Abstract
Insulin resistance (IR) and obesity represent two major factors closely associated with gout. Triglyceride-glucose body mass index (TyG-BMI), calculated from fasting triglyceride and fasting glucose, has recently been proposed as a surrogate indicator of insulin resistance, potentially offering a simplified method for assessing IR-related risk. The prevalence of gout in the U.S. is higher than in the rest of the world, and the condition is even worse in people with obesity and IR. Therefore, this study aims to explore the correlation of the new index with gout. In this cross-sectional study, 13,307 participants were enrolled from the NHANES database, spanning six survey periods from 2007 to 2008 to 2017–2018. Multivariable logistic regression was used to evaluate the association between TyG-BMI and gout in U.S. adults (> 20 years) and to estimate odds ratios. Restricted cubic spline (RCS) analysis and subgroup analysis were conducted to visualize the results and assess potential differences across specific subgroups. The fully adjusted multivariable logistic regression revealed that a 20-unit increase in TyG-BMI was linked to a 15% higher prevalence of gout (OR = 1.15; 95% CI: 1.11, 1.19). Compared with the lowest TyG-BMI tertile group, the participants in the highest showed a 169% higher prevalence of gout (OR = 2.69, 95% CI: 1.80, 4.00). This association remained consistent and robust across different models and subgroups. No significant interactions were observed between TyG-BMI and stratified variables. TyG-BMI is strongly associated with gout in U.S. adults, indicating the potential application of the new index as a supplementary tool in gout detection. More longitudinal studies are needed to confirm the causal relationship.
Similar content being viewed by others
Introduction
Gout is considered a common inflammatory arthritis, characterized by the deposition of monosodium urate (MSU) crystals in joint tissues, mostly caused by elevated serum urate (hyperuricemia)1. Beyond its role as a joint disease, gout is increasingly recognized as a complex condition involving systemic inflammation and metabolic dysregulation, with symptoms extending beyond local MSU crystal deposition2. According to the latest data from Global Burden of Disease (GBD), in 2019, the United States had the highest burden of gout, with a prevalence of approximately 1752 cases per 100,000 individuals (95% UI: 1507.1, 2016.7), surpassing other recorded countries3.
Apart from its high gout rate, the U.S. also ranks among the highest for the prevalence of diabetes and obesity4,5. Previous studies have explored the association between obesity and insulin resistance (IR) and the development of gout, suggesting that these factors may increase gout risk. For obesity, there is an elevated burden of gout among both overweight and obese adults (defined by BMI) in America6. Furthermore, Yang et al. found that obesity may have causal effects on hyperuricemia and gout7. Supporting this, another study observed that weight reduction surgery can lead to significant drop in serum urate levels8.
The triglyceride–glucose (TyG) index is a novel marker of IR, calculated from fasting triglycerides and fasting glucose. It is well-known now that IR serves as the risk factor of plenty of diseases, not merely type 2 diabetes mellitus (T2DM), but also obesity, heart and kidney diseases, etc9. This new index appears to have significant potential for disease detection, identification, and prediction10,11,12,13.
Building upon this, the TyG-BMI index integrates TyG with BMI, thus capturing both insulin resistance and obesity-related metabolic risk. Several studies have demonstrated its superiority over either TyG or BMI alone in predicting conditions like T2DM and cardiovascular diseases14,15,16. Zhou et al. also found that TyG-BMI performs better than the other two indices in terms of assessing hyperuricemia17.
However, to our knowledge, the relationship between TyG-BMI and gout has remained unclear. Therefore, the purpose of this cross-sectional study is to investigate the association between TyG-BMI and gout prevalence, offering new insights into the potential of this composite marker.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative, cross-sectional survey conducted by the Centers for Disease Control and Prevention (CDC) to assess the health and nutritional status of adults and children in the United States. Data for this study were obtained from the NHANES database (https://www.cdc.gov/nchs/nhanes/index.html).
