Abstract
This study aims to evaluate the impact of pulmonary infections and antibiotic use on the recurrence of myocardial infarction (MI) in patients. A total of 3807 MI patients were included in this study. The effects of pulmonary infections and different antibiotics on recurrent MI were investigated using multivariable logistic regression and propensity score matching (PSM) analysis. Kaplan-Meier survival curves were used to compare the risk of recurrent MI between patients with and without pulmonary infections. In the multivariable logistic regression analysis, pulmonary infections significantly increased the risk of recurrent MI in patients with non-ST-segment elevation myocardial infarction (NSTEMI) (odds ratio [OR] = 1.47, 95% confidence interval [CI]: 1.22–1.79, P < 0.0001) and ST-segment elevation myocardial infarction (STEMI) (OR = 1.43, 95% CI: 1.15–1.80, P = 0.0016). PSM analysis showed that, without adjusting for antibiotic use, pulmonary infections significantly increased the risk of recurrent MI (NSTEMI: OR = 1.41, 95% CI: 1.12–1.79, P = 0.004; STEMI: OR = 1.48, 95% CI: 1.13–1.95, P = 0.0051). However, after adjusting for antibiotic use, the impact of pulmonary infections on recurrent MI was no longer significant (NSTEMI: OR = 0.91, 95% CI: 0.57–1.45, P = 0.691; STEMI: OR = 1.06, 95% CI: 0.80–1.41, P = 0.6925). Different antibiotics had significant effects on the risk of recurrent MI: quinolone antibiotics were associated with an increased risk, while cephalosporin antibiotics and metronidazole were associated with a decreased risk. Pulmonary infections significantly increase the risk of recurrent MI in patients, and antibiotic use can modify this effect. Clinically, the use of antibiotics and management of pulmonary infections should be carefully considered to optimize treatment strategies for MI patients.
Similar content being viewed by others
Introduction
Myocardial infarction (MI) is one of the leading causes of death and disability worldwide1. Despite significant advancements in acute phase treatment, MI patients still face a high risk of recurrent myocardial infarction (RMI)1,2. RMI not only increases patient mortality and complication rates but also significantly elevates healthcare resource consumption and economic burden3,4. Therefore, exploring the factors influencing RMI is of great clinical importance.
Pulmonary infection is a common complication among MI patients during hospitalization, which may result from an immune response triggered by infection or hospital-acquired infections due to the hospital environment and treatments5. Studies have shown that pulmonary infection may increase the risk of cardiovascular events by exacerbating systemic inflammatory responses and cardiac load6,7. However, research on the impact of pulmonary infection on RMI in MI patients is limited, and results are controversial8.
Antibiotics are the primary treatment for pulmonary infections, but different types of antibiotics may have varying effects on the cardiovascular system. For instance, some antibiotics may improve outcomes through anti-inflammatory and immunomodulatory effects, while others may increase cardiovascular risk due to drug interactions and side effects9,10. Therefore, understanding the impact of different antibiotics on RMI in MI patients can help optimize antibiotic treatment strategies and improve clinical outcomes.
This study aims to systematically evaluate the impact of pulmonary infections and antibiotic use on RMI in MI patients through multivariable logistic regression and propensity-score matching (PSM) analysis. We included 3807 MI patients and analyzed the relationship between pulmonary infections, different antibiotic uses, and RMI risk, aiming to provide evidence-based guidance for clinical practice and optimize management strategies for MI patients.
Methods
Study design and population
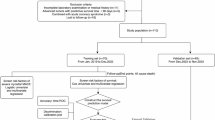
This retrospective cohort study included 12,857 patients admitted to a tertiary hospital for chest pain and diagnosed with acute coronary syndrome (ACS) from January 2015 to March 2023. Among these, 4,120 patients were diagnosed with myocardial infarction (MI), including both ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI). Exclusion criteria included: 8,737 patients with unstable angina (UA), 148 in-hospital deaths, 68 patients with mechanical complications, 38 patients with other non-cardiac diseases with a life expectancy of less than six months, and 59 patients lacking critical data. Ultimately, 3,807 MI patients were included in the analysis, comprising 2,224 NSTEMI and 1,583 STEMI patients (see Fig. 1).
Data collection
Patient demographics, medical history, clinical features, laborato ry test results, and treatment information were collected through the electronic medical record system. The primary exposure factors were the presence or absence of pulmonary infection and the use of antibiotics. The diagnosis of pulmonary infection was based on clinical symptoms, imaging studies, and microbiological test results. Antibiotic use was documented in terms of specific drug types and duration of treatment. The outcome event was the occurrence of recurrent myocardial infarction, confirmed through follow-up of outpatient and inpatient records.
