixed afternoon times. Relevant indices were measured after 30 days of drought stress. P. bournei belongs to the genus Machilus in the Lauraceae family.
Sample materials
All chemicals and solvents employed in this study were of analytical or HPLC grade. Ultrapure water, methanol, and acetonitrile were supplied by Thermo Fisher Scientific (Waltham, MA, USA). The MDA assay kit was obtained from Shenzhen Boyaoyang Technology Co., Ltd. (Shenzhen, China). P. bournei seeds were provided by the Guizhou Academy of Forestry Sciences.
Measurement of plant leaf morphology
Leaf area was determined using the LI3000 instrument (LI-COR, USA). Following the measurement of leaf area, the leaves were subjected to a killing process at 105 °C for 30 min, then dried further at 60 °C for 48 h before dry weight was recorded. Specific Leaf Area (SLA) was calculated as the ratio of the leaf’s single-sided area to its dry weight.
Paraffin section of leaf tissue
The mature leaves of Phoebe sheareri were selected, and 0.2 cm × 0.4 cm rectangular segments were excised from both sides of the midrib. Immediately following sampling, the segments were fixed in FAA fixative (a mixture of glacial acetic acid, formaldehyde, and 70% ethanol in a ratio of 90:5:5). After adding 5 ml of glycerol, the samples were placed in a refrigerator at 4 °C for at least 24 h. Conventional paraffin sectioning techniques were employed, as described by Brown et al.23.
The determination of chlorophyll content
The collected P. bournei leaves were washed, dried, and weighed. A sample of 0.1 g of the leaves was finely chopped and placed in a glass tube, to which 5 ml of 100% acetone was added. The tube was sealed and immersed in a dark environment for 36 h until the leaves turned white. The supernatant was then collected and analyzed using a V756CRT UV–Vis spectrophotometer (UV spectrophotometer, Shanghai Youke Instrument & Meter Co., Ltd., China) to measure the absorbance (A) at wavelengths of 662 nm, 645 nm, and 470 nm. The following formulas were used to calculate the concentrations:
where Chlorophyll a (Chl a) represents the concentration of chlorophyll a, Chlorophyll b (Chl b) represents the concentration of chlorophyll b, Car stands for carotenoids, and Chl(a + b) denotes the total chlorophyll content.
The measurement of photosynthetic rate
The measurements of chlorophyll content were conducted using a Li-6400 XT portable photosynthesis system (LI-COR Inc., Lincoln, NE, USA) under a light intensity of 800 μmol m−2 s−1. The instrument operated in a closed-loop system, with the light intensity set at 800 μmol m−2 s−1, the leaf fan set to "Fast," and the flow rate maintained at 500 μmol m−2 s−1. Additionally, the leaf temperature was set at 25 °C and the relative humidity at 75%. Aseptic CO2 from a factory-sealed cylinder was used as the carbon dioxide source at a concentration of 400 μmol m−2 s−1. The photosynthetic parameters, including net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (Gs), and transpiration rate (Tr), were measured within a 30 min timeframe.
Determination of malonic dialdehyde (MDA) content
The determination of MDA content was carried out using the thiobarbituric acid assay. For each sample, 0.5 g of seedling leaves was taken. The procedure was conducted as described by Hodges24. Each sample was replicated three times biologically. The optical density (OD) values were measured at wavelengths of 450 nm, 532 nm, and 600 nm, and the MDA content was subsequently calculated.
Determination of soluble protein content
The soluble protein concentration was quantified by the Bradford assay, utilizing bovine serum albumin (BSA) as a standard. Initially, 0.2 g of leaf tissue was finely ground in 10 mL of distilled water. Following this, the homogenate was centrifuged at 5000 × g for 10 min, and the resulting supernatant was used as the protein extract. Next, 0.1 mL of the protein extract was diluted with 0.9 mL of distilled water and 5 mL of Coomassie Brilliant Blue G-250 dye. The mixture was then thoroughly mixed and allowed to react for 2 min. The absorbance at 595 nm was then recorded. The protein concentration was determined by comparing to a standard curve constructed from known concentrations of BSA.
Transcriptome sequencing
Transcriptome sequencing and subsequent data analysis were carried out as previously described25. Briefly, total RNA was isolated from each sample (refer to the Materials section) using the RNAprep Pure Plant Kit (Tiangen, Beijing, China). RNA quality was assessed with a Nanophotometer Spectrophotometer (IMPLEN, California, USA) and an Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Library preparation was performed with the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, CA, USA), and sequencing was conducted by OE Biotech Co., Ltd. (Shanghai, China) on an Illumina HiSeq X Ten platform, yielding 150 bp paired-end reads. Raw reads were cleaned using Trimomatic26, and clean reads were aligned to the Phoebe genome with HISAT227. Fragments per kilobase million (FPKM) values for each gene were calculated using Cufflinks28, and read counts were obtained with HTSeq-Count28. Differential expression analysis was performed using the DESeq R package29, with p-values ≤ 0.05, fold change (FC) ≤ 0.5 or ≥ 2, and FPKM ≥ 2 as criteria for identifying significantly differentially expressed genes (DEGs). Principal component analysis (PCA) of the expression profiles was conducted using the R PCA function.
Real-time PCR (qRT-PCR)
To corroborate the findings from the transcriptomic analysis, qRT-PCR was carried out. A selection of four highly up-regulated and four highly down-regulated differentially expressed genes (DEGs) was made from the transcriptomic sequencing data based on their differential expression patterns. The gene OF25210, known for its stable expression across all treatments, was designated as the reference gene. Total RNA was isolated from phoebe samples using the Plant RNA Extraction Kit (DNase I) from Kangwei Biotech (Jiangsu, China). Impurities were eliminated with the HiFiScript gDNA Removal RT MasterMix (Kangwei Biotech, Jiangsu, China), followed by cDNA synthesis with the HiFiScript cDNA Synthesis Kit (Kangwei Biotech, Jiangsu, China). Real-time fluorescence quantitative PCR (qRT-PCR) was performed using the UltraSYBR One Step qRT-PCR Kit (Kangwei Biotech, Jiangsu, China). The relative expression levels were determined using the 2−ΔΔCt method30, with the expression level of the reference gene set to 1. The fold change in expression for other genes relative to the reference gene was then calculated. The primers utilized in this study are detailed in Table S1.