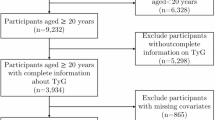
A total of 59,842 adult participants (aged ≥ 20 years) were initially considered. Exclusion criteria included: (1) missing BMI data (height or weight) (n = 7151), (2) missing fasting triglyceride or fasting glucose measurements (laboratory tests) (n = 34,799), (3) missing gout status information (n = 3083), and (4) gout records marked as ‘Refused’ or ‘Unknown’ by participants (n = 11). Additionally, participants with missing or unknown/refused covariate records for coronary heart disease (CHD), hypertension, education, marital status, and smoking exposure (n = 1491) were excluded. In this context, “missing” refers to the absence of survey data (i.e., no questionnaire completed), while “unknown” and “refused” represent specific response categories within the NHANES survey. For CHD, hypertension, education, marital status, and smoking exposure, the proportion of participants with “unknown” or “refused” responses was minimal, justifying their exclusion. However, for the variable “Drinking,” the proportion of “refused” responses was substantial (approximately 10%), potentially due to cultural factors or social stigma influencing participants’ willingness to provide answers. Given this significant proportion, participants with missing “Drinking” data were not excluded; instead, the “Unknown/Refused” category was retained as a separate group for analysis. After applying these criteria, a final sample of 13,307 individuals was included in the analysis, as illustrated in the flowchart (Fig. 1).
Since TyG-BMI is the primary exposure and gout status is the outcome, only participants with complete TyG-BMI and confirmed gout status (‘Yes’ or ‘No’) were included in the analysis.
TyG-BMI
The TyG-BMI index is calculated by multiplying the TyG index with BMI:
\({\text{TyG}} - {\text{BMI }}={\text{ TyG }} \times {\text{ BMI}}\)
where.
\({\text{BMI }}={\text{ Weight}}\;({\text{kg}}){\text{/Heigh}}{{\text{t}}^2}\;({{\text{m}}^2})\)
and.
\({\text{TyG }}={\text{ }}\ln \left[ {{\text{fasting}}\;{\text{triglycerides}}\;({\text{mg/dl}}){\text{ }} \times {\text{ fasting }}\;{\text{glucose}}\;({\text{mg/dl}})/2} \right]\)
The logarithmic transformation used in its calculation helps mitigate skewness in triglyceride and glucose distributions, enhancing its stability and reliability in clinical research. Furthermore, fasting glucose and triglyceride values were obtained from Laboratory Data (LBXGLU and LBXSTR), while BMI was computed using weight and height data from the Body Measures (BMX) dataset.
Gout
A questionnaire called the medical conditions section (MCQ) was performed to collect medical conditions data from participants. MCQ160n is a question about gout status, where interviewees were asked, “Has a doctor or other health professional ever told {you/SP} that {you/s/he} had gout?” If the answer was “yes”, which was coded as 1 in the dataset, then the participant was marked as “gout”.
Covariables
Based on previous research, common demographic, socioeconomic, lifestyle, and health-related variables were included as covariates in our study18,19,20,21. From the Demographics dataset, we obtained age (years), sex (male or female), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), marital status (married, widowed, divorced, separated, never married, living with partner), the ratio of family income to poverty (PIR, categorized as < 1.3, 1.3–3.5, > 3.5), and education (less than high school, high school graduate, some college, college graduate or above). Lifestyle factors included smoking exposure (≥ 100 cigarettes in a lifetime, SMQ dataset) and alcohol exposure (≥ 12 alcoholic drinks/year, ALQ dataset). For variables with “unknown/refused” responses, we excluded participants for coronary heart disease (CHD), hypertension, education, marital status, and smoking exposure due to their small proportion (< 10%), but retained alcohol consumption “unknown/refused” responses (≈ 10%) as a separate category due to potential cultural or social influences. Clinical conditions, including CHD, hypertension, and diabetes, were assessed via self-reported physician diagnoses from the Medical Conditions (MCQ) and Diabetes (DIQ) datasets. Participants with missing covariate data were excluded, except for alcohol consumption as noted.
