Abstract
Salinity is a significant environmental stress impacting plant growth and metabolism in irrigated areas of arid and semi-arid regions; the use of biostimulants is an effective alternative to enhance plant productivity under saline conditions. This study examined the effects of salinity stress by applying various electrical conductivities (0.5, 1.5, 2.5, 3.5, and 4.5 dS m− 1) of irrigation water combined with foliar application of the in combination with foliar application of biostimulant VIUSID Agro (0, 0.3, and 0.6 mL L− 1) on the growth, productivity, physiological, and biochemical responses of bell pepper plants in a greenhouse. Conducted in a factorial design with a completely randomized layout and five replications, and the results indicated that salinity caused a significant reduction in growth, decreased chlorophyll levels, and increased malondialdehyde levels, osmoregulators, and antioxidant enzyme activity. Plants treated with biostimulant demonstrated relatively superior growth parameters, such as plant height, dry matter, leaf area and number of leaves, as well as fruit quality, such as fruit size, firmness, ascorbic acid and soluble solids. Both doses of the biostimulant effectively mitigated the effects of salt stress by maintaining higher chlorophyll levels (15% increase), enhancing photosynthetic performance (20% increase), and improving fruit size and quality, as well as leaf water status, ultimately leading to better crop performance. The biochemical mechanisms through which these effects occur include enhanced osmoregulation and increased antioxidant activity. The foliar application of the biostimulant was an effective strategy to enhance the tolerance of bell pepper plants under salt stress conditions and can serve as a sustainable solution for agricultural production in saline irrigation water.
Similar content being viewed by others
Introduction
Climate change and adverse local conditions impact agriculture, jeopardizing food production1. Changes in the climate has intensified abiotic stresses, such as water and soil salinity, directly affecting agricultural crops and, consequently, reducing food production, especially in arid and semi-arid regions2. Semi-arid regions, such as the Brazilian Northeast, suffer from recurrent periods of drought, resulting in the accumulation of salt in the soil and groundwater due to the scarcity of rainfall and high evapotranspiration. Despite the high salt concentration, groundwater is often used for agriculture purposes, making salt stress inevitable in these regions2. Most horticultural crops are sensitive to salinity, and growth is affected in salinized soils, where an accumulation of ions occurs due to continuous irrigation3. This is what happens with bell pepper (Capsicum annuum L.), for example, which is considered a moderately salt-sensitive crop4.
Crops growing in saline environments are subject to osmotic stress, nutritional disorders, toxicity, and reduced productivity5. Plant responses to salt stress are diverse and involve many different biochemical and physiological processes6. Decrease in leaf surface expansion rate, stomatal closure, decreased photosynthesis and transpiration rates, and reduction in growth are some of the responses to salt stress7. Another consequence of salt stress is the excessive production of reactive oxygen species (ROS), which can have cytotoxic action, causing damage to cellular structures, such as chlorophyll disintegration, ionic extravasation, lipid peroxidation, inhibition of protein synthesis, and cell death8. As an important response to stress factors, plants have specific adaptive physiological and molecular responses, for example, osmotic adjustment (OA) and enhanced antioxidant capacity9. Another crucial plant tolerance strategy under salt stress is the activation of the defense system against oxidative damage10.
Several management techniques have been evaluated to mitigate the deleterious effects of salts in agricultural production, among which the use of biostimulants stands out11, biologically derived compounds that cover a diverse range of substances, including microbial inoculants, humic acids, and amino acids (AAs), stands outs12,13. Biostimulants stimulate plant growth, development, and resilience to abiotic stresses, stimulating natural processes in plants13,14, improving nutrient uptake and assimilation efficiency, the overall quality of the agricultural product15. These compounds fortify plants against osmotic imbalance, ionic toxicity, and oxidative damage induced by salinity stress, reinforcing the plant’s intrinsic defense mechanisms, amplifying its ability to withstand and adapt to salinity11,13,14. The application of biostimulants represents an alternative to synthetic chemicals to mitigate salt stress, being an environmentally correct option for sustainable agriculture, being increasingly used to increase agricultural production16,17.
Studies show that the use of AAs as biostimulants has positive effects on plant growth and development under stressful conditions7,16,18. Amino acids have a beneficial role in plant development by providing organic nitrogen and as protein units, which play a crucial role in glutamine biosynthesis16 and as plant growth regulators19. Under salinity conditions, amino acids regulate ion transport, control stomatal opening, enzyme activation, and the maintenance of redox homeostasis16,20. Glycine, for example, is widely applied in plant nutrition, producing a wide range of amino-chelated fertilizers21. Arginine is a precursor in polyamine synthesis and molecule signaling, and is one of the most multifunctional amino acids with the highest N/C ratio22. The application of amino acids is an important tool for mitigating the effects of salt stress because they are essential molecules for plant metabolites in protein synthesis and other vital cellular functions.
Salt stress negatively impacts the growth and value of many agricultural products, including bell peppers, which are considered a moderately salt-sensitive crop, with a salinity threshold averaging 1.5 dS m− 14. Research on the use of amino acid-based biostimulants to mitigate salt stress in bell peppers is limited, and existing studies often yield conflicting result regarding their effectiveness. The use of biostimulants has a positive effect on improving productive performance and tolerance to salinity in some crops23,24, and in the search for more sustainable practices in current agricultural production. While some experiments with other horticultural crops suggest that various biostimulants can enhance salt tolerance by improving growth parameters, osmoregulators, and antioxidative enzyme activities, the variability in results raises questions about the consistency and reliability of these findings. This research aims to clarify the physiological and biochemical changes; and the yield and quality of the fruit, aspect of great importance for commercial purposes and human consumption25, under salt stress while testing the hypothesis that foliar application of an amino acid-based biostimulant (including glycine, arginine, and tryptophan) can alleviate the adverse effects of salt stress on bell pepper plants in greenhouse conditions. However, the variability in previous studies highlights the need for further investigation to establish consistent benefits, particularly for organic production in both field and commercial greenhouse settings.
Materials and methods
Geographic location and experimental design
This study was conducted in a greenhouse at the Department of Plant Science and Environmental Sciences, Universidade Federal da Paraíba (UFPB), Areia, Paraíba, Brazil (6°58’04’’S, 35°42’58’’W and altitude: 508 m). The region has an As’ climate (Köppen classification: tropical with dry summers)26, characterized by: hot, dry summers (average temperature: 28 °C; rainfall: <50 mm/month); rainy winters (average temperature: 22 °C; rainfall > 300 mm/month).
Greenhouse conditions (temperature and relative humidity) were monitored hourly using a calibrated digital thermohygrometer (Incoterm, Model 76666.02.0.00, USA). During the 3-month experimental period (February-2022 to July-2022): mean temperature: 23.42 ± 1.26 °C (range: 20.16–26.68 °C); mean relative humidity: 81.36 ± 8.71% (range: 66.66–96.07%). Figure 1 illustrates daily fluctuation in these parameters.
The experiment followed a completely randomized design arranged in a 5 × 3 factorial scheme, with five replications, totaling 75 experimental units (pots). Each pot (11 dm³ capacity, filled with 10 kg of soil) represented an individual plot, containing one plant per pot. Salinity treatments consisted of: Control (0.5 dS m− 1), four elevated levels: 1.5, 2.5, 3.5, and 4.5 dS m− 1. Biostimulant treatments (VIUSID Agro, foliar-applied) included: 0 (control), 0.3, and 0.6 mL L− 1 in distilled water27. VIUSID Agro contains: amino acids mixtures: free amino acids (7% m/m), glycine (2.5% m/m), arginine (2.4% m/m), aspartic acid (1.6% m/m), and tryptophane (0.5% m/m) (All reported to enhance plant growth under stress18); vitamins and minerals. Biocatalytically processed to increase efficacy without altering molecular properties, thereby improving biological activity and antioxidant reactivity.