Results
The effect of actual exogenous nitrogen on the growth of P. bournei seedlings under drought stress
As shown in Fig. 1A and B, exogenous nitrogen application significantly mitigated the impact of drought stress on P. bournei seedlings. The severity of wilting and chlorosis in P. bournei seedlings treated with nitrogen (NT) was significantly lower than that in the CK (control group). Under drought stress conditions, the fresh weight and dry weight of NT seedlings increased by 29.27% and 512.99%, respectively, compared to CK (Fig. 1C). Furthermore, the SPAD (relative chlorophyll content) in the leaves of P. bournei seedlings after nitrogen treatment increased by 35.03%, compared to the control (Fig. 1B). Additionally, exogenous application also improved the thickness of P. bournei leaves under drought stress conditions, with the NT leaves being 19% thicker than those of CK (Fig. 1C). Evidently, exogenous nitrogen application can enhance the adaptability of P. bournei leaves to drought stress to a certain extent, thereby mitigating the adverse effects of drought stress on plant growth. Figure 1D depicts the paraffin sections of leaf tissue cross-sections from P. bournei seedlings, comparing the N and CK treatment groups. The fence tissue thickness, spongy tissue thickness, leaf thickness, and the ratio of palisade to spongy tissue in the NT treatment were all significantly greater than those in the CK treatment. Specifically, the respective metrics of the NT treatment exceeded those of the CK by 29.13%, 8.42%, 10.77%, and 18.87%, respectively, while all parameter metrics of the CK treatment were significantly lower than those of the NT treatment (Fig. 1E).
Phenotype of P. bournei seedlings under drought stress (A, B); including dry weight, fresh weight, SPAD value, and leaf thickness (C); Dcanning electron microscopy (SEM) images of cross-sections of the leaves of P. bournei seedlings (D); thickness, Sponge tissue thickness, Palisade/Sponger (E). CK: P. bournei seedlings grown under drought stress (control); NT: P. bournei seedlings treated with nitrogen and then grown under drought stress. Significant differences are indicated by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Bars represent the standard deviation of the mean (n = 6).
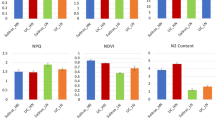
Effects of exogenous nitrogen application on physiological indices of P. bournei seedlings under drought stress.
As shown in Fig. 2, under drought stress conditions, the application of exogenous nitrogen to P. bournei seedlings effectively increased the soluble sugar content in their leaves by 7.9%, with a significant difference (P < 0.05). Additionally, nitrogen treatment significantly mitigated the accumulation of membrane lipid peroxidation induced by drought, with the MDA accumulation in the NT group being 77.06% lower than that in the CK group.
Physiological indices of P. bournei seedling leaves under drought stress. CK: P. bournei seedlings grown under drought conditions (control); NT: P. bournei seedlings grown under drought conditions after being treated with nitrogen; significant differences are indicated by asterisks (*, for P < 0.05; **, for P < 0.01; ***, for P < 0.001). The bar graphs represent the standard deviation of the mean values (n = 6).
Transcriptional changes in P. bournei seedlings under drought stress
The leaf materials of P. bournei were divided into two groups, CK and NT, with three biological replicates in each group. In total, 24.55 GB of Clean Reads sequencing data were obtained, ranging from 2.86 to 3.87 GB per sample. The Q30 score of each sample were greater than 96%, indicating that the data were reliable for subsequent analysis. Subsequently, RNA-seq analysis was conducted. The normalized FPKM expression levels of DEGs were extracted to construct a hierarchical clustering heatmap (Fig. 3a), revealing substantial transcriptional alterations in the leaves of P. bournei under drought stress when nitrogen was applied. Through differential expression analysis between CK and NT, a total of 2598 DEGs were identified. Compared to the control group CK, there were a total of 1373 DEGs that showed upregulated differential expression and 1225 DEGs that showed downregulated differential expression in NT (Fig. 3B). The results of PCA (Principal Component Analysis) indicated that the first principal component (PC1) accounted for 54.52% of the total variance, while the PC2 (second principal component) explained 19.44% of the total variance. The CK group and the NT group could be significantly distinguished by PC1 and PC2 (Fig. 3C).
Transcriptome data analysis of P. bournei seedlings leaves under drought stress. CK: P. bournei grown in a drought environment (control); NT: P. bournei seedlings grown in a drought environment after treatment with nitrogen. (A) Heatmap of correlation coefficients between DEGs identified under drought stress conditions, constructed by selecting the top 50 DEGs with the highest differential expression levels. (B) Comparative analysis of DEGs between NT and CK after drought stress treatment. (C) PCA score plot, where each point represents an independent biological replicate sample. (D) GO enrichment analysis of enriched metabolic pathways in P. bournei seedlings under drought treatment. (E) KEGG enrichment analysis of enriched metabolic pathways in P. bournei seedlings under drought treatment. The color of the circles indicates the adjusted P-value (Padj), and the size of the circles is proportional to the number of DEGs involved in the pathway enrichment.
To gain a deeper understanding of the transcriptome of P. bournei seedlings under drought stress, enrichment analyses of DEGs were performed using GO and KEGG. The application of exogenous nitrogen resulted in significant enrichment differences in various biological pathways in P. bournei seedlings under drought stress (Fig. 3D and E).