Statistical analysis
In this study, extreme values in fasting blood glucose and triglyceride levels were managed using Winsorization, a statistical technique that limits outliers by capping them at specified percentiles to reduce their influence on the analysis. Specifically, values beyond the 1st and 99th percentiles were capped using this method. The TyG-BMI was designated as an exposure variable, and TyG-BMI tertiles were used to divide participants into three groups to present their characteristics: T1 [142,217], T2 (217,270], and T3 (270,460]. Each group contained 4436 or 4437 individuals. The group with the lowest TyG-BMI (T1) served as the reference. Likewise, according to gout status (gout or non-gout), two groups were formed to outline the basic differences within the population. The Kruskal–Wallis rank sum test, Pearson’s Chi-squared test were employed to assess the significance of differences. Those covariates with significant p-value would be selected for adjustments of the subsequent multivariable regression.
Multivariable logistic regression was used to investigate the association between the TyG-BMI index and gout across four models. The crude model was unadjusted, while adjusted models 1, 2, and 3 were controlled with covariables, as mentioned above, to different degrees. Specifically, Model 1 was adjusted for age, sex, and race. Building on this, Model 2 was further adjusted to include additional covariates, namely marital status, hypertension, diabetes, and coronary heart disease (CHD), in addition to the variables from Model 1. Subsequently, Model 3 was adjusted to account for an even broader set of factors, incorporating PIR, education level, smoking exposure, and alcohol exposure, alongside age, sex, race, hypertension, diabetes, and CHD.
Further analysis was made by converting the continuous TyG-BMI to a categorical variable (tertiles). Tertile 1 (T1) was set as the reference to test for potential trends in the association. Additionally, a bar chart and restricted cubic spline (RCS) curve were utilized to illustrate the continuous and categorical results of the multivariable logistic regression, respectively. And then, to evaluate the discriminatory performance of TyG-BMI, TyG, and BMI in identifying gout, we conducted a receiver operating characteristic (ROC) curve analysis. The ROC curve is a graphical plot that illustrates the diagnostic ability of a binary classifier system as its discrimination threshold is varied. The area under the curve (AUC) was calculated to assess the overall predictive ability, with higher values indicating better discrimination.
Additionally, we determined the optimal threshold for each index based on the Youden index, which maximizes the sum of sensitivity and specificity. Sensitivity and specificity were extracted at these optimal cutoff points to further compare the classification performance of each metric. Additionally, subgroup analyses were conducted to examine the robustness of the results. Interaction analysis was performed to assess the heterogeneity of associations across different subgroups.
In this study, all estimates and regression models were weighted using the appropriate NHANES sample weights, thereby providing unbiased and accurate effects for the overall population. In terms of software, subgroup analyses were conducted using EmpowerStats 2.0 (https://www.empowerstats.net/en/), a graphical statistical software that allows for efficient subgroup analysis and convenient selection of confounding variables (details on confounder adjustment can be found in the logistic regression section). All other analyses were performed in R version 4.4.1 (2024-06-14 ucrt) using the survey, tableone, and tidyverse packages. ROC curves and AUC estimations were generated with the pROC package. A P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 presents the overall characteristics of the study sample. After applying complex survey analysis, 13,307 participants represented a weighted population of 205,957,734.04. Among them, 48.2% were male, and 67.6% were Non-Hispanic White. A notable proportion of U.S. adults were drinkers (60.4%) and smokers (44.1%). Table 1 also shows a significant increase in the prevalence of gout with rising TyG-BMI levels, from 1.7% in the T1 group to 6.4% in the T3 group (P < 0.001). Additionally, conditions such as hypertension, diabetes, and CHD were more prevalent in populations with higher TyG-BMI levels (P < 0.002).