Soil preparation
The soil (not sterilized) used to conduct the experiment was sieved to remove clods and unwanted materials and weighed to obtain of 10 kg of substrate for each pot. According to the analysis, the soil presented the following characteristics: pH = 7.2; electrical conductivity = 0.5 dS m− 1 at 25 °C; SO4 − 2 = 0.95 dS m− 1 at 25 °C; Ca ++ = 1.72 mmolc L− 1; Mg++ = 2.71 mmolc L− 1, Na+ = 0.61 mmolc L− 1; K+ = 1.17 mmolc L− 1; CO3 − 2 = 0.00 mmolc L− 1; HCO3 = 17.1 mmolc L− 1; Cl− = 9.8 mmolc L− 1; sodium adsorption ratio = 0.41 mmolc L− 1; percentage of exchangeable sodium = 0.0%, classified as non-saline and non-sodic. Regarding the physical analysis, the soil was classified as: sand (2–0.05 mm) = 876; silt (0.005–0.002 mm, g kg− 1) = 99; clay (< 0.002 mm) = 25, textural class = sand. For the chemical analysis: pH (H2O: 1:2.5) = 6.9; P (mg dm− 3) = 33.04; S (mg dm− 3) = -; K+ (mg dm− 3) = 96,74; Na+ cmolc dm− 3 = 0.05; H+Al+ 3 cmolc dm− 3 = 1.60; Al+ 3 cmolc dm− 3 = 0.00; Ca+ 2 cmolc dm− 3 = 2.52 Mg+ 2 cmolc dm− 3 = 1.18; SB cmolc dm− 3 = 4.00; CTC cmolc dm− 3 = 5.60; MO g kg− 1 = 21.36. P, K, Na: Mehlich extractor 1; H + Al: calcium acetate extractor 0.5 M, pH 7.0; Al, Ca, Mg: KCl extractor 1 M; SB: Sum of tradable bases CEC: cation exchange capacity; OM: organic matter – Walkley-Black.
Plant material and execution of the experiment
The plant materials of the species Capsicum annuum L., cultivar Magistral (Seminis Company) used in this study were obtained from an agricultural store called Agrocenter, located in Campina Grande, Paraíba, Brazil. After the purchase, the seeds were placed to germinate in a commercial greenhouse of Agrocenter, specialized in the production of seedlings in trays, located in the municipality of São Sebastião de Lagoa de Roça, Paraíba, Brazil (7°06’00.0”S, 35°52’56.4”W)—where expanded polystyrene trays with 200 cells filled with Mecplant commercial substrate were used for seedling development. The conduction of the plant material was carried out in compliance with the applicable ethical and regulatory guidelines, ensuring the authenticity of the material. In the experiment, there was only one cultivar of bell pepper. All the plants were the same age and at the same stages of development.
The sowing date was on February 21, 2022 and they emerged 7 days after sowing. When the seedlings reached four to six definitive leaves, approximately 35 days after sowing (DAS), they were transplanted into pots with a capacity of 11 dm2, filled with 10 kg of soil, one plant per pot.
Fertilization was carried out with N (100 mg kg− 1), P2O5 (300 mg kg− 1) and K2O (150 mg kg− 1 according to Novais et al.28, and fertilizing urea (45% N), monoammonium phosphate (MAP, 11% N, 52% P2O5), and potassium chloride (KCl, 60% K2O), respectively. Fertilization with N and P2O5 was divided into five applications (15, 30, 45, 60, and 75 DAS), fertilization with K2O was divided into three applications (45, 60 and 75 DAS). To meet the micronutrient needs of the plants, 2.5 g L− 1 of a commercial product was applied every two weeks via foliar, with the following composition: N (15%), P2O5 (15%), K2O (15%), Ca (1%), Mg (1.4%), S (2.7%), Zn (0.5%), B (0.05%), Fe (0.5%), Mn (0.05%), Cu (0.5%) and Mo (0.02%). Micronutrients were applied uniformly in all treatments, but at different times than biostimulant applications.
The experiment was conducted for over 110 days, with biostimulant applications starting at 14 DAS and saline irrigation starting at 50 DAS. The first application was carried out at 7 days after seedling emergence (14 DAS) and the other applications were carried out an intervals of 7 days, in the late afternoon, until the beginning of fruit harvest, which took place at 90 DAS, totaling 11 applications. The application was made with sprayers with a capacity of 350 mL, in which the biostimulant was diluted in distilled water using 1000 µL micropipettes. Each plant received six sprays per application, and the volume of biostimulant solution applied per plant was 1.8 µL in 6 mL of distilled water for the 0.3 mL L− 1 dose and 3.6 µL for the 0.6 mL L− 1 dose. During the application of the biostimulant, to avoid contamination of neighboring plants, each plant was isolated at the time of foliar application. The cultural practices consisted of manual weeding, whenever necessary, staking of the plants using bamboo stakes, and preventive application of chemical fungicide of the triazole group.
Irrigation with saline water was started at 50 DAS. Sodium chloride (NaCl) was used to induce salt stress at concentrations of 0, 960, 1600, 2240 and 2880 mg L− 1 in water collected from a well to achieve conductivities of 0.5 (control); 1.5; 2.5; 3.5 and 4.5 dS m− 1, whose values were verified with the aid of a digital conductivity meter (Model TDS&EC meter (hold)). The electrical conductivity of the NaCl solutions was obtained according to the equation of Rhoades et al.29. After the preparation, the saline water solutions were stored in 100 L plastic drums and the ECs were checked weekly with the conductivity meter to avoid possible changes in salinity. According to Table 1, the water supply used as a control treatment in this study had an EC of 0.5 dS m− 1.
Irrigations were carried out every other day, in the late afternoon (5 pm), following a two-day irrigation frequency in which each treatment received its own irrigation volume, corrected for each irrigation, based on the water consumption of the plants in the previous irrigation, dividing the estimated volume by the factor 0.9. Thus, soil moisture was restored to field capacity, obtaining a leaching fraction (LF) of approximately 0.1 for all treatments, as follows: \(\:Vi=\frac{Va-Vd}{1-LF}\), where: Vi – volume of water to be applied in irrigation, in mL; Va – volume of water applied in the previous irrigation, in mL; Vd – volume of water drained after the previous irrigation, in mL. The drained water was collected in the morning of the day after irrigation, measuring the leachate volume, from collectors installed in each pot. As it was in a controlled environment, no seasonal effects influenced the irrigation water. Chemical analysis of the irrigation water is shown in Table 1.
Growth, production components and physico-chemical attributes
Plant height, stem diameter, number of leaves and leaf area were measured weekly after the start of treatments. The leaf area was determined according to Reis et al.30. After the beginning of fruiting, successive fruit harvests were carried out every three days, obtaining the number of fruits (fruits per plant), the average fruit weight (AFW), firmness, ascorbic acid content (AAC) and solid soluble content (SS).
The estimated yield of bell pepper fruits was obtained by multiplying the mean number of fruits per plant by the mean weight of the fruit per plant, given in kg per plant− 1.
At the end of the crop cycle, the plants were collected, separated into leaves, stems, and roots, and placed to dry in an oven at 65 °C until a constant mass, thus obtaining the dry mass of each part of the plant.
The fruit width and fruit length were determined by measuring five fruits at random for each treatment, with the aid of a digital caliper (Electronic Digital Caliper Carbon Fiber Caliper 150 mm).
The physico-chemical characteristics of the fruits were carried out at the Phytopathology Laboratory of the Universidade Federal da Paraíba (UFPB). The firmness analysis of the fruits was performed after the harvest of the fruits, and the chemical attributes were performed in the first days of storage. The following parameters were evaluated: fruit firmness, ascorbic acid content, and soluble solids (SS).
The firmness of the fruit was determined by means of resistance to penetration by the use of a digital penetrometer (Magness Taylor Pressure Tester), pressed at two opposite ends in the median region of the unpeeled fruits and the results were expressed in Newtons (N). Ascorbic acid content was determined by ascorbic acid content (mg 100 g− 1 of pulp) through titrology, according to Strohecker and Henning31. The SS content was determined directly from the crushed pulp with a digital refractometer (Milwaukee MA871 Digital Brix/Sugar) and expressed in ºBrix32.
Gas exchange, chlorophyll fluorescence, chlorophyll index, and relative water content
Gas exchange and chlorophyll fluorescence evaluations were performed weekly between 40 and 60 days after transplanting (DAT). The leaves used in the measurement were five leaves from the middle third of the plant (middle leaves). Gas exchange was measured between 8 and 11 am with an infrared gas analyzer IRGA (LI-6400XT, LI-COR, Nebraska, USA) with an airflow of 400 µmol s− 1, and a natural light source. The following parameters were obtained: net CO2 assimilation rate (A, µmol CO2 m− 2 s− 1), stomatal conductance (gs, mol H2O m− 2 s− 1), CO2 concentration in the intercellular spaces (Ci, µmol CO2 mol− 1), transpiration (E, mmol H2O m− 2 s− 1), leaf temperature (TF, °C), water use efficiency (WUE = A/E), intrinsic water use efficiency (iWUE = A/gs) and instantaneous carboxylation efficiency (iCE = A/Ci).