In the GO analysis, the top 30 enriched pathways for DEGs were primarily related to plant photosynthesis-related pathways (photosynthesis; light reaction photosynthesis; photosynthetic electron transport chain); plant hormone signaling-related pathways (response to abscisic acid; regulation of signal transduction; intracellular signal transduction; hormone-mediated signaling pathway); protein metabolism-related pathways (regulation of protein metabolic process; regulation of cellular protein metabolic process); carbohydrate metabolism-related pathways (carbohydrate metabolic process; cellular carbohydrate metabolic process); and stress tolerance and response-related pathways (defense response to bacteria; immune system process; response to water; response to water deprivation) (Fig. 3D).
In the KEGG enrichment analysis, the top 5 enriched pathways were Biosynthesis of Cofactors, Biosynthesis of Amino Acids, mRNA Surveillance Pathway, Proteasome, and Photosynthesis (Fig. 3E). The transcriptome analysis data of 10 genes were validated through qRT-PCR (Fig. S1). The consistency between the omics and qRT-PCR data strengthened the validity of the findings in this study.
DEGs involved in plant hormone metabolism and hormone signal transduction
Plant hormones play a crucial role in regulating plant tolerance to drought stress. In this study, a total of 58 DEGs involved in plant hormone metabolism and hormone signal transduction were identified (Fig. 4A). These DEGs were enriched in signaling pathways such as "response to abscisic acid", "regulation of signal transduction", and "hormone-mediated signaling pathway". The expression profile analysis of these plant hormone-related genes revealed that the majority of them (44) were highly expressed in the NT group, while a smaller proportion of DEGs (14) were highly expressed in the control CK group. This indicates that exogenous nitrogen can regulate plant hormone metabolism in stress-exposed materials. It was found that the expression levels of JA genes encoding jasmonic acid ZIM-domain proteins, such as OF06200 (JAZ1), OF23762 (JAZ2), and OF23206 (JAZ3), were significantly higher in the NT group compared to the CK group. Furthermore, the expression of several transcription factor genes involved in plant hormone synthesis and stress tolerance responses, such as OF22206 (MYBP), OF04128 (MYB), OF12761 (HD-ZIP2), OF24220 (WRKY), OF18152 (NAC01), OF13438 (NACO2), and OF14818 (HD-ZIP1), was significantly upregulated in the NT group.
Pathway analysis of DEGs in the transcriptome of P. bournei seedling leaves under drought stress. CK: P. bournei seedlings grown under drought conditions (control); NT: P. bournei seedlings grown under drought conditions after treatment with nitrogen; (A) DEGs involved in plant hormone metabolism and hormone signaling; (B) Photosynthesis-related DEGs; (C) Carbohydrate-related DEGs.
DEGs involved in photosynthesis
According to GO and KEGG enrichment analysis, photosynthesis plays a significant role in the drought stress response of P. bournei seedlings. In this study, a total of 13 DEGs involved in photosynthesis were identified, belonging to the gene families of PFP, TRY, LQY, FTSH, FRO, CURT, PETF, ATPF, PETA, CRRSP, and MEN, respectively. Figure 4B illustrates the expression profiles of genes encoding photosynthesis-related proteins in the CK and NT treatments. Notably, compared to the control, the FRO gene (encoding Ferric Reduction Oxidase), the ATPF1G gene (encoding Mitochondrial ATP Synthase Inhibitor Factor), and the CRRSP2 gene (encoding Plasmodesmata-located Protein) were all significantly upregulated in the NT treatment (Fig. 4B).
DEGs involved in carbohydrate metabolism
Compared to the control group, under drought conditions, the application of exogenous nitrogen to P. bournei seedlings resulted in the differential expression of a total of 17 genes related to carbon metabolism (Fig. 4C). After the application of exogenous nitrogen, 10 genes, namely PWD, GBE1, GAPA, PFKA, RFS, ISA, GLGC, PGK and ALDO, exhibited upregulated expression. Conversely, 7 genes, including GUX, RX9, MIOX, HCT, BAM, MPFP, and ERNI, demonstrated downregulated expression.
Discussion
The application of exogenous nitrogen promotes the growth of P. bournei and enhances the tolerance of seedling leaves to drought stress
Due to its exceptionally high economic value, P. bournei occupies an important position in meeting the global demand for premium timber. However, P. bournei is highly sensitive to drought, which has become one of the major factors limiting its growth31. With the intensification of climate change, it is anticipated that the intensity and frequency of droughts will continue to increase in the future32. Therefore, developing appropriate management measures to enhance plants’ WUE (water use efficiency) and thus increase their tolerance to water deficit is crucial for mitigating the negative impacts of drought33. Research indicates that appropriate nitrogen application can effectively alleviate the inhibition of drought stress on plant growth and significantly reduce the negative effects of drought34,35. In this experiment, exogenous nitrogen significantly mitigated the effects of drought stress on P. bournei seedlings (Fig. 1). Following nitrogen treatment, the thickness of the seedling leaves significantly increased, and both the fresh weight and dry weight were also significantly higher than those of the control group (Fig. 1B). Furthermore, nitrogen treatment elevated the relative chlorophyll content in the leaves (SPAD) by 35.03%, which could be attributed to the upregulation of certain genes within the photosynthetic pathway (Fig. 4B). Plant drought resistance is closely associated with leaf anatomical structures36. The current study revealed that the palisade tissue thickness, spongy tissue thickness, and the palisade-to-spongy ratio in the leaves of P. bournei seedlings treated with nitrogen were significantly greater than those in the control group (CK). In drought stress conditions, the application of exogenous nitrogen may alter the anatomical structure of leaves and their stomatal regulatory abilities, thereby reducing water loss and enhancing plant growth performance under arid conditions37.
Physiological responses of P. bournei seedlings to exogenous nitrogen under drought stress conditions
Drought stress leads to a homeostatic imbalance between the production and scavenging of ROS, triggering oxidative stress and the accumulation of ROS38,39. When plants are subjected to drought stress, osmolytes such as soluble proteins, soluble sugars, proline, and betaine rapidly accumulate within the plant body. These osmolytes serve to decrease the cellular osmotic potential, thereby maintaining water status and conferring resistance against the accumulation of reactive oxygen species40,41. Furthermore, these substances stabilize membranes, proteins, and other subcellular structures under osmotic pressure42. This study indicates that under the application of exogenous nitrogen, the MDA content in the leaves of P. bournei seedlings significantly decreased, suggesting that nitrogen alleviated the oxidative damage to plant cells under drought stress. Additionally, the soluble protein content in the leaves of P. bournei seedlings after nitrogen application was also significantly higher than that of the control, indicating that nitrogen enhanced ROS detoxification in the leaves under severe drought stress (Fig. 2).