Table 2 compares the characteristics of individuals with and without gout. Those diagnosed with gout were older (median age 62.00 vs. 46.00 years, P < 0.001) and more likely to be male (65.5% vs. 47.5%, P < 0.001). A higher prevalence of CHD (11.6% vs. 3.1%), diabetes (25.1% vs. 8.8%), and hypertension (71.2% vs. 31.0%) was observed in the gout population (P < 0.001). Regarding laboratory indicators, gout patients had higher levels of fasting plasma triglycerides (median 125.42 vs. 99.00 mg/dL, P < 0.001) and fasting plasma glucose (median 109.00 vs. 100.00 mg/dL, P < 0.001), resulting in an elevated TyG-BMI value (median 246.0 vs. 191.0, P < 0.001).
Relationships of TyG‑BMI with gout prevalence
Table 3 presents the multivariate logistic regression results for the association between TyG-BMI and gout. Each 20-unit increase in TyG-BMI was associated with a 15% higher likelihood of gout (OR 1.15; 95% CI: 1.11, 1.19; P < 0.001) in the fully adjusted model (Model 3). This association was significant across all models: OR 1.16 (95% CI: 1.12, 1.20) in the unadjusted model, OR 1.19 (95% CI: 1.15, 1.23) in Model 1, and OR 1.15 (95% CI: 1.10, 1.19) in Model 2 (P < 0.001 for all). Compared to the lowest TyG-BMI tertile (T1), the highest tertile (T3) showed a 169% increased prevalence of gout in Model 3 (OR 2.69; 95% CI: 1.80, 4.00; P < 0.001), with a consistent trend across models (T2: OR 1.54, 95% CI: 1.00, 2.38; T3: OR 2.71, 95% CI: 2.51, 5.47 in unadjusted model; P for trend < 0.001). Figure 2a illustrates a restricted cubic spline (RCS) curve, showing a positive association between TyG-BMI and gout prevalence. Figure 2b, a bar chart of logistic regression results, visually supports the increasing odds ratios across tertiles (T1: 142,217; T2: 217,270; T3: 270,460), with a P-value for trend < 0.0001.
The discriminatory ability of TyG-BMI, TyG, and BMI for gout
As shown in Table 4; Fig. 3, TyG-BMI exhibited the highest AUC. However, the absolute AUC values for all three indices remain modest, all slightly above 0.6, indicating that none of them serve as highly accurate standalone diagnostic tools for gout. Therefore, even though TyG-BMI may offer diagnostic value, its clinical utility for gout screening likely requires combination with other diagnostic markers to improve accuracy and robustness in real-world applications. More detail of the logistic regression results between TyG, BMI and gout can be founded in supplement material.
Subgroup analysis
According to the results presented in Fig. 4, the association between TyG-BMI and gout prevalence remained consistent across all examined subgroups, with higher TyG-BMI levels corresponding to an increased risk of gout. None of the interaction terms were statistically significant (all P for interaction > 0.05), indicating no meaningful differences in this association between subgroups. Interestingly, even though the difference is not significant, the observed association appeared weaker in participants with comorbidities such as CHD, hypertension, or diabetes. For example, in individuals without CHD, the OR for gout per 20-unit increase in TyG-BMI was 1.17 (95% CI: 1.13–1.21), whereas in those with CHD, the OR was 1.09 (95% CI: 1.00–1.20). A similar pattern was observed in the hypertension and diabetes subgroups.
This may be explained by a marginal effect, wherein individuals with chronic conditions such as CHD, hypertension, or diabetes may already have elevated baseline metabolic risk, reducing the additional impact of TyG-BMI on gout prevalence; metabolic disturbances common in these populations may also weaken this association; or it was the small sample size in these subgroups that led to wider confidence intervals and effect estimates closer to null.
Discussion
Prior works have reported the correlation of obesity and insulin resistance with gout, demonstrating that these factors may contribute to an elevated risk of gout6,7,8,22,23. In this study, we examined the prevalence of gout in relation to the TyG-BMI. The main finding of our study is that the new index is positively associated with the probability of having gout. This association remains robust and statistically significant even after adjusting for various covariables. Stratified by age, gender, race, and other chronic conditions, subgroup analyses indicate that this relationship was consistent across different populations.