Chlorophyll a was determined with an unmodulated fluorometer (Opti-Sciences Inc.- Model OS-30p, Hudson, USA) on dark-adapted leaves for 30 min using leaf tweezers. Through this fluorometer the quantum yield of photosystem II (Fv/Fm) was measured. Chlorophyll index (chlorophylls a, b, and total chlorophyll) was measured in the third developed leaf (from the apex to the base) by the non-destructive method with the aid of a portable electronic chlorophyll meter (ClorofiLog, model CFl 1030, Porto Alegre, Brazil). For this, the mean of three measures made on the middle third of the leaf blade was considered, avoiding the central vein. Values were expressed as Falker Chlorophyll Index (FCI).
The relative water content (RWC) in the leaf was performed according to Gomes et al.33. Leaf discs were taken at eleven and fifteen after water suspension and nine days after rewatering. The RWC was calculated using five leaf disc (from LEAF 1) that were cut and sequentially weighed (fresh mass, FM) maintained under light for 24 h in a flask with water to achieve saturation (turgid mass, TM), and oven-dried at 80 °C for 48 h (dry mass, DM). The relative water content was calculated using the formula, \(\:\text{R}\text{W}\text{C}\:\left(\text{\%}\right)=\left[\frac{\text{F}\text{M}-\text{D}\text{M}}{\text{T}\text{M}-\text{D}\text{M}}\right]\text{*}100\). The RWC was performed in the period between 40 and 60 DAT.
Organic components: total soluble sugars, amino acids, proline, and protein content
The extraction and quantification of the organic components were carried out at the Plant Ecophysiology Laboratory, Universidade Federal de Alagoas (UFAL). Leaf samples were collected at 85 DAS. Extraction of soluble metabolites for quantification of total soluble sugars, amino acids, and proline was obtained from 100 mg of lyophilized leaf plant material, using 5 mL of the MCW mixture (methanol: choloform: water, 12:5:3, v/v/v), as described by Bieleski and Turner34. The samples were homogenized for 2 min in a vortex and kept under constant agitation for 30 min at room temperature (25 °C). They were then centrifuged at 10,000 × g for 10 min at 4 °C. The upper aqueous phase was carefully collected and stored under refrigeration (ultra-freezer at − 20 °C) for later quantification of soluble metabolites.
Quantification of total soluble sugars was performed using the phenol-sulfuric colorimetric method of Dubois et al.35, with adaptations. Soluble sugars were extracted from 100 mg of leaf tissue, homogenized in 5 mL of 80% (v/v) ethanol. The samples were kept in a water bath at 100 °C for 1 h to facilitate sugar solubilization. They were then centrifuged at 3,000 × g for 10 min, and the supernatant was collected and stored for analysis. For the reaction, 500 µL aliquots of the extract were transferred to test tubes and mixed with 500 µL of 5% phenol and 2.5 mL of concentrated sulfuric acid. The samples were homogenized for 30 s and incubated for 10 min at room temperature for color development. After the tubes were cooled to room temperature, readings were performed in a spectrophotometer at 490 nm. The standard curve was constructed using dilutions of a glucose stock solution (0.1 mg mL− 1), resulting in concentrations ranging from 0 to 100 µg mL− 1. The results were expressed in µmol g− 1 FM, using the standard curve equation to calculate the concentrations.
Free amino acids were performed by the ninhydrin method, adapted from Yemm and Cocking36, based on the reaction between ninhydrin and the free α-amino groups of amino acids, forming a violet-colored compound, whose absorbance is measured in a spectrophotometer at 570 nm. For quantification, 250 µL of the MCW extract were added to hermetically sealed test tubes containing 250 µL of 0.2 M sodium citrate buffer (pH 5.0), 100 µL of 5% ninhydrin solution in methyl cellosolve and 500 µL of 0.2 mM KCN in methyl cellosolve. The samples were incubated in a water bath at 100 °C for 15 min. After cooling in an ice bath for 10 min, 1.5 mL of 60% ethanol were added to stabilize the color developed. After that, the samples were shaken with a vortex mixer and the tubes remained for 20 min at room temperature for readings with the aid of a spectrophotometer at 570 nm absorbance. A solution containing 500 µL of extraction buffer and 1.7 mL of ninhydrin reagent was used as a blank. The standard curve was constructed with an amino acid solution containing arginine, glycine, glutamic acid and phenylalanine, prepared in different dilutions, ranging from 0 to 0.3 µmol mL− 1. The results were expressed in µmol of amino acids per gram MF.
Proline quantification was performed according to the method described by Rena and Masciotti37. For the reaction, 450 µL aliquots of the MCW extract were added to test tubes containing 1 mL of acidic ninhydrin (1.25 g of ninhydrin dissolved in 30 mL of glacial acetic acid and 20 mL of 6 M phosphoric acid) and 1 mL of glacial acetic acid. The mixture was homogenized and incubated in a water bath at 90 °C for 1 h. After this period, the tubes were immediately cooled in an ice bath for 10 min. Then, 2 mL of toluene were added to each tube, followed by vigorous shaking for 1 min. After separation of the phases, the upper phase containing the chromophore was warmed to room temperature and carefully removed, and its absorbance was determined in a spectrophotometer at 520 nm. The blank consisted only of toluene. The proline concentration (µmol g− 1 FM) was established from the L-proline standard curve, prepared from a 1.0 mM proline stock solution, with dilutions ranging from 0 to 0.25 µmol mL− 1.
Protein quantification was performed by the Bradford method38, using the protein extract obtained by the MCW extraction protocol34. After extraction of the compounds, the precipitate obtained by centrifugation was reserved for protein extraction. The extraction medium consisted of a 0.1 N NaOH solution, prepared by dissolving sodium hydroxide in ultrapure water. Ten milliliters (10 mL) of this solution were added to the Falcon tube containing the precipitate, and resuspension was performed using a glass rod, followed by vigorous shaking for 20 s. The tubes were stored under refrigeration for 24 h. After this period, the tubes were centrifuged at 4,000 × g for 9 min, and the supernatant containing soluble proteins was collected and stored in an ultra-freezer (-20 °C) until analysis. The Bradford reagent was prepared according to a standard protocol, dissolving the dye in a solution containing ethanol and phosphoric acid, followed by filtration and storage under refrigeration. To determine protein concentration, standard curves were prepared using bovine serum albumin (BSA) solutions at different known concentrations (0–100 µg mL). To read the samples, each sample (75 µL) was pipetted into test tubes, followed by the addition of 2000 µL of Bradford reagent and 425 µL of water, totaling 2500 µL of solution. Readings were taken on a spectrophotometer at 595 nm (Thermo Scientific Genesys 10 UV Scanning), approximately 10 to 20 min after addition of the reagent. The results were expressed as mg of soluble protein g− 1 of fresh mass (FM). The protein concentration in the samples was determined by interpolating the absorbance values on the BSA standard curve.
Antioxidant enzymes: catalase and ascorbate peroxidase activity
The samples for biochemical analysis were collected from the same leaves (middle leaves) in which gas exchange was measured (between 40 and 60 DAT). Leaf samples (100 mg) were ground in 2 mL of extraction buffer containing 50 mM phosphate buffer, pH 7.5, containing 100 mg polyvinylpolypyrrolidine and deionized water for CAT, and 2 mM EDTA, 20 mM sodium ascorbate, 0.1% triton X 100 at for APX.
Catalase activity (CAT) was determined according to the methodology adopted by Havir and Mchale39, with some modifications. We used a reaction mixture containing 334 µL of potassium phosphate buffer (pH 7.5), 250 µL of 100 mM H2O2, 50 µL of plant extract and 1366 µL of deionized water, final volume of 2 mL. The decrease in absorbance at 240 nm was followed spectrophotometrically for 1 min using an extinction coefficient (ɛ) of 36 mM− 1 cm− 1. Catalase activity was calculated as: \(\:\text{C}\text{A}\text{T}=\:\:\frac{\varDelta\:\text{A}}{{\upvarepsilon\:}\:\text{x}\:\text{l}}\:\text{x}\:\:{\text{V}}_{\text{t}\text{o}\text{t}\text{a}\text{l}\:}\:\text{x}\:\frac{1}{{\text{V}}_{\text{s}\text{a}\text{m}\text{p}\text{l}\text{e}}}\), where ΔA = change in absorbance per minute at 240 nm, ε = molar extinction coefficient of H2O2 (39.4 mM−1 cm−1), l = path length of the cuvette (1 cm), Vtotal total reaction volume (mL) and Vsample = volume of the enzyme extract used in the assay (mL).