Transcriptional responses of P. bournei seedlings to exogenous nitrogen under drought stress conditions
Transcriptome analysis has been widely proven to be of significant value in analyzing plant genome expression profiles under different developmental and environmental conditions43. The study results indicate that in arid environments, the number of differentially expressed genes (DEGs) upregulated in nitrogen-treated Phoebe bournei seedlings exceeds that of downregulated DEGs (Fig. 3B). These DEGs are significantly enriched in pathways such as plant hormone signaling, photosynthesis, carbohydrate metabolism, and stress tolerance responses (Fig. 3E). Research suggests that hormones such as abscisic acid, jasmonic acid, ethylene, and cytokinins can regulate stomatal conductance, chlorophyll content, and osmotic pressure, enhancing plant tolerance to drought44. Additionally, under drought stress, plants dissipate excess light energy as heat through the NPQ mechanism of the photosynthetic pathway, protecting photosynthetic structures45. In this experiment, after nitrogen treatment, the maximum photochemical efficiency of PSII and the potential activity of PSII in P. bournei seedling leaves were significantly increased (Fig. 2). The research by Tauzin and Giardina also showed that under stress conditions, plants can adjust carbohydrate allocation by regulating sucrose production and activate the construction of defense systems46. These findings align with the results of studies on forest plants such as Populus simonii, Salix psammophila, and Populus adenopoda8,47,48.
Plant hormone metabolism is involved in the response of P. bournei seedlings to exogenous nitrogen under drought stress conditions
Plant hormones play a crucial regulatory role in plant responses to stress adversity49. JA, as an important plant hormone, plays a role when plants are subjected to drought stress. By activating a series of signal transduction pathways, it regulates stomatal opening and closing to reduce water evaporation, while also inducing the activation of the antioxidant system and the accumulation of osmolytes within the plant. This enhances the plant’s adaptability to drought conditions and improves its survival chances under water-deficit conditions50,51. The results of this study indicate that nitrogen treatment activated the "response to abscisic acid; regulation of signal transduction; hormone-mediated signaling pathway," significantly upregulating genes such as OF06200 (JAZ1), OF23762 (JAZ2), and OF23206 (JAZ3) that are involved in the metabolic regulation of jasmonic acid (JA). JAZ proteins contribute to drought tolerance in Arabidopsis thaliana by regulating photosynthesis, redox reactions, as well as amino acid, plant hormone, and defense metabolite production52. In addition, we observed that numerous transcription factors potentially associated with jasmonic acid (JA) synthesis were upregulated in Arabidopsis thaliana (NT), including MYBBP, MYB, HDZIP1, HDZIP2, WRKY, and NAC01 (Fig. 4A). Previous research has demonstrated that under drought stress, overexpression of MYB genes in Arabidopsis can systematically upregulate JA biosynthesis genes, activate the JA signaling pathway, and thereby enhance plant drought resistance53; WRKY transcription factors participate in the biosynthesis of secondary metabolites in plants under drought stress and are also implicated in JA signaling54; NAC transcription factors can respond to abiotic stress signals and enhance plant drought tolerance by regulating JA synthesis in Arabidopsis55.
Transcription factors, serving as key regulatory elements in plant hormone metabolic pathways, can recognize and bind to the promoter regions of genes related to hormone biosynthesis, thereby activating or inhibiting the expression of these genes. This precise regulation of plant hormone synthesis rates and levels fulfills the physiological needs of plants at different growth and development stages and under environmental stress conditions56. This regulatory mechanism involves intricate signal transduction pathways and gene expression regulatory networks, ensuring a precise match between plant hormone synthesis and plant growth as well as stress responses57. Under drought stress conditions, the expression of transcription factors OF22206 (MYBP), OF04128 (MYB), OF12761 (HD-ZIP2), OF24220 (WRKY), OF18152 (NAC01), OF13438 (NAC02), and OF14818 (HD-ZIP1) was significantly upregulated in nitrogen-treated (NT) plants. Previous studies have shown that in Populus euphratica, MYB genes contain various cis-regulatory elements in their promoter regions that are related to development, light response, plant hormone response, and environmental stress response58. Under drought stress, the Populus MYB gene (PtoMYB142) enhances drought tolerance by regulating wax biosynthesis59. HD-ZIP (Homeodomain-leucine zipper) proteins are plant-specific transcription factors that contain both a HD (homeodomain) and a LZ (leucine zipper) domain. In Arabidopsis thaliana, HD-ZIP proteins primarily mediate plant stress tolerance by regulating the expression of downstream stress-related genes through an ABA-mediated signaling pathway, as well as regulating plant growth and development60. Studies using expression silencing experiments have demonstrated that the transcription factor GhNAC2 plays a role in cotton’s response to drought stress by affecting the expression of genes related to drought stress. Meanwhile, the transcription factor WRKY is believed to participate in Arabidopsis thaliana’s response to drought stress by regulating stomatal movement61. Nitrogen treatment caused the DEGs in leaves to concentrate in pathways related to secondary metabolite biosynthesis, transport, and catabolism, carbohydrate metabolism, and signal transduction metabolism.