There is ample evidence supporting a strong causal relationship between obesity and gout24,25. For instance, Lu et al. found that weight gain in adulthood significantly increases the risk of gout, which aligns with our findings26. In obese individuals, bariatric surgery has been shown to reduce serum urate levels, thereby significantly lowering the incidence of gout. This finding further reinforces the role of adiposity in the pathogenesis of gout8. Indeed, visceral fat may play a key role in the effect of obesity on gout. In particular, visceral fat has been implicated in the development of gout through mechanisms such as increased production of proinflammatory cytokines (e.g., TNF-α and IL-6)27. This chronic, low-grade inflammation can, in turn, trigger IR in obese individuals, increasing their vulnerability to T2DM and metabolic syndrome28. These metabolic disturbances, such as elevated blood pressure and blood glucose levels, are closely linked to the development of gout through the elevation of serum urate levels23. Specifically, components of metabolic syndrome, including hypertension, dyslipidemia, and hyperglycemia, significantly heighten the risk of gout. While obesity is a well-established risk factor for the initial onset of gout, its relationship with recurrent gout attacks remains less clear, with some studies suggesting a paradoxically weak or even null association29.
On the other hand, the relationship between IR and gout is relatively complicated and remains controversial. It’s uncertain whether there is a bidirectionally causal effect between them30. Hyperinsulinemia is commonly observed in individuals with IR. McCormick et al. using bidirectional Mendelian randomization, found that it is hyperinsulinemia to lead to hyperuricemia, which in turn contributes to gout, but not vice versa23. Pathophysiological research suggests that IR induces hyperuricemia mainly by affecting sodium reabsorption in the kidney, the major organ responsible for uric acid excretion. Specifically, IR enhances proximal tubular sodium reabsorption, which indirectly facilitates urate reabsorption; this elevates serum uric acid levels31,32. Interestingly, such effects may change after the onset of diabetes. A retrospective study reported that compared with individuals with no diabetes, diabetic patients show a lower risk of gout incidence33. Furthermore, using the TyG-BMI to evaluate IR and obesity, Gou et al.34 found a positive correlation between hyperuricemia and TyG-BMI in both US and Chinese populations (Q4, OR 6.67, 95% CI: 5.55, 8.02). However, it is notable that not all cases of hyperuricemia finally lead to gout2. The progression from hyperuricemia to clinical disease is influenced by multiple factors, including genetic susceptibility, inflammatory response, and metabolic status. Thus, our findings may support the utility of TyG-BMI as a supplementary tool for identifying individuals with gout.
To our knowledge, this paper is the first to represent a comprehensive investigation of the association between the TyG-BMI index and gout. Given the complex sampling design of NHANES and the weighted analysis we conducted, the conclusions of this study can be generalized to the broader U.S. population. This research, therefore, helps to provide more evidence for the link between IR, obesity, and gout, suggesting the potential application of this novel index for assessing gout in the public.
Admittedly, the present study has certain limitations, primarily due to its cross-sectional design, which precludes the establishment of a causal relationship between the TyG-BMI and gout. Such design also raises the possibility of reverse causality, where gout itself may influence TyG-BMI levels. Thus, a high-quality cohort is needed to validate any causal links. Additionally, even though variables such as income and education level have been taken into account, confounding bias may still persist. For example, sugar-lowering and lipid-lowering medications were not included as control variables in this study. These medications can influence plasma triglycerides and glucose levels, which in turn affect the TyG-BMI index. Moreover, self-reported gout diagnoses may differ from actual morbidity, as individuals with elevated urate levels might not experience sufficient inflammation or pain to seek medical attention, thus remaining undiagnosed. Alternatively, socioeconomic factors, such as financial constraints, may deter individuals from consulting a doctor for tolerable discomfort, contributing to morbidity discrepancy. Finally, BMI has been widely used in research to define obesity and overweight. However, compared with several other indicators, such as waist-to-hip ratio (WHR), which could provide a more accurate assessment of obesity, BMI may not adequately capture visceral fat distribution. Some studies had reported that visceral fat obesity, rather than subcutaneous fat, is highly associated with primary gout. This association persists even among individuals who are classified as non-obese according to BMI standards; that is, an increase in visceral fat is a critical factor in metabolic abnormalities leading to gout35,36.