APX activity was determined according to the methodology adopted by Nakano and Asada40, with some modifications. The reaction mixture contained 334 µL of potassium phosphate buffer (pH 7.5), 20 µL of 10 mM sodium ascorbate, 20 µL of 10 mM H2O2, 50 µL of enzyme extract, and 1576 µL of deionized water, final volume 2 mL. The decrease in absorbance through 290 nm for 1 min was measured spectrophotometrically. The ascorbate extinction coefficient was 2.8 mM− 1 cm− 1 at 290 nm, and APX activity expressed as Micromoles ascorbate oxidized min− 1 mg− 1 protein and was calculated by using formula: \(\:\text{A}\text{P}\text{X}=\:\frac{\varDelta\:\text{A}}{{\upvarepsilon\:}\:\text{x}\:\text{l}}\:\:\text{x}\:{\text{V}}_{\text{t}\text{o}\text{t}\text{a}\text{l}\:\:}\text{x}\:\frac{1}{{\text{V}}_{\text{s}\text{a}\text{m}\text{p}\text{l}\text{e}}}\), where ΔA = change in absorbance per minute at 290 nm, ε = molar extinction coefficient of ascorbic acid (2.8 mM⁻¹ cm⁻¹), l = path length of the cuvette (1 cm), Vtotal total reaction volume (mL) and Vsample = volume of the enzyme extract used in the assay (mL).
Malondialdehyde content and electrolyte leakage
The lipid peroxidation was determined, according to Dhindsa41, with modifications. Fresh leaf samples (150 mg) were homogenized in 2.5 mL of 0.1% (w: v) trichloroacetic acid (TCA). The homogenate was centrifuged at 15,000 × g for 10 min at 4 °C. 500 µL aliquot of the supernatant was mixed with 2 mL of a solution containing 20% TCA and 0.5% thiobarbituric (TBA). The samples were heated at 90 °C for 15 min and immediately cooled in an ice bath. After centrifugation (10,000 × g for 10 min at 4 °C), the absorbance of the supernatant was measured at 600 nm and 532 nm. The MDA concentration was calculated using an extinction coefficient of 155 mM− 1 cm− 142. \(\:\text{M}\text{D}\text{A}=\:\frac{\varDelta\:\text{A}}{{\upvarepsilon\:}\:\text{x}\:\text{l}}\:\text{x}\:{\text{V}}_{\text{e}\text{x}\text{t}\text{r}\text{a}\text{c}\text{t}}\:\text{x}\:\frac{1}{{\text{V}}_{\text{s}\text{a}\text{m}\text{p}\text{l}\text{e}}}\), where ΔA = difference in absorbance at 532 nm and 600 nm (to correct turbidity), ε = molar extinction coefficient of the MDA-TBA complex (155 mM−1 cm−1), l = path length of the cuvette (1 cm), Vextract = total extract volume (mL) and Vsample = volume of sample used in the assay (mL).
The electrolyte leakage (EL) was determined as described by Houimli et al.43, with modifications. Leaves disks (about 1 cm²) were washed with deionized water and incubated in 20 mL of deionized water at 25 °C for 24 h. The initial electrical conductivity (EC1) was read out, and then leaf disks kept in solution at 120 °C for 20 min. The final conductivity (EC2) was measured after cooling to room temperature. Electrolyte leakage (EL) was calculated as: \(\:\text{EL=}\left(\frac{\text{EC1}}{\text{EC2}}\right)\text{x\:100}\). EL was performed in the period between 40 and 60 DAT. The experiment conduction in resume is show in the Fig. 2.
Data analysis
Data were initially subjected to the Shapiro-Wilk and Levene tests to verify the assumptions of normality and homogeneity of variance, respectively. Experimental data were analyzed using two-way ANOVA to assess the effects of salinity levels and biostimulant doses. When significant effects were observed (p < 0.05), quantitative factor (salinity levels) was analyzed using quadratic polynomial regression, while quantitative factor (biostimulant doses) was compared using Tukey test at 5%. All analyses were performed by using the SISVAR software (version 5.6), considering five repetitions per treatment. When necessary, data were transformed into logarithm or Box-Cox to meet the assumption of normality. Missing data were handled by excluding incomplete cases. Data were presented in graphs as means ± standard deviation (SD) of five repetitions (n = 5).
Results
Growth, production components and physico-chemical attributes
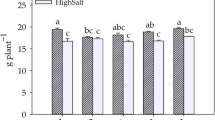
All growth indicators were impacted by salt stress and as salt concentration increased; these characteristics declined. There was a significant effect (p ≤ 0.001) (Table 2) between salinity levels and biostimulant doses for the dry matter of leaves, stems, roots, and total (Fig. 3). All biostimulant treatments increased biomass accumulations, regardless of saline treatments. All biostimulant treatments increased leaf dry matter among the salt levels of 0.5, 1.5, 3.5, and 4.5 dS m− 1, with increases of 64.0% in the 0.6 mL dose and 79.2% in the 0.3 mL dose at the level of 0.5 dS m− 1, and 145.3% in the 0.3 mL dose and 180.7% in the 0.6 mL dose, in the EC of 4.5 dS m− 1 (Fig. 3A). In terms of dry matter in the stem, there was a significant difference (p ≤ 0.001) between treatments. The biostimulant treatment 0.3 mL dose showed greater increases in the ECs of 1.5, 3.5, and 4.5 dS m− 1. The 0.6 mL dose provided increases of 7.5% in the EC of 1.5 dS m− 1, 35.4% in the EC of 3.5 dS m− 1, and 37% in the EC of 4.5 dS m− 1, compared to the control (Fig. 3B). The accumulation of dry matter in the root was greater in plants that received biostimulant, in all electrical conductivities. Highlight for the biostimulant treatment 0.3 mL dose, which showed greater accumulation in the EC of 2.5 dS m− 1, with an increase of 100.4% compared to the control (Fig. 3C). In comparison to control conditions, plants treated with the 0.3 mL dose of the biostimulant had greater accumulations of total dry matter, with emphasis on ECs 1.5 (44.5%) and 3.5 dS m− 1 (73.1%). Plants treated with the 0.6 mL dose of the biostimulant, the greatest increases were in ECs 2.5 dS m− 1 (51.6%) and 4.5 dS m− 1 (60.6%) (Fig. 3D).
Leaf (A), stem (B), root (C) and total dry matter (D) accumulation of Capsicum annuum L. in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
There was a significant interaction (p ≤ 0.001) (Table 3) between salinity and biostimulant for estimated yield, number of fruits, and fruit weight (Fig. 4). High salinity significantly impaired the yield. With increasing salinity, there was reduction of 97% in estimated fruit yield. Compared to the control conditions, plants that received the biostimulant treatment increased yield; treatments with 0.3 mL and 0.6 mL of biostimulant showed higher yield (203% and 198%) at EC 3.5 dS m− 1 (Fig. 4A). The number of fruits showed reduction of 68.9% and 84.3%, even with the application of the biostimulant; however, when comparing the control plants and the plants that received the application of the biostimulant at each salt level, an increase of 121.7% was observed in the EC of 4.5 dS m− 1 for the 0.6 mL dose, and 50% in the EC of 1.5 dS m− 1 for the 0.3 mL dose (Fig. 4B). Salinity led to a decrease in fruit weight; but the biostimulant treatments had a positive effect, with a 32% increase in the EC of 3.5 dS m− 1 at both doses of the product, in relation to the control conditions (Fig. 4C).
Estimated yield (A), number of fruits (B), and fruit weight (C) of Capsicum annuum L. in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
Salinity affected growth parameters in this study. There was a significant effect (p ≤ 0.001) of salinity and biostimulant doses (Table 4). Plant height, stem diameter, leaf area, and number of leaves were negatively affected by salinity (Fig. 5). Salt stress considerably decreased plant height (Fig. 5A) and stem diameter (Fig. 5B) in plants not treated with the biostimulant; however, there was a greater decrease in leaf area in the biostimulant treatment at the 0.6 mL dose (48.58%) (Fig. 5C). The number of leaves was affected in plants under salinity conditions (Fig. 5D). Different biostimulant concentration applications evidenced the helpful impacts on plant growth, with emphasis on the 32.81% increase in both doses applied at the EC of 3.5 dS m− 1, and number of leaves was increase 47.71% in the EC of 2.5 dS m− 1 (0.6 mL dose).
Plant height (A), stem diameter (B), leaf area (C), and number of leaves (D) of Capsicum annuum L. in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
There was a significant interaction (p ≤ 0.001) between salinity and biostimulant (Table 5). The physico-chemical attributes of the fruits (Figs. 6 and 7) were significantly affected by salinity. The 0.3 mL treatment was the most effective for the width (+ 26.6%) and length (+ 31.8%) parameters of the fruits under 4.5 dS m− 1 (Fig. 6A, B). There was a significant increase in fruit firmness at the 0.6 mL dose, with increases of 147.4% at 4.5 dS m− 1, and 131% at 3.5 dS m− 1 (Fig. 7A). The ascorbic acid content increased with salinity, with emphasis on the 0.3 mL dose at 4.5 dS m− 1 (+ 166%) (Fig. 7B). Soluble solids increased progressively with salinity, especially in fruits treated with the 0.3 mL dose (58%) at EC 2.5 dS m− 1 (Fig. 7C).