Photosynthesis is involved in the response of P. bournei seedlings to exogenous nitrogen under drought stress conditions
Photosynthesis is the foundation for biomass accumulation. When crop plants are exposed to stress conditions, the leaf photosynthetic system undergoes corresponding changes to adapt to growth under adverse conditions62. Under drought conditions, the lack of water in plants can inhibit chlorophyll biosynthesis and even accelerate the decomposition of existing chlorophyll63. Nitrogen application generally stimulates chlorophyll synthesis in plants and increases the nitrogen content in plant leaves, thereby effectively regulating photosynthetic performance and mitigating the photodamage caused by drought stress to plants64. In this experiment, the relative chlorophyll content in the leaves of P. bournei treated with nitrogen increased by 35.03% compared to the control group (Fig. 1B).
Research indicates that short-term or mild drought stress affects plant photosynthesis by limiting the supply of CO2 through stomatal factors, whereas long-term or severe drought stress impacts photosynthesis through non-stomatal factors such as inhibiting the photosynthetic activity of mesophyll cells10,65. We subjected P. bournei seedlings to a sustained 30-day drought treatment. The application of nitrogen maintained higher levels of stomatal conductance, transpiration rate, and intercellular carbon dioxide concentration in the leaves of P. bournei seedlings. Additionally, nitrogen effectively enhanced their net photosynthetic rate, maximum photochemical efficiency of PSII, and potential activity of PSII. Therefore, exogenous nitrogen application sustained the photosynthesis of P. bournei leaves, not only by improving gas exchange under drought conditions but also by playing a crucial role in maintaining the photosynthetic efficiency of the leaves (Fig. 2).
Apart from adapting to drought stress through physiological mechanisms, numerous studies have also revealed the significant role of transcriptional regulation of photosynthesis in plants’ response to drought stress. This regulatory mechanism allows plants to adjust their photosynthetic performance by altering the expression of related genes, thereby adapting to the challenges posed by drought conditions66,67. In this study, it was found that after nitrogen treatment, the genes FRO, ATPF1G, and CRRSP2 were significantly upregulated, while the genes PFP, TRY, LQY, FTSH, CURT, PETF, PETA, and MEN were significantly downregulated in the NT group. The FRO gene encodes Ferric Reduction Oxidase, which plays a crucial role in organisms. It not only participates in iron redox reactions, facilitating respiration and chlorophyll biosynthesis, but also its mediated rapid changes in iron oxidation states are essential for regulating cellular functions, electron transport, and various metabolic processes68,69. The cyclic electron transport pathway of PSI is considered one of the most important mechanisms for regulating the production ratio of ATP/NADPH. ATPase, located on the chloroplast thylakoid membrane, is used by plants to synthesize ATP in order to maintain this ratio balance70,71. In this experiment, the upregulation of ATPF1G, which encodes a subunit protein related to the F-type ATPase, indicates that exogenous nitrogen application promotes the synthesis of ATPase, thereby maintaining an appropriate ATP/NADPH ratio and facilitating the dark reactions of photosynthesis in P. bournei. CRRSP2 encodes a plasmodesmata-localized protein. Current research has clearly shown that plant polysaccharide callose deposits on plasmodesmata, regulating their pore size and function72. Under stress conditions, callose is rapidly synthesized and deposited, which can block sieve plates, reducing or preventing the transport of substances between cells, thereby serving as a defense mechanism in response to stress73. The upregulation of CRRSP2 may enhance the plant’s regulatory capacity of plasmodesmata and the level of callose synthesis, enabling it to more efficiently cope with environmental pressures. Plasmodesmata-localized proteins may also block sieve plates by depositing callose at plasmodesmata, reducing the loss of essential substances. Additionally, the upregulation of CRRSP2 contributes to maintaining or regulating intercellular communication and resource sharing under stress conditions, which is crucial for plant survival and subsequent growth recovery in adverse environments. However, the specific mechanisms still require detailed subsequent research.
Carbohydrates, as the products of photosynthesis in plants and indispensable substrates in respiration, play a vital role as energy sources and carbon supplies during plant growth and development. Furthermore, carbohydrates exhibit important regulatory functions when plants encounter stress conditions, and their storage, transport, and decomposition mechanisms are crucial for plants to effectively respond to and adapt to environmental stresses. These stored carbohydrates can be mobilized at critical times to provide necessary energy support for plants, ensuring their survival and recovery under adverse environmental conditions74. Nitrogen treatment resulted in the upregulation of 10 DEGs related to carbohydrate biosynthesis processes, namely PWD, GBE1, GAPA, PFKA, ISA, GLGC, PGK, RFS and ALDO. Concurrently, it led to the downregulation of 7 DEGs, including GUX, RX9, MIOX, HCT, BAM, MPFP, and ERNI. Among them, PWD encodes Phosphoglucan, Water Dikinase, which is involved in the phosphorylation process of carbohydrates and regulates starch degradation75. GAPA encodes glyceraldehyde-3-phosphate dehydrogenase, which plays a crucial role in processes such as glycolysis and carbon assimilation in photosynthesis. Studies have shown that glyceraldehyde-3-phosphate dehydrogenase positively regulates drought stress resistance in tobacco76. PFK encodes ATP-dependent 6-phosphofructokinase, which catalyzes the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate, participating in the first step of glycolysis77. The application of exogenous nitrogen can regulate the expression of genes related to carbohydrate metabolism in plants, thereby enhancing their adaptive capacity to drought stress. It is speculated that these genes regulate the synthesis, accumulation, and decomposition of carbohydrates in P. bournei, providing the plant with abundant energy and carbon sources to maintain normal physiological functions under drought conditions.
Drought stress can affect the chemical composition of plant cell walls, such as increasing the proportion of components like cellulose, hemicellulose, and pectin, to enhance the structural stability and elasticity of the cell wall78. GUX encodes xylan α-glucuronosyltransferase, which is involved in the synthesis of hemicellulosic xylan in plant cell walls and plays a crucial role in plant development, growth, and defense responses against pathogens79. Hormone-responsive elements, growth and development-responsive elements, and stress-responsive elements have been identified within the cis-acting elements of the GUX promoter80. MIOX catalyzes the oxidative cleavage of inositol to produce D-glucuronic acid. MIOX is involved in the biosynthesis of UDP-GlcA (UDP-glucuronic acid), providing nucleotide sugars for cell wall polymers81. Thus, we speculate that the application of nitrogen may alleviate the changes in cell wall structure in leaves under drought stress.