Handling of missing data
Missing data were addressed using multiple imputation techniques. For continuous variables, missing values were imputed using the mean imputation method, and for categorical variables, the mode imputation method was used. If core variables had missing values, those cases were excluded from the analysis. A sensitivity analysis was performed to assess the impact of imputation on the results, ensuring the robustness of our findings.
Statistical analysis
Baseline characteristics
Descriptive statistics were used to compare baseline characteristics between patients with and without pulmonary infection. Categorical variables were compared using the chi-square test or Fisher’s exact test, while continuous variables were compared using the t-test or Mann-Whitney U test.
Multivariable logistic regression analysis
Multivariable logistic regression models were constructed to assess the impact of pulmonary infection and antibiotic use on the risk of recurrent myocardial infarction. Multivariable logistic regression models were constructed using stepwise regression to assess the impact of pulmonary infection and antibiotic use on the risk of recurrent myocardial infarction. Model A adjusted only for basic demographic variables. Model B further adjusted for sex and age. Model C additionally adjusted for other potential confounding factors, including BMI, LVEF, NT-proBNP, troponin T, LDL, PCI, Killip classification, atrial fibrillation, valvular heart disease, previous MI, hypertension, diabetes, COPD, renal insufficiency, stroke, antiplatelet drugs, number of antiplatelet drugs, statins, beta-blockers, novel oral anticoagulants, and warfarin. Model D further adjusted for antibiotic use.
To assess the validity of the logistic regression model and check for any assumptions violations, we conducted residual analysis. Residuals vs. Fitted Values plot was used to evaluate the homoscedasticity of the residuals, while Q-Q plot was employed to test the normality of the residuals.To assess the validity of the logistic regression model, we performed several diagnostic tests. The normality of residuals was evaluated using the Shapiro-Wilk test. Homoscedasticity was tested using the Breusch-Pagan test. Multicollinearity among the predictors was assessed using the Variance Inflation Factor (VIF), with a threshold of 10 indicating significant multicollinearity.
Propensity score matching (PSM)
A 1:1 propensity score matching method was used to reduce the impact of potential confounders. Matching variables included sex, age, BMI, LVEF, NT-proBNP, troponin T, LDL, PCI, Killip classification, atrial fibrillation, valvular heart disease, previous MI, hypertension, diabetes, COPD, renal insufficiency, stroke, antiplatelet drugs, number of antiplatelet drugs, statins, beta-blockers, novel oral anticoagulants, and warfarin. After matching, the incidence of recurrent myocardial infarction was compared between patients with and without pulmonary infection using the chi-square test or Fisher’s exact test.
Kaplan-Meier survival analysis
The Kaplan-Meier method was used to plot survival curves and compare the cumulative incidence of recurrent myocardial infarction between patients with and without pulmonary infection. Differences between groups were compared using the log-rank test.
Analysis of the impact of antibiotic use on recurrent myocardial infarction
The impact of different antibiotics (e.g., quinolones, cephalosporins, new beta-lactams, penicillins, aminoglycosides, nitroimidazoles) on the risk of recurrent myocardial infarction was assessed using multivariable logistic regression models, adjusting for potential confounders.
All statistical analyses were performed using R software version 4.2.0 (http://www.R-project.org). A two-sided P-value of < 0.05 was considered statistically significant.
Results
Baseline characteristics of patients
This study included a total of 3,807 myocardial infarction (MI) patients, of whom 2,224 did not experience recurrent myocardial infarction (RMI) and 1,583 did. The baseline demographic and clinical characteristics of the patients are presented in Table 1.
In terms of gender distribution, the proportion of males was 72.65% in the RMI group and 70.41% in the non-RMI group, with no statistically significant difference (P = 0.133). The mean age of patients in the RMI group was 65.56 years, while in the non-RMI group, it was 66.42 years, a statistically significant difference (P = 0.032). The mean body mass index (BMI) showed no significant difference between the RMI and non-RMI groups (19.74 ± 3.11 vs. 19.71 ± 3.04, P = 0.786).
Regarding medical history, there were no statistically significant differences between the RMI and non-RMI groups for atrial fibrillation (9.22% vs. 8.63%, P = 0.528), valvular heart disease (19.08% vs. 18.84%, P = 0.854), hypertension (65.00% vs. 67.63%, P = 0.091), diabetes (31.84% vs. 34.67%, P = 0.068), chronic obstructive pulmonary disease (COPD) (21.29% vs. 20.50%, P = 0.557), renal insufficiency (22.99% vs. 21.18%, P = 0.182), and stroke (16.42% vs. 16.86%, P = 0.721). However, the incidence of previous MI was significantly lower in the RMI group compared to the non-RMI group (3.41% vs. 7.46%, P < 0.001).