Fruit width (A) and fruit length (B) of Capsicum annuum L. in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
Fruit firmness (A) ascorbic acid content (B) and total soluble solids (C) of Capsicum annuum L. in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
Gas exchange, chlorophyll fluorescence, chlorophyll index, relative water content, and electrolyte extravasation
There was a significant interaction (p ≤ 0.001) (Tables 6 and 7) between salinity levels and biostimulant doses for gas exchange (Fig. 8) and photosynthetic efficiency (Figs. 9 and 10). The CO2 assimilation rate (A) was severely reduced in plants under salt stress conditions; however, there was an increase in the biostimulant treatment, with increases of 65.93% in EC 4.5 dS m− 1 in the 0.3 mL treatment (Fig. 8A). The 0.6 mL treatment promoted an increase in A at ECs 1.5, 3.5, and 4.5 dS m− 1. The intercellular CO2 concentration was higher in plants under salt stress, mainly at ECs 1.5 dS m− 1 and 4.5 dS m− 1 in the biostimulant treatment, indicating more efficient stomatal closure (Fig. 8C and D). The water use efficiency (A/gs and A/E) and carboxylation (A/Ci) were significantly higher in the biostimulant treatment (Fig. 8E, F, G). Under salinity, the most favorable to increase A/gs, A/E, and A/Ci was the 0.3 mL treatment, however, there was no significant difference between the biostimulant treatments in the ECs.
Net Co2 assimilation rate (A), intercellular CO2 concentration (B), stomatal conductance (C), transpiration (D), intrinsic (E) and instantaneous water use efficiency (F), instantaneous carboxylation efficiency (G) of Capsicum annuum in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
Chlorophyll index a (A), b (B), total (C), and chlorophyll ratio a/b (D) of Capsicum annuum in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
Quantum yield of photosystem II (Fv/Fm) of Capsicum annuum in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
The biostimulant treatment improved the chlorophyll a, b, and total contents, especially at 3.5 dS m− 1 (Fig. 9A, B, C). The results show higher chlorophyll levels at EC 3.5 dS m− 1, 24.27% for chlorophyll a, 96.96% for chlorophyll b, and 26.3% for total chlorophyll. The chlorophyll a/b ratio was higher in plants under salt stress conditions without biostimulant (Fig. 9D). Salt stress decreased Fv/Fm, but these effects were reversed by all applied biostimulant concentrations (Fig. 10), especially at EC 1.5 dS m− 1 (0.792). There was a significant interaction between salinity levels and biostimulant doses for RWC and organic components. The results presented in Fig. 11 showed a significant decrease in RWC in bell pepper under salinity conditions, but it was favored by the biostimulant treatment, with increases of 9.87% at 3.5 dS m− 1.
Relative water content (RWC) of Capsicum annuum in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
Organic components: total soluble sugars, amino acids, proline, and protein content
There was a significant interaction (p ≤ 0.001) between salinity and biostimulant (Table 8). Salt stress increased organic solute contents (Fig. 12), especially when associated with the biostimulant. Soluble sugar contents increased significantly in plants exposed to salinity, with increases of up to 22.01% at 4.5 dS m− 1 in the 0.6 mL biostimulant treatment (Fig. 12A). The high salinity concentration provided significant increases in the total amino acid content (Fig. 12B), mainly in the 0.3 mL treatment (+ 162.92%). Foliar spraying of biostimulant, especially in the 0.3 mL treatment, further increased proline accumulation (4742.18%) (Fig. 12C), compared to the control and untreated stressed plants. Total protein levels exhibited a significant increase, with a highlight on 3.5 dS m− 1 (0.3 mL) (Fig. 12D). These results indicated that foliar application of amino acid mixtures plays an important role in discovering an alternative approach to increase salt tolerance in plants.
Content of total soluble sugars (A), amino acids (B), proline (C) and total soluble proteins (D) of Capsicum annuum in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
Antioxidant enzymes and oxidative damage
Salt stress caused significant increases in catalase and ascorbate peroxidase activities, lipid peroxidation, and electrolyte leakage (Fig. 13). There was a significant interaction between salinity and biostimulant (p ≤ 0.001) (Table 8). CAT activity (Fig. 13A) increased in stressed plants when compared to the control; plants treated with 0.3 mL showed higher CAT activities at ECs 1.5 and 3.5 dS m− 1 with increases of 50% and 45%, compared to plants without biostimulant. Plants in the 0.6 mL treatment showed higher APX activities (Fig. 13B) at all salinity levels when compared to the control, with an increase of 47.94% at EC 3.5 dS m− 1.
Activity of antioxidant enzymes catalase (CAT, A) and ascorbate peroxidase (APX, B), malondialdehyde content (MDA, C) and electrolyte leakage (EL, D) of Capsicum annuum in response to different salinity levels and biostimulant doses. *The values with the different alphabetical letters differ significantly, based on the Tukey test at 5%. Data represent the average of 5 replication as mean, and each replication represents one plant.
According to Fig. 13C, lipid peroxidation (MDA) increased significantly in bell pepper under saline conditions. However, MDA decreased significantly in plants stressed with biostimulant treatment. Reductions of 22.96% were observed at higher saline concentrations (3.5 dS m− 1 and 4.5 dS m− 1), indicating lower lipid leakage increased significantly in stressed plants; however, the application of the biostimulant led to a significant decrease in EL% in stressed plants under both concentrations, with emphasis on the EC of 3.5 dS m− 1 with a reduction of 27.5%.
Discussion
Salinity is one of the main abiotic stresses that limit the growth and production of cultivated plants44,45. It has been reported that saline stress considerably reduces growth, physiology, biochemistry, and productivity parameters4,34,46, which is parallel to our result with bell pepper. Salinity stress can cause ionic toxicity, lower cellular water content, lower osmotic potential and, a decrease in photosynthetic activity and membrane stability. However, it was found that biomass accumulation was improved with the application of biostimulant47, which is the same as our result but the difference between their study and ours is the biostimulant and the crop. Similarly, to our results, other authors48,49,50 also stated that the application of biostimulants is beneficial for the vegetative stage. Biostimulants modulate plant responses to abiotic stresses through complex interactions at the molecular level, influencing gene expression, protein levels and metabolite profiles51. This multi-faceted approach allows plants to better resist environmental challenges and maintain productivity in adverse conditions. These finding are in line with our results.
Growth, weight and dry biomass decreased under salinity, demonstrating that bell pepper is sensitive to salt stress52. High levels of salinity promote a decline in plant biomass, which may be related to osmotic stress, oxidative stress, ion imbalance reducing shoot growth and leaf expansion, reducing internode growth and accelerating leaf abscission53. However, it was found that treatment with exogenous AAs (alanine, arginine, glutamine, glycine, methionine and proline) mitigated the detrimental effects of salinity, improving fresh weight, dry biomass and plant growth16, a result similar to ours, with a different crop. The higher crop yield with the application of the biostimulant may be the result of better root growth and better nutrients. Studies show that roots are actively involved in the release of carbon dioxide and ethylene, in the efficient retention of water and potassium and play a key role in promoting overall plant growth and productivity54.
Our results indicate a significant increase in weight, fruit number, and fruit yield in non-stressed bell peppers treated with the biostimulant. Although there is a wealth of research on the beneficial effects of biostimulants on fruit production and number under non-stressed conditions48,55,56, there is no relevant information demonstrating that these products can increase production in any berry plant species under salinity stress conditions.
Fruit firmness, ascorbic acid content and soluble solids were evaluated by the effect of biostimulant application and salt stress conditions which is not previously reported. Dobrek et al.57 reported that biostimulant application has a beneficial effect on turgidity and cell wall components, factors related to fruit firmness58,59, which represents an important indicator of fruit quality. Vitamin C is one of the antioxidants that reduces the negative effects of lipid peroxidation caused by saline stress25,60, decreasing as electrolyte leakage increases. Our results demonstrate a decrease in vitamin C content by exposure to salinity. In bell pepper, vitamin C values can vary between 73.64 and 213.52 mg of ascorbic acid per 100 g of product over 12 days of storage61. In our experiment, ascorbic acid values ranged from 47.5 to 128 mg per 100 g. The synthesis and construction of bioactive compounds, such as ascorbic acid, can be correlated with the indirect or direct consequences of the application of biostimulants on the formation of antioxidants in plant tissues, as they can promote the activity of certain enzymes involved in the homeostasis of antioxidant cells, or an indirect effect, such as potassium accumulation, can lead to increased antioxidants in fruits60. Reports show that the soluble solids content in bell pepper increases with the application of biostimulants24,55, similar to what was observed in our experiments, and may represent an additional benefit of the product to improve the organoleptic quality characteristics of a crop such as bell pepper, in addition to the increase in fruit production, as observed by Ikuyinminu et al.3.