Conclusion
Physiological and phenotypic analysis of P. bournei seedling leaves under drought stress conditions revealed that, compared to the control, nitrogen treatment significantly alleviated leaf wilting, increased leaf biomass and chlorophyll content, and improved plant growth performance under drought stress. The application of nitrogen fertilizer further increased the soluble sugar content in the leaves while reducing the MDA content. In addition, the application of exogenous nitrogen also had a positive impact on the photosynthetic performance of P. bournei seedling leaves under drought stress. Simultaneously, nitrogen treatment significantly increased the net photosynthetic rate, stomatal conductance, transpiration rate, and intercellular CO2 concentration in the seedling leaves. Transcriptome analysis revealed that the application of nitrogen fertilizer mainly concentrated the DEGs in leaves on pathways such as photosynthesis, carbohydrate metabolism, and plant hormone signal transduction. Through the regulation of these pathways, nitrogen fertilizer aids P. bournei in adapting to drought conditions, mitigating the adverse effects of drought stress on its growth, and thus enhancing its survival rate and recovery ability (Fig. 5).
Schematic representation of the physiological and molecular mechanisms of nitrogen alleviating drought stress. Parts with a red background indicate upregulated genes, while those with a blue background indicate downregulated genes. Arrows represent regulatory relationships, with dashed arrows signifying that the relationship lacks direct evidence in this study and is considered speculative.
Data availability
Sequence data that support the findings of this study have been deposited in the National Library of MedicineNational(National Center for Biotechnology Information,NCBI) with the primary accession code SRA data: PRJNA1209408″. These data werederived from the following resourcesavailable in the public domain: https://www.ncbi.nlm.nih.gov/sra/PRJNA1209408.
References
Beyel, V. & Brüggemann, W. Differential inhibition of photosynthesis during pre-flowering drought stress in Sorghum bicolor genotypes with different senescence traits. Physiol. Plant. 124, 249–259 (2005).
Brodribb, T. J. & Holbrook, N. M. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 132, 2166–2173 (2003).
Takahashi, F., Kuromori, T., Sato, H. & Shinozaki, K. Regulatory gene networks in drought stress responses and resistance in plants. In Survival Strategies in Extreme Cold and Desiccation Vol. 1081 (eds Iwaya-Inoue, M. et al.) 189–214 (Springer, 2018).
Jiao, Z. et al. 5-Aminolevulinic acid pretreatment mitigates drought and salt stresses in poplar plants. Forests 12, 1112 (2021).
Garavillon-Tournayre, M. et al. Integrated drought responses of black poplar: How important is phenotypic plasticity?. Physiol. Plant. 163, 30–44 (2018).
Ying, L. et al. Population structure and dynamic characteristics of Phoebe zhennan S. Lee in karst areas of Chongqing. J. Ecol. https://doi.org/10.5846/stxb202106171607 (2022).
Nie, R., Wei, X., Jin, N., Su, S. & Chen, X. Response of photosynthetic pigments, gas exchange and chlorophyll fluorescence parameters to light quality in Phoebe bournei seedlings. Plant Growth Regul. 103, 675–687 (2024).
Zhang, S., Xu, X., Sun, Y., Zhang, J. & Li, C. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric. 17, 336–347 (2018).
Zhi, Q.-Q. et al. Physiological and transcriptome analyses reveal tissue-specific responses of Leucaena plants to drought stress. Plant Physiol. Biochem. 214, 108926 (2024).
Gupta, A., Rico-Medina, A. & Caño-Delgado, A. I. The physiology of plant responses to drought. Science 368, 266–269 (2020).
Anderegg, W. R. L., Anderegg, L. D. L. & Huang, C. Testing early warning metrics for drought-induced tree physiological stress and mortality. Glob. Change Biol. 25, 2459–2469 (2019).
Ghotbi-Ravandi, A. A., Shahbazi, M., Shariati, M. & Mulo, P. Effects of mild and severe drought stress on photosynthetic efficiency in tolerant and susceptible barley (Hordeum vulgare L.) genotypes. J. Agron. Crop Sci. 200, 403–415 (2014).
Munn-Bosch, S. & Peuelas, J. Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 217, 758–766 (2003).
Xiong, L. & Zhu, J.-K. Molecular and genetic aspects of plant responses to osmotic stress. Plant, Cell Environ. 25, 131–139 (2002).
Nazir, M. M. et al. Exogenous calcium oxide nanoparticles alleviate cadmium toxicity by reducing Cd uptake and enhancing antioxidative capacity in barley seedlings. J. Hazard. Mater. 438, 129498 (2022).
Yu, Z. et al. How plant hormones mediate salt stress responses. Trends Plant Sci. 25, 1117–1130 (2020).
Smékalová, V., Doskočilová, A., Komis, G. & Šamaj, J. Crosstalk between secondary messengers, hormones and MAPK modules during abiotic stress signalling in plants. Biotechnol. Adv. 32, 2–11 (2014).
Riemann, M. et al. exploring jasmonates in the hormonal network of drought and salinity responses. Front. Plant Sci. https://doi.org/10.3389/fpls.2015.01077 (2015).
Yang, J. et al. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 141, 164–171 (2019).
Liu, Y. et al. Dynamic responses of accumulation and remobilization of water soluble carbohydrates in wheat stem to drought stress. Plant Physiol. Biochem. 155, 262–270 (2020).
Zhao, Y., Wei, X., Ji, X. & Ma, W. Endogenous NO-mediated transcripts involved in photosynthesis and carbohydrate metabolism in alfalfa (Medicago sativa L.) seedlings under drought stress. Plant Physiol. Biochem. 141, 456–465 (2019).
He, W., Liu, H., Qi, Y., Liu, F. & Zhu, X. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Change Biol. 26, 3627–3638 (2020).