Regarding clinical condition at admission, the left ventricular ejection fraction (LVEF) was significantly lower in the RMI group compared to the non-RMI group (51.47% vs. 53.70%, P < 0.001). Levels of N-terminal pro-B-type natriuretic peptide (NTproBNP) were significantly higher in the RMI group than in the non-RMI group (4110.36 ± 8008.28 pg/mL vs. 3210.11 ± 6690.12 pg/mL, P < 0.001). Troponin T levels were also higher in the RMI group (3.17 ± 2.91 ng/mL vs. 2.89 ± 2.86 ng/mL, P = 0.004). Low-density lipoprotein (LDL) levels were significantly higher in the RMI group compared to the non-RMI group (2.81 ± 0.93 mmol/L vs. 2.70 ± 0.96 mmol/L, P < 0.001).
Regarding MI type, the proportion of non-ST-segment elevation myocardial infarction (NSTEMI) was significantly lower in the RMI group compared to the non-RMI group (47.50% vs. 67.90%), while the proportion of ST-segment elevation myocardial infarction (STEMI) was significantly higher in the RMI group (52.50% vs. 32.10%, P < 0.001). The incidence of pulmonary infection was also significantly higher in the RMI group compared to the non-RMI group (32.15% vs. 24.64%, P < 0.001).
In terms of treatment, the proportion of patients undergoing percutaneous coronary intervention (PCI) was significantly higher in the RMI group compared to the non-RMI group (84.65% vs. 76.44%, P < 0.001). The use of antibiotics was also significantly higher in the RMI group (33.35% vs. 25.58%, P < 0.001). There were no significant differences between the two groups regarding the use of antiplatelet drugs, statins, and beta-blockers.
In summary, patients with recurrent myocardial infarction exhibited significant differences in age, history of previous myocardial infarction, LVEF at admission, NTproBNP levels, troponin T levels, LDL levels, type of myocardial infarction, and incidence of pulmonary infection compared to those without recurrence. These differences suggest that patients with recurrent myocardial infarction may have higher risk characteristics.
Hypothesis testing and assumption checks
Supplementary Table 1 presents the results of hypothesis testing. The Shapiro-Wilk test for normality indicated that the residuals do not follow a normal distribution (P-value < 0.05). The Breusch-Pagan test for homoscedasticity showed the presence of heteroscedasticity (P-value < 0.05), meaning that the variance of the residuals changes with the fitted values. These results suggest that while the residuals are not normally distributed and there is heteroscedasticity in the model, these issues are typically addressed through model adjustments, and the analysis was conducted accordingly.
Multicollinearity assessment and VIF results
Supplementary Table 2 presents the VIF values for all variables. Most variables have low VIF values (below 10), indicating no significant multicollinearity. However, Beta-lactam antibiotics (VIF = 11.68) and Use of antibiotics (VIF = 10.78) have VIF values greater than 10. These variables, however, were not included in the multivariable regression analysis. Instead, Use of antibiotics was analyzed separately, so their impact on the model is minimal.
Model diagnostics: residuals and normality check
In Supplementary Fig. 1, the Residuals vs. Fitted Values plot is presented to assess the fit of the logistic regression model. The plot shows that the residuals (differences between observed and predicted values) are randomly distributed around zero, indicating that the model does not exhibit patterns of heteroscedasticity or misspecification. The absence of any discernible trend or curvature supports the validity of the model’s assumptions regarding homoscedasticity.
Supplementary Fig. 2 shows the Q-Q plot of the residuals from the logistic regression model. This plot is used to test the normality of the residuals. The points closely align with the diagonal line, suggesting that the residuals are approximately normally distributed, which is a key assumption for logistic regression. Minor deviations from the line are expected, but no significant departure from normality is observed.
Multivariable logistic regression analysis
Table 2 presents the impact of pulmonary infection on outcomes based on multivariable logistic regression analysis.In the multivariable logistic regression analysis, the adverse outcome being analyzed was defined as the occurrence of RMI in patients with MI.
In Model A, which considers only the impact of pulmonary infection, results show that pulmonary infection significantly increases the risk of adverse outcomes in both NSTEMI (non-ST-segment elevation myocardial infarction) and STEMI (ST-segment elevation myocardial infarction) patients. Specifically, pulmonary infection was associated with a 1.47-fold increase in the risk of adverse outcomes in NSTEMI patients (OR = 1.47, 95% CI: 1.22–1.79, P < 0.0001), a 1.43-fold increase in STEMI patients (OR = 1.43, 95% CI: 1.15–1.80, P = 0.0016), and a 1.46-fold increase in the overall population (OR = 1.46, 95% CI: 1.26–1.69, P < 0.0001).