Exposure of the plants to salinity compromised leaf gas exchange parameters, reducing A, Ci, E and gs52, but our results show that biostimulant treatment attenuated the negative effects of salinity stress. Reports show that the use of biostimulants revealed the intricate mechanisms underlying their action, including the activation of plant defense pathways, modulation of phytohormone signaling, increased nutrient absorption and utilization51,62, and induced epigenetic changes, leading to improved stress tolerance and adaptive responses in plants12,63. The use of amino acid mixtures as biostimulants against the negative effects of stresses, including salt stress, was related to the role of these molecules in protecting proteins and photosystems7. Amino acids can act as important osmolytes to balance cell osmotic potential and control ion transport and the opening of stomata. Peña Calzada et al.7 showed that the application of amino acids (arginine, tryptophan, aspartate and glycine) improved the photosynthetic activity of soybean.
Our findings indicate a lower internal CO2 concentration under salt stress, demonstrating that stomata opening and photosynthesis of these plants are in balance. Studies show that this fact may be associated with the amino acids in the biostimulant, which, by acting as osmolytes, promote the control of stomatal opening, regulation of transport, enzyme activation, detoxification of heavy metals, maintenance of redox homeostasis and gene expression19,64, an important function in conditions of salt stress. Our study found the biostimulant treatment was effective in increasing the intrinsic and instantaneous water use efficiencies and the carboxylation efficiency, demonstrating the proper functioning of the photosystem. According to D’Amato and Del Buono65 the use of amino acids increases the formation of coenzymes and the photosynthesis process in plants under stress conditions, allowing for improved efficiency in the use of water and nutrients, stimulating plants to combat the harmful effects of biotic and abiotic stresses. Glycine is the main amino acid in chlorophyll synthesis, playing an important role in photosynthesis18, which can help maintain photosynthetic efficiency under stress, supporting overall plant growth and productivity12,66, confirming our findings.
Salinity reduced chlorophyll index (a, b and total), but biostimulant treatment alleviated the deterioration of photosynthetic pigments, results similar to those observed by Abdelkader et al.16 and Peña Calzada et al.7. According to ur Rehman et al.67 the chlorophyll a/b ratio is generally around 3 and is related to the amounts of chlorophyll associated with the photosystems, and an increase in this ratio indicates environmental changes and stress or external stimuli. Our results show significant increases in the chlorophyll a/b ratio in plants exposed to salinity. However, the biostimulant attenuated this increase65,68. Osmotic stress caused by salinity results in lower photosynthetic performance (Fv/Fm)67. The results show that quantum efficiency was reduced in plants subjected to salt stress, but biostimulant treatments were less affected, as amino acids have a potential role in the elimination reactive oxygen species, reducing the oxidative damage caused by salt stress in the photosynthetic apparatus7,69.
Osmotic adjustment is an effective strategy for plants to resist salt-induced osmotic stress7. In this study, plants with biostimulant application had higher RWC than that control plants, suggesting that the biostimulant alleviated the salt-induced osmotic stress, at least partially to maintain a higher photosynthetic rate, the same observed by Alfosea-Simón et al.70, in tomato. When exposed to high concentrations of salts, plants may present reduced turgor in their leaves. RWC is a critical indicator, reflecting the water retention capacity and hydration status of a plant, directly influencing its tolerance to salt stress. The amino acids glycine and tryptophan, present in the product, improve the water relations of plants, helping to retain water within the leaves, thus preventing further turgor loss7.
Plants under salt stress synthesize and accumulate osmotically active organic substances, mainly sugars and amino acids, which help to alleviate osmotic stress23. The accumulation of these osmoprotectants helps to maintain cell turgor, creating a gradient force for water uptake and stabilizing membranes and proteins against the denaturing activity of harmful solutes and salts. In our study, the organic components contents were increased in plants treated with the biostimulant, suggesting good adaptation and osmotic adjustment of the plants, which is in accordance with the observations of previous researches7,71. A relevant research72 indicated that proline, for example, plays a vital role in stabilizing the Rubisco enzyme and stimulating its functionality even in the presence of salt. Glycine, the simplest amino acid in nature and most rapidly absorbed by plants, functions as an osmoregulator of the cytosol and cellular compartments73. These osmoprotectants are involved in signaling and regulating plant responses to stresses, such as salinity, playing adaptive roles in regulating osmotic adjustment and protecting subcellular structures in stressed plants.
The activities of the enzymes catalase (CAT) and ascorbate peroxidase (APX) increased with the addition of the salt dose. Higher antioxidant activity is generally considered an indicator of salt tolerance in plants23. ROS generation is stimulated by salt stress and is neutralized by intracellular antioxidants under normal conditions. However, during salt stress, excessive accumulation of ROS incites oxidative stress, interrupting normal metabolism, degrading proteins, and acting on nucleic acid mutation. The antioxidant enzyme system, which comprises enzymes such as CAT and APX, acts to combat oxidative damage induced by salinity, eliminating H2O2, for example72. Pepper plants treated with the biostimulant showed higher enzymatic activity of the enzymes CAT and APX. Protein oxidation by ROS is generally irreversible, but this does not apply to proteins rich in sulfur-containing amino acids (arginine, proline, tryptophan), since in addition to the thiol group (-SH), they also contain a carbonyl group, which functions as an inhibitor of proteolytic attack18. Thus, the application of exogenous amino acids helped eliminate ROS and reduced oxidative stress under saline conditions, protecting cellular structures, as already demonstrated by Zuzunaga-Rosas et al.23.
MDA and EL were increased under salt stress conditions. Previous study demonstrated that application of biostimulant leads to maintaining the low contents of MDA and EL, enhancing the antioxidant capacity in response to stresses7, confirming the function of AAs as protective molecules against oxidative damage, presumably due to the increase in the activity of antioxidant enzymes18. MDA is a crucial physiological indicator used to assess the integrity and permeability of cell membranes, which can be disrupted by abiotic stress53. The increase in MDA results from oxidative damage in organelles, including chloroplast and mitochondria, induced by salinity. The increased in EL may be the result of the negative impact of salinity on the cytoplasmic membrane and permeability process46. Thus, the results confirmed the previous studies that biostimulant-based amino acid played an important role in alleviating the stress tolerance.
In general, the parameters evaluated were inhibited by salt stress treatment. In the present research, the alleviation of short-term salt stress by the application of biostimulant-based amino acid in pepper plants and fruits was investigated. The results suggest that the studied biostimulant has great potential to improving salt tolerance in crops, mainly produced in semiarid regions, and field studies and long-term research are necessary to further confirm its performance in the future. Therefore, this study presents practical applications, especially the effect of biostimulants in organic and/or hydroponic crops may contribute considerably to the better use of these products, in smaller quantities than commercially recommendations, being expanded to other crops, seeking the use of more sustainable agricultural practices and in the management of crops under stress conditions, including salt stress.
Conclusion
The results of this study provide new insights into the effects of salinity and the use of a biostimulant-based amino acids on the growth, fruit quality, physiology, and biochemistry of bell pepper plants, which are considered a moderately salt-sensitive crop, even with the application of saline doses above the tolerated level (1.5 dS m− 1). The results indicate that both doses of the biostimulant (0.3 and 0.6 mL L− 1) had significant effects on gas exchange, maintained higher levels of chlorophyll, improved photosynthetic performance, and improved plant water status, leading to better crop yield. The response of the plant to the applied salt doses was also influenced by the biostimulant in processes related to oxidative adjustment and antioxidant capacity/activity. The doses of the biostimulant were effective in attenuating salt stress, with the dose of 0.3 mL L− 1 being the most indicated for cost savings in conditions of production with saline water. The results demonstrate that this method has the potential to enhance plant resistance to saline stress while significantly increase crop yield. Similar investigations can be conducted on different crops, using the findings of this study as a benchmark. The results of this study have implications for agricultural production, especially in salinized regions, and for the development of low-cost agricultural practices aimed at more sustainable agriculture in the management of economically important crops.
Data availability
All data generated during this study are included in this published article and supplementary information file. Supplementary material: General raw data.
References
Gowdy, J. Our hunter-gatherer future: Climate change, agriculture and uncivilization. Fut 115, 102488. https://doi.org/10.1016/j.futures.2019.102488 (2020).
Sousa, V. F. O. et al. Castor bean cake increases osmoprotection and oil production in Basil (Ocimum Basilicum) under saline stress. Sci. Horticult. 309, 111687. https://doi.org/10.1016/j.scienta.2022.111687 (2023).