Brown, R. C., Lemmon, B. E. & Mullinax, J. B. Immunofluorescent staining of microtubules in plant tissues: improved embedding and sectioning techniques using polyethylene glycol (PEG) and Steedman’s wax. Botanica Acta 102, 54–61 (1989).
Hodges, D. M., DeLong, J. M., Forney, C. F. & Prange, R. K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611 (1999).
Liu, Q. et al. Systematic analysis of photo/sko-regulated germination and post-germination development of shallow photodormant seeds in Nicotiana tabacum L. Front. Plant Sci. 13, 1042981 (2023).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Trapnell, C. et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Love, M. I., Soneson, C. & Patro, R. Swimming downstream: statistical analysis of differential transcript usage following Salmon quantification. F1000Res 7, 952 (2018).
Willems, E., Leyns, L. & Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 379, 127–129 (2008).
Tariq, A. et al. Phosphorus fertilization of Phoebe zhennan seedlings under drought reduces nitrogen assimilation. J. Plant Nutr. 45, 2228–2238 (2022).
Ellison, E., Baker, L. & Wilson, A. IPCC special report meeting: Climate change around the globe. Weather 75, 293–294 (2020).
Asensio, V. et al. Potassium fertilization increases hydraulic redistribution and water use efficiency for stemwood production in Eucalyptus grandis plantations. Environ. Exp. Bot. 176, 104085 (2020).
Prasertsak, A. & Fukai, S. Nitrogen availability and water stress interaction on rice growth and yield. Field Crop Res. 52, 249–260 (1997).
Teixeira, E. I. et al. The impact of water and nitrogen limitation on maize biomass and resource-use efficiencies for radiation, water and nitrogen. Field Crop Res. 168, 109–118 (2014).
Li, Y. et al. Responses in growth and anatomical traits of two subtropical tree species to nitrogen addition, drought, and their interactions. Front. Plant Sci. 12, 709510 (2021).
Lu, M. et al. Anatomy and transcriptome analysis in leaves revealed how nitrogen (N) availability influence drought acclimation of Populus. Trees 33, 1003–1014 (2019).
Qi, J. et al. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. JIPB 60, 805–826 (2018).
Samanta, S., Seth, C. S. & Roychoudhury, A. The molecular paradigm of reactive oxygen species (ROS) and reactive nitrogen species (RNS) with different phytohormone signaling pathways during drought stress in plants. Plant Physiol. Biochem. 206, 108259 (2024).
De Souza Rodrigues, T. et al. Elevated CO2 increases biomass of Sorghum bicolor green prop roots under drought conditions via soluble sugar accumulation and photosynthetic activity. Physiol. Plant. 175, e13984 (2023).
Wang, W. et al. Seed priming with protein hydrolysate promotes seed germination via reserve mobilization, osmolyte accumulation and antioxidant systems under PEG-induced drought stress. Plant Cell Rep. 41, 2173–2186 (2022).
Khan, M. A. et al. Molecular physiology for the increase of soluble sugar accumulation in citrus fruits under drought stress. Plant Physiol. Biochem. 203, 108056 (2023).
Ding, Z. et al. Extensive post-transcriptional regulation revealed by transcriptomic and proteomic integrative analysis in cassava under drought. J. Agric. Food Chem. 67, 3521–3534 (2019).
Yoshida, T. & Fernie, A. R. Hormonal regulation of plant primary metabolism under drought. J. Exp. Bot. 75, 1714–1725 (2024).
Qiao, M., Hong, C., Jiao, Y., Hou, S. & Gao, H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants 13, 1808 (2024).
Tauzin, A. S. & Giardina, T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. https://doi.org/10.3389/fpls.2014.00293 (2014).
Cai, Z. et al. Differences in phytohormone and flavonoid metabolism explain the sex differences in responses of Salix rehderiana to drought and nitrogen deposition. Plant J. 114, 534–553 (2023).
Kim, T.-L., Lim, H., Denison, M. I. J. & Oh, C. Transcriptomic and physiological analysis reveals genes associated with drought stress responses in Populus alba × Populus glandulosa. Plants 12, 3238 (2023).
Waadt, R. et al. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 23, 680–694 (2022).
Awan, S. A. et al. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol. Plant. 172, 809–819 (2021).
Rahman, Md. M. et al. Jasmonic acid priming augments antioxidant defense and photosynthesis in soybean to alleviate combined heat and drought stress effects. Plant Physiol. Biochem. 206, 108193 (2024).
Meng, L. et al. Comparative proteomics and metabolomics of JAZ7-mediated drought tolerance in Arabidopsis. J. Proteomics 196, 81–91 (2019).
Zhou, Y. et al. A novel sweetpotato transcription factor gene IbMYB116 enhances drought tolerance in transgenic Arabidopsis. Front. Plant Sci. 10, 1025 (2019).
Li, P., Li, X. & Jiang, M. CRISPR/Cas9-mediated mutagenesis of WRKY3 and WRKY4 function decreases salt and Me-JA stress tolerance in Arabidopsis thaliana. Mol. Biol. Rep. 48, 5821–5832 (2021).
Fang, L. et al. Expression of Vitis amurensis NAC26 in Arabidopsis enhances drought tolerance by modulating jasmonic acid synthesis. EXBOTJ 67, 2829–2845 (2016).
Khoudi, H. SHINE clade of ERF transcription factors: A significant player in abiotic and biotic stress tolerance in plants. Plant Physiol. Biochem. 195, 77–88 (2023).
Wang, Y. et al. Integrative transcriptome and metabolome analysis reveals the mechanism of exogenous melatonin alleviating drought stress in maize roots. Plant Physiol. Biochem. 199, 107723 (2023).
Zhao, X. et al. Genome-wide analysis of specific PfR2R3-MYB genes related to paulownia witches’ broom. Genes 14, 7 (2022).
Song, Q. et al. PtoMYB142, a poplar R2R3-MYB transcription factor, contributes to drought tolerance by regulating wax biosynthesis. Tree Physiol 42, 2133–2147 (2022).