Conclusion
The present study reveals that TyG-BMI is strongly correlated with gout in U.S. adults. Specifically, each 20-unit increase in TyG-BMI correlates with an approximately 15% higher probability of gout (OR 1.15; 95% CI: 1.11, 1.19). These results highlight the importance of managing obesity and IR as strategies for potentially preventing or mitigating gout. While TyG-BMI exhibited a slightly higher AUC than TyG or BMI, the difference was minimal, and its predictive value remains modest. Thus, TyG-BMI may offer some diagnostic value but should not be considered a standalone screening tool. Further longitudinal studies are needed to confirm these findings, assess causality, and evaluate the potential integration of TyG-BMI with other diagnostic markers.
Data availability
The datasets for this study can be found in the NHANES (https://www.cdc.gov/nchs/nhanes/index.html).
References
Dalbeth, N., Gosling, A. L., Gaffo, A. & Abhishek, A. Gout. Lancet 397, 1843–1855 (2021).
Zhang, W. Z. Why does hyperuricemia not necessarily induce gout? Biomolecules 11, 280 (2021).
Han, T., Chen, W., Qiu, X. & Wang, W. Epidemiology of gout—Global burden of disease research from 1990 to 2019 and future trend predictions. Ther. Adv. Endocrinol. Metab. 15, 20420188241227295 (2024).
Boutari, C. & Mantzoros, C. S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 133, 155217 (2022).
Hossain, M. J., Al-Mamun, M. & Islam, M. R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 7, e2004 (2024).
Juraschek, S. P., Miller, E. R. & Gelber, A. C. Body mass index, obesity, and prevalent gout in the United States in 1988–1994 and 2007–2010. Arthritis Care Res. 65, 127–132 (2013).
Simental-Mendía, L. E., Rodríguez-Morán, M. & Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 6, 299–304 (2008).
Mills, D. W., Woolley, D. M., Ammori, B. J., Chinoy, H. & Syed, A. A. Changes in serum urate levels after bariatric surgery in patients with obesity: An observational study. Obes. Surg. 34, 1737–1741 (2024).
Lee, S. H., Park, S. Y. & Choi, C. S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 46, 15–37 (2022).
Paramanathan, T., Sandrasegarampillai, B., Arasaratnam, V. & Thirunavukarasu, K. The discriminative ability of the triglyceride-glucose index to identify metabolic syndrome among adults of the Northern Sri Lankan population. BMC Endocr. Disord. 24, 101 (2024).
Wan, H., Cao, H. & Ning, P. Superiority of the triglyceride glucose index over the homeostasis model in predicting metabolic syndrome based on NHANES data analysis. Sci. Rep. 14, 15499 (2024).
Nabipoorashrafi, S. A. et al. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. NMCD 32, 2677–2688 (2022).
Yin, J. L. et al. Triglyceride-glucose index and health outcomes: An umbrella review of systematic reviews with meta-analyses of observational studies. Cardiovasc. Diabetol. 23, 177 (2024).
Lyu, L. et al. Association between triglyceride glucose-body mass index and long-term adverse outcomes of heart failure patients with coronary heart disease. Cardiovasc. Diabetol. 23, 162 (2024).
Song, B. et al. Triglyceride glucose-body mass index and risk of incident type 2 diabetes mellitus in Japanese people with normal glycemic level: A population-based longitudinal cohort study. Front. Endocrinol. 13, 907973 (2022).
Wang, X. et al. Triglyceride glucose-body mass index and the risk of diabetes: A general population-based cohort study. Lipids Health Dis. 20, 99 (2021).