Ikuyinminu, E., Goñi, O. & O’Connell, S. Enhancing irrigation salinity stress tolerance and increasing yield in tomato using a precision engineered protein hydrolysate and Ascophyllum nodosum-derived biostimulant. Agron 12, 809. https://doi.org/10.3390/agronomy12040809 (2022).
Kacjan Marši´c, N. et al. Ferjanˇ ciˇ C, B. Physiological and biochemical responses of ungrafted and grafted bell pepper plants (Capsicum annuum L. Var. Grossum (L.) Sendtn.) grown under moderate salt stress. Plants 10, 314. https://doi.org/10.3390/plants10020314 (2012).
Etesami, H. & Noori, F. Soil salinity as a challenge for sustainable agriculture and bacterial-mediated alleviation of salinity stress in crop plants, Saline Soil-Based Agriculture by Halotolerant Microorganisms, in (eds Kumar, M., Etesami, H. & Kumar) V. (Springer), 1–22, https://doi.org/10.1007/978-981-13-8335-9_1 (2019).
Fricke, W. Energy costs of salinity tolerance in crop plants: Nighttime transpiration and growth. New Phytol. 225, 1152–1165. https://doi.org/10.1111/nph.15773 (2020).
Peña Calzada, K. et al. G. E. Exogenous application of amino acids mitigates the deleterious effects of salt stress on soybean plants. Agron. 12, (2014). https://doi.org/10.3390/agronomy12092014 (2022).
Ahmad, R. et al. in Oxidative Stress and Antioxidant Defense Mechanisms in Plants Under Salt Stress. 191–205 (eds Tolerance, P. A. S., Hasanuzzaman, M., Hakeem, K., Nahar, K. & Alharby, H.) (Springer, 2019).
Linh, N. T., Cham, L. T. T. & Thang, V. N. Effects of salinity stress on the growth, physiology, and yield of soybean (Glycine max (L.) Merrill). Vietnam J. Agric. Sci. 4, 1043–1055. https://doi.org/10.31817/vjas.2021.4.2.05 (2021).
Ren, J. et al. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agron 10, 663. https://doi.org/10.3390/agronomy10050663 (2020).
Bulgari, R., Franzoni, G. & Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agron 9, 306. https://doi.org/10.3390/agronomy9060306 (2019).
Khalid, F. et al. Plant biostimulants: Mechanisms and applications for enhancing plant resilience to abiotic stresses. J. Soil. Sci. Plant. Nutr. 24, 6641–6690. https://doi.org/10.1007/s42729-024-01996-3 (2024).
Singh, N. et al. Salt stress and its eco-friendly management using biostimulants in grain legumes: A review. Discov. Agric. 3 https://doi.org/10.1007/s44279-024-00150-y (2025).
Melito, S. et al. Root-promoting biostimulant enhances salinity tolerance in wild and cultivated rocket salads. J. Soil. Sci. Plant. Nutr. 24, 6268–6282. https://doi.org/10.1007/s42729-024-01960-1 (2024).
Ikiz, B., Dasgan, H. Y., Balik, S., Kusvuran, S. & Gruda, N. S. The use of biostimulant as a key to sustainable hydroponic lettuce farming under saline water stress. BMC Plant. Biol. 24, 808. https://doi.org/10.1186/s12870-024-05520-8 (2024).
Abdelkader, M. et al. Monitoring role of exogenous amino acids on the proteinogenic and ionic responses of lettuce plants under salinity stress conditions. Hort 9, 626. https://doi.org/10.3390/horticulturae9060626 (2023).
Mandal, S. et al. Biostimulants and environmental stress mitigation in crops: A novel and emerging approach for agricultural sustainability under climate change. Environ. Resear. 233, 116357. https://doi.org/10.1016/j.envres.2023.116357 (2023).
Matysiak, K. et al. Effect of exogenous application of amino acids L-arginine and glycine on maize under temperature stress. Agron 10, 769. https://doi.org/10.3390/agronomy10060769 (2020)., DOI: t-.
Sun, W., Shahrajabian, M. H., Kuang, Y. & Wang, N. Amino acids biostimulants and protein hydrolysates in agricultural sciences. Plants 13, 210. https://doi.org/10.3390/plants13020210 (2024).
Cheng, Y., Tian, Q. & Zhang, W. H. Glutamate receptors are involved in mitigating effects of amino acids on seed germination of Arabidopsis thaliana under salt stress. Environ. Exp. Bot. 130, 68–78. https://doi.org/10.1016/j.envexpbot.2016.05.004 (2016).
Zargar Shooshtari, F., Souri, M. K., Hasandokht, M. R. & Jari, S. K. Glycine mitigates fertilizer requirements of agricultural crops: Case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric. 7 (19), 1–10. https://doi.org/10.1186/s40538-020-00185-5 (2020).
Sun, Y. et al. L-Arginine alleviates the reduction in photosynthesis and antioxidant activity induced by drought stress in maize seedlings. Antioxidant 12 (2), 482. https://doi.org/10.3390/antiox12020482 (2023).
Zuzunaga-Rosas, J. et al. Use of a biostimulant to mitigate the effects of excess salinity in soil and irrigation water in tomato plants. Plants 12, 1190. https://doi.org/10.3390/plants12051190 (2023).
Ntanasi, T. et al. Plant biostimulants enhance tomato resilience to salinity stress: Insights from two Greek landraces. Plants 13, e1404. https://doi.org/10.3390/plants13101404 (2024).
Habibi, N. et al. Potential benefits of seed priming under salt stress conditions on physiological, and biochemical attributes of micro-tom tomato plants. Plants 12, 2187. https://doi.org/10.3390/plants12112187 (2023).
Alvares, C. A., Stape, J. L., Sentelhas, P. C., Gonçalves, J. L. M. & Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Zeitsch. 22, 711–728. https://doi.org/10.1127/0941-2948/2013/0507 (2013).
Peña Calzada, P., Fernandéz, J. C. R. & Meléndrez, J. F. El viusid agro uma alternativa En El incremento de La producción de tomate (Solanum lycopersicum L). Rev. Carib Cienc. Soc. (2016).
Novais, R. F., Neves, J. C. L. & Barros, N. F. Ensaio em ambiente controlado, in Métodos de pesquisa em ambiente controlado eds A. J. Oliveira, W. E. Garrido, J. D. Araujo, J. D, S. Lourenço (Embrapa), 189–273 (1991).
Rhoades, J. D., Kandiah, A. & Mashali, A. M. Uso De Águas Salinas Para Produção Agrícola (UFPB:Campina Grande, 2000).
Reis, L. S., Azevedo, C. A. V., Albuquerque, A. W. & Junior, J. F. Leaf area index and productivity of tomatoes under greenhouse conditions. Agriambi 17, 386–391. https://doi.org/10.1590/S1415-43662013000400005 (2013).
Strohecker, R. & Henning, H. M. Análises De Vitaminas: Métodos Comprovados (Paz Montolvo: Madrid, 1967).
IAL – Instituto Adolfo Lutz. Normas Analíticas Do Instituto Adolfo Lutz: Métodos físico-químicos Para Análise De Alimentos (IAL:São Paulo, 2008).
Gomes, M. D. M. D. A. et al. Brassinosteroid analogue affects the senescence in two Papaya genotypes submitted to drought stress. Theor. Experim Plant. Physiol. 25, 186–195 (2013).
Houimli, S. M., Denden, M. & Mouhandes, B. D. Effects of 24-epibrassinolide on growth, chlorophyll, electrolyte leakage and proline by bell pepper plants under NaCl-stress. EurAsian J. BioSci. 4, 96–104. https://doi.org/10.5053/ejobios.2010.4.0.12 (2010).
Bieleski, R. L. & Turner, A. Separation and Estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal. Biochem. 17, 278–293. https://doi.org/10.1016/0003-2697(66)90206-5 (1966).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. https://doi.org/10.1021/ac60111a017 (1956).
Yemm, E. W., Cocking, E. C. & Ricketts, R. E. The determination of amino acids with ninhydrin. Analyst 80, 209–213. https://doi.org/10.1039/AN9558000209 (1955).
Rena, A. B. & Masciotti, G. Z. The effect of dehydration on nitrogen metabolism and growth of bean cultivars (Phaseolus vulgaris L). Rev. Ceres. 23, 288–301 (1976).
Bradford, M. M. The dye-binding assay for protein. Anal. Biochem. 72, 248–254 (1976).
Havir, E. A. & Mchale, N. A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant. Physiol. 84, 450–455. https://doi.org/10.1104/pp.84.2.450 (1987).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232 (1981).
Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Bioph. 125, 189–198. https://doi.org/10.1016/0003-9861(68)90654-1 (1968).
Dhindsa, R. S. Inhibition of protein synthesis by products of lipid peroxidation. Phytochem 21, 309–313. https://doi.org/10.1016/S0031-9422(00)95257-9 (1982).
Hassanein, R. A., Abdelkader, A. F. & Faramawy, H. M. Moringa leaf extracts as biostimulants-inducing salinity tolerance in the sweet Basil plant. Egypt. J. Bot. 59, 303–318. https://doi.org/10.21608/EJBO.2019.5989.1242 (2019).
Lorenzo, P., Souza-Alonso, P., Guisande-Collazo, A. & Freitas, H. Influence of Acacia dealbata link bark extracts on the growth of Allium cepa L. plants under high salinity conditions. J. Sci. Food Agric. 99, 4072–4081. https://doi.org/10.1002/jsfa.9637 (2019).
ALKahtani, M. D. F. et al. A. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with Chitosan and plant growth promoting rhizobacteria. Agron 10, 1180. https://doi.org/10.3390/agronomy10081180 (2020).
Lucini, L. et al. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 182, 124–133. https://doi.org/10.1016/j.scienta.2014.11.022 (2015).
Rouphael, Y. et al. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 226, 353–360. https://doi.org/10.21273/HORTSCI12200-17 (2017a).
Alfosea-Simón, M. et al. Effect of foliar application of amino acids on the salinity tolerance of tomato plants cultivated under hydroponic system. Sci. Hortic. 272, 109509. https://doi.org/10.1016/j.scienta.2020.109509 (2020a).
Malécange, M. et al. Biostimulant properties of protein hydrolysates: Recent advances and future challenges. Int. J. Mol. Sci. 24, 9714. https://doi.org/10.3390/ijms24119714 (2023).
Johnson, R., Joel, J. M. & Puthur, J. T. Biostimulants: The futuristic sustainable approach for alleviating crop productivity and abiotic stress tolerance. J. Plant. Growth Reg. 43, 659–674. https://doi.org/10.1007/s00344-023-11144-3 (2024).
Veloso, L. L. S. A. et al. Attenuation of salt stress on the physiology and production of bell peppers by treatment with Salicylic acid. Semina 42, 2751–2768. https://doi.org/10.5433/1679-0359.2021v42n5p2751 (2021).
Zamani, E. et al. Comparative morphological, physiological, and biochemical traits in sensitive and tolerant maize genotypes in response to salinity and Pb stress. Sci. Rep. 14, 31036. https://doi.org/10.1038/s41598-024-82173-5 (2024).
Habibi, N., Tayobong, R. R. P., Parneel, Terada, N., Sanada, A. & Koshio, K. Novel insights into seed priming for tomato plants: Restoring root vitality in the face of salt stress. Hort Environ. Biotech. 66, 361–380. https://doi.org/10.1007/s13580-024-00651-1 (2025).
Colla, G., Cardarelli, M., Bonini, P. & Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience Horts. 52, 1214–1220. https://doi.org/10.21273/HORTSCI12200-17 (2017).
Shukla, P. S. et al. Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant. Sci. 10, e655. https://doi.org/10.3389/fpls.2019.00655 (2019).
Drobek, M., Frąc, M. & Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 10, 433. https://doi.org/10.3390/agronomy9060335 (2020).
Huang, Y., Lu, R. & Chen, K. Prediction of firmness parameters of tomatoes by portable visible and near-infrared spectroscopy. J. Food Eng. 222, 185–198. https://doi.org/10.1016/j.jfoodeng.2017.11.030 (2018).
Cozzolino, E. et al. Appraisal of biodegradable mulching films and vegetal-derived biostimulant application as eco-sustainable practices for enhancing lettuce crop performance and nutritive value. Agronomy 10, 427. https://doi.org/10.3390/agronomy10030427 (2020).
Rouphael, Y. et al. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Horticult. 226, 353–360. https://doi.org/10.1016/j.scienta.2017.09.007 (2017b).
Rinaldi, M. M., Sandri, D., Ribeiro, M. O. & Amaral, A. G. Physico-chemical and nutritional characteristics of bell pepper produced in the field and through a hydroponic production system. Food Sci. Technol. 28, 558–563. https://doi.org/10.1590/S0101-20612008000300009 (2008).
Keya Tudu, C., Dey, A., Pandey, D. K., Panwar, J. S. & Nandy, S. Role of plant derived extracts as biostimulants in sustainable agriculture: A detail study on research advances, bottlenecks and future prospect. New. Fut. Develop. Microb. Biotech. Bioeng. 159–179. https://doi.org/10.1016/b978-0-323-85579-2.00017-4 (2022).
Khokhar, A. et al. Genetic modification strategies for enhancing plant resilience to abiotic stresses in the context of climate change. Func. Integr. Genom. 23, 283. https://doi.org/10.1007/s10142-023-01202-0 (2023).
Shahrajabian, M. H., Chaski, C., Polyzos, N. & Petropoulos, S. A. Biostimulants application: A low input cropping management tool for sustainable farming vegetables. Biom 11, 698. https://doi.org/10.3390/biom11050698 (2021).
D’Amato, R. & Del Buono, D. Use of a biostimulant to mitigate salt stress in maize plants. Agron 11, 1755. https://doi.org/10.3390/agronomy11091755 (2021).
Rakkammal, K., Maharajan, T., Ceasar, S. A. & Ramesh, M. Biostimulants and their role in improving plant growth under drought and salinity. Cereal Res. 51, 61–74. https://doi.org/10.1007/s42976-022-00299-6 (2023).
ur Rehman, H., Alharby, H. F., Bamagoos, A. A., Abdelhamid, M. T. & Rady, M. M. Sequenced application of glutathione as an antioxidant with an organic biostimulant improves physiological and metabolic adaptation to salinity in wheat. Plant. Physiol. Biochem. 158, 43–52. https://doi.org/10.1016/j.plaphy.2020.11.041 (2021).
Sonobe, R., Yamashita, H., Mihara, H., Morita, A. & Ikka, T. Estimation of leaf chlorophyll A, B and carotenoid contents and their ratios using hyperspectral reflectance. Remote Sens. 12, 3265. https://doi.org/10.3390/rs12193265 (2020).
Rizwan, M. et al. Correction to: effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 41, 72. https://doi.org/10.1007/s11738-019-2863-4 (2019).
Alfosea-Simón, M. et al. Application of biostimulants containing amino acids to tomatoes could favor sustainable cultivation: Implications for tyrosine, lysine, and methionine. Sustainab 12, 9729. https://doi.org/10.3390/su12229729 (2020b).
Liu, B., Peng, X., Han, L., Hou, L. & Li, B. Effects of exogenous spermidine on root metabolism of cucumber seedlings under salt stress by GC-MS. Agron 10, 459. https://doi.org/10.3390/agronomy10040459 (2020).
Maryum, Z. et al. R. An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cotton. Front. Plant. Sci. 13, 1–22. https://doi.org/10.3389/fpls.2022.907937 (2022).
Baqir, R. P., Zeboon, N. H. & Al-Behadili, A. A. J. The role and importance of amino acids within plants: a review. Plant. Arch. 19 (2), 1402–1410 (2019).
Acknowledgements
To the Plant Ecophysiology Laboratory, Universidade Federal de Alagoas (UFAL), Phytopathology Laboratory of the Universidade Federal da Paraíba (UFPB), Instituto Nacional de Ciência e Tecnologia em Agricultura Sustentável no Semiárido Tropical (INCTAgris), contract CNPQ/INCT: 406570/2022-1.
Funding
This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (PhD’s scholarship). Mônica Danielly de Mello Oliveira is supported by CAPES PDPG-FAPIII/FAPESQ (Fundação de Apoio à Pesquisa do Estado da Paraíba) under the contract 88887.929825/2023-00, edict Nº 9/2023.
Author information
Authors and Affiliations
Contributions
A.L.J.S.: Writing – performed the experiment, total analysis and statistical analysis and writing the manuscript. O.R.F.: writing the manuscript. E.B.C.: writing the manuscript. C.F.L.: Writing – writing the manuscript. A.S.M.: writing the manuscript. M.D.M.O.: designed the experiment and writing the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Research involving plants
The authors confirmed that all methods in regards to plants were carried out in accordance with relevant guidelines in the method section.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Silva, A.L.J., de Farias, O.R., Corrêa, É.B. et al. Biostimulant modulate the physiological and biochemical activities, improving agronomic characteristics of bell pepper plants under salt stress. Sci Rep 15, 14969 (2025). https://doi.org/10.1038/s41598-025-99414-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99414-w