Li, Y. et al. The roles of HD-ZIP proteins in plant abiotic stress tolerance. Front. Plant Sci. 13, 1027071 (2022).
Qiao, Z., Li, C.-L. & Zhang, W. WRKY1 regulates stomatal movement in drought-stressed Arabidopsis thaliana. Plant Mol Biol 91, 53–65 (2016).
Yuan, W. et al. Electrical and photosynthetic response of Rosa chinensis under drought stress. Biosys. Eng. 236, 248–257 (2023).
Hu, J. et al. The effects of high temperature, drought, and their combined stresses on the photosynthesis and senescence of summer maize. Agric. Water Manag. 289, 108525 (2023).
Raese, J. T., Drake, S. R. & Curry, E. A. Nitrogen fertilizer influences fruit quality, soil nutrients and cover crops, leaf color and nitrogen content, biennial bearing and cold hardiness of ‘golden delicious’. J. Plant Nutr. 30, 1585–1604 (2007).
Kruse, J. et al. Optimization of photosynthesis and stomatal conductance in the date palm Phoenix dactylifera during acclimation to heat and drought. New Phytol. 223, 1973–1988 (2019).
Chen, K., Huang, Y., Liu, C., Liang, Y. & Li, M. Transcriptome profile analysis of Arabidopsis reveals the drought stress-induced long non-coding RNAs associated with photosynthesis, chlorophyll synthesis, fatty acid synthesis and degradation. Front. Plant Sci. 12, 643182 (2021).
El-Khouly, M. E., El-Mohsnawy, E. & Fukuzumi, S. Solar energy conversion: From natural to artificial photosynthesis. J. Photochem. Photobiol. C 31, 36–83 (2017).
Suzuki, M. et al. Accumulation of starch in Zn-deficient rice. Rice 5, 9 (2012).
Vigani, G., Morandini, P. & Murgia, I. Searching iron sensors in plants by exploring the link among 2′-OG-dependent dioxygenases, the iron deficiency response and metabolic adjustments occurring under iron deficiency. Front. Plant Sci. https://doi.org/10.3389/fpls.2013.00169 (2013).
Kobayashi, R., Yamamoto, H., Ishibashi, K. & Shikanai, T. Critical role of cyclic electron transport around photosystem I in the maintenance of photosystem I activity. Plant J. 118, 2141–2153 (2024).
Yamori, W. & Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 67, 81–106 (2016).
Li, Z., Liu, S.-L., Montes-Serey, C., Walley, J. W. & Aung, K. PLASMODESMATA-LOCATED PROTEIN 6 regulates plasmodesmal function in Arabidopsis vasculature. Plant Cell https://doi.org/10.1093/plcell/koae166 (2024).
Li, N. et al. The multifarious role of callose and callose synthase in plant development and environment interactions. Front. Plant Sci. 14, 1183402 (2023).
Collins, A. D. et al. Foliar respiration is related to photosynthetic, growth and carbohydrate response to experimental drought and elevated temperature. Plant Cell Environ. 44, 3623–3635 (2021).
Baunsgaard, L. et al. A novel isoform of glucan, water dikinase phosphorylates pre-phosphorylated alpha-glucans and is involved in starch degradation in Arabidopsis. Plant J. 41, 595–605 (2005).
Kappachery, S., Baniekal-Hiremath, G., Yu, J. W. & Park, S. W. Effect of over-and under-expression of glyceraldehyde 3-phosphate dehydrogenase on tolerance of plants to water-deficit stress. Plant Cell Tiss. Organ. Cult. 121, 97–107 (2015).
Chandel, N. S. Glycolysis. Cold Spring Harb. Perspect. Biol. 13, a040535 (2021).
Ahl, L. I. et al. Dynamics of intracellular mannan and cell wall folding in the drought responses of succulent Aloe species. Plant, Cell Environ. 42, 2458–2471 (2019).
Mortimer, J. C. et al. Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc. Natl. Acad. Sci. U.S.A. 107, 17409–17414 (2010).
Li, L., Tang, J., Wu, A., Fan, C. & Li, H. Genome-wide identification and functional analysis of the GUX gene family in Eucalyptus grandis. IJMS 25, 8199 (2024).
Kanter, U. et al. The inositol oxygenase gene family of Arabidopsis is involved in the biosynthesis of nucleotide sugar precursors for cell-wall matrix polysaccharides. Planta 221, 243–254 (2005).
Funding
This work was supported in full by grants from Research on the Technology of Phoebe zhennan for sexual Reproduction and Strong seedling and the Transformation Technology of Low quality and Low Efficiency Forest (Guizhou) (GuoJia Zhong Dian Yan Fa Ji Hua [2017 YFD0601102] sub-topic), Status evaluation and protection techniques of the native Phoebe zhennan population in Guizhou province (Qian Ke He Zhi Cheng [2023] YiBan187), the projects of Key funding Project from Anshun Technical College (Contract No. X202302) and Natural science research project of Education Department of Guizhou Province (No. Qian jiao ji [2024] 309).
Author information
Authors and Affiliations
Contributions
Jing An: wrote the main manuscript text; Honghao Huo: prepared the conceptualization and editing; Qiyuan Liu: prepared figures; Yunli Jiang: editing; Hong Luo: writing—editing; Hao Yupei: review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experimental research and field studies on plants described in this work, including the collection of plant material, were conducted in accordance with relevant institutional, national, and international guidelines, legislation, and ethical standards. Permissions for plant collection and/or use of cultivated/wild species were obtained where required, and voucher specimens (Voucher Number XF20140728-087) have been deposited in Guizhou University. This study did not involve endangered or protected species unless explicitly authorized by the appropriate regulatory bodies.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
An, J., Huo, H., Liu, Q. et al. Physiological and molecular mechanisms of nitrogen in alleviating drought stress in Phoebe bournei. Sci Rep 15, 14684 (2025). https://doi.org/10.1038/s41598-025-99312-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99312-1