Zhou, S. et al. Association of obesity, triglyceride-glucose and its derivatives index with risk of hyperuricemia among college students in Qingdao, China. Front. Endocrinol. 13, 1001844 (2022).
Li, T., Zhang, H., Wu, Q., Guo, S. & Hu, W. Association between triglyceride glycemic index and gout in US adults. J. Health Popul. Nutr. 43, 115 (2024).
Hayward, R. A. et al. The association of gout with socioeconomic status in primary care: A cross-sectional observational study. Rheumatol. Oxf. Engl. 52, 2004–2008 (2013).
Nieradko-Iwanicka, B. The role of alcohol consumption in pathogenesis of gout. Crit. Rev. Food Sci. Nutr. 62, 7129–7137 (2022).
Clarson, L. et al. Increased cardiovascular mortality associated with gout: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 22, 335–343 (2015).
Asma Sakalli, A., Küçükerdem, H. S. & Aygün, O. What is the relationship between serum uric acid level and insulin resistance? A case-control study. Medicine (Baltim). 102, e36732 (2023).
McCormick, N. et al. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional Mendelian randomization. Arthritis Rheumatol. Hoboken NJ. 73, 2096–2104 (2021).
Yang, Y. et al. The role of obesity, type 2 diabetes, and metabolic factors in gout: A Mendelian randomization study. Front. Endocrinol. 13, 917056 (2022).
Evans, P. L. et al. Obesity, hypertension and diuretic use as risk factors for incident gout: A systematic review and meta-analysis of cohort studies. Arthritis Res. Ther. 20, 136 (2018).
Bai, L., Zhou, J. B., Zhou, T., Newson, R. B. & Cardoso, M. A. Incident gout and weight change patterns: A retrospective cohort study of US adults. Arthritis Res. Ther. 23, 69 (2021).
Ellulu, M. S., Patimah, I., Khaza’ai, H., Rahmat, A. & Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. AMS. 13, 851–863 (2017).
Wondmkun, Y. T. & Obesity Insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. Targets Ther. 13, 3611–3616 (2020).
Nguyen, U. S. D. T. et al. Obesity paradox in recurrent attacks of gout in observational studies: Clarification and remedy. Arthritis Care Res. 69, 561–566 (2017).
Li, C., Hsieh, M. C. & Chang, S. J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 25, 210–216 (2013).
Ter Maaten, J. C. et al. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin. Sci. Lond. Engl. 1979 92, 51–58 (1997).
Muscelli, E. et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am. J. Hypertens. 9, 746–752 (1996).
Rodríguez, G., Soriano, L. C. & Choi, H. K. Impact of diabetes against the future risk of developing gout. Ann. Rheum. Dis. 69, 2090–2094 (2010).
Gou, R. et al. Association between triglyceride glucose index and hyperuricemia: A new evidence from China and the united States. Front. Endocrinol. 15 (2024).
Wang, Y. et al. Association of obesity based on different metabolic status with risk of gout occurrence in patients: A national study. Endocr. Metab. Immune Disord. Drug Targets 24, 973–980 (2024).
Lee, J. et al. Visceral fat obesity is highly associated with primary gout in a metabolically obese but normal weighted population: A case control study. Arthritis Res. Ther. 17, 79 (2015).
Author information
Authors and Affiliations
Contributions
R.Z. and R.L. contributed to the study design, data collection, and data analysis and interpretation, with revisions guided by L.Z.H.Z. was responsible for manuscript drafting.J.L. and S.L. assisted with data collection and provided critical revisions to the manuscript.S.Y. offered statistical support and contributed to data analysis.C.Z. supervised the study and provided critical feedback on the manuscript.D.Y. and L.Z. provided overall guidance, reviewed, revised, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, R., Lin, R., Zhang, H. et al. Association between TyG-BMI and gout in US adults: evidence from NHANES 2007–2018. Sci Rep 15, 15534 (2025). https://doi.org/10.1038/s41598-025-99379-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99379-w