After adjusting for sex and age in Model B, pulmonary infection still significantly increased the risk of adverse outcomes in both NSTEMI and STEMI patients. Pulmonary infection was associated with a 1.48-fold increase in the risk of adverse outcomes in NSTEMI patients (OR = 1.48, 95% CI: 1.22–1.80, P < 0.0001), a 1.47-fold increase in STEMI patients (OR = 1.47, 95% CI: 1.17–1.85, P = 0.0009), and a 1.48-fold increase in the overall population (OR = 1.48, 95% CI: 1.28–1.72, P < 0.0001).
In Model C, further adjustments were made for sex, age, BMI, LVEF, NTproBNP, troponin T, LDL, PCI, Killip classification, atrial fibrillation, valvular heart disease, previous MI, hypertension, diabetes, COPD, renal insufficiency, stroke, antiplatelet drugs, number of antiplatelet drugs, statins, beta-blockers, novel oral anticoagulants, and warfarin. The results still showed that pulmonary infection significantly increased the risk of adverse outcomes in NSTEMI and STEMI patients. Pulmonary infection was associated with a 1.35-fold increase in the risk of adverse outcomes in NSTEMI patients (OR = 1.35, 95% CI: 1.09–1.66, P = 0.0052), a 1.50-fold increase in STEMI patients (OR = 1.50, 95% CI: 1.18–1.92, P = 0.0011), and a 1.42-fold increase in the overall population (OR = 1.42, 95% CI: 1.21–1.66, P < 0.0001).
In Model D, which further adjusted for antibiotic use, the impact of pulmonary infection on the risk of adverse outcomes in NSTEMI and STEMI patients was reduced. Pulmonary infection was not significantly associated with an increased risk of adverse outcomes in NSTEMI patients (OR = 1.23, 95% CI: 0.95–1.58, P = 0.1164) or STEMI patients (OR = 1.21, 95% CI: 0.91–1.61, P = 0.2004). However, in the overall population, pulmonary infection still significantly increased the risk of adverse outcomes (OR = 1.22, 95% CI: 1.01–1.47, P = 0.0429).
In summary, the multivariable logistic regression analysis showed that pulmonary infection significantly increases the risk of adverse outcomes in MI patients, especially when antibiotic use is not adjusted for.
Propensity score matching analysis
Table 3 shows the baseline characteristics of patients with and without pulmonary infection before and after propensity score matching (PSM).In the PSM analysis, adverse outcomes refer to the RMI in patients.
Before PSM, there were significant differences in several baseline characteristics between the pulmonary infection group (N = 1057) and the non-pulmonary infection group (N = 2750). The proportion of males was significantly lower in the pulmonary infection group (68.21% vs. 72.55%, P = 0.008), and the mean age was higher (69.24 ± 11.49 years vs. 64.84 ± 12.17 years, P < 0.001). The pulmonary infection group had a lower BMI (19.41 ± 3.07 kg/m2 vs. 19.84 ± 3.06 kg/m2, P < 0.001) and higher rates of atrial fibrillation (13.43% vs. 7.13%, P < 0.001), valvular heart disease (22.33% vs. 17.64%, P < 0.001), diabetes (40.11% vs. 30.95%, P < 0.001), renal insufficiency (33.68% vs. 17.42%, P < 0.001), and stroke (22.80% vs. 14.33%, P < 0.001).
There were also significant differences in clinical conditions at admission. The pulmonary infection group had a lower left ventricular ejection fraction (LVEF) (49.95 ± 12.46% vs. 53.85 ± 11.49%, P < 0.001), higher NTproBNP levels (5801.94 ± 9238.93 pg/ml vs. 2732.12 ± 6161.71 pg/ml, P < 0.001), higher troponin T levels (3.16 ± 2.97 ng/mL vs. 2.95 ± 2.85 ng/mL, P = 0.037), and lower low-density lipoprotein (LDL) levels (2.67 ± 0.95 mmol/L vs. 2.77 ± 0.95 mmol/L, P = 0.002). There was no significant difference in the proportion of NSTEMI and STEMI patients between the groups (58.47% vs. 59.78%, P = 0.460), but the pulmonary infection group had a higher proportion of patients with higher Killip class (P < 0.001).
Regarding treatment, the pulmonary infection group had a lower proportion of patients undergoing PCI (73.60% vs. 82.25%, P < 0.001) and lower use of antiplatelet drugs (98.30% vs. 99.16%, P = 0.020) and statins (98.11% vs. 99.05%, P = 0.017). The use of novel oral anticoagulants was higher in the pulmonary infection group (4.73% vs. 2.44%, P < 0.001).
After PSM, 1,026 patients were matched in each group. Post-matching, there were no significant differences in most baseline characteristics between the groups. The proportion of males (67.8% vs. 68.6%, P = 0.740) and mean age (69.03 ± 11.54 years vs. 68.20 ± 11.87 years, P = 0.109) were similar. There were no significant differences in BMI (19.42 ± 3.06 kg/m2 vs. 19.36 ± 3.05 kg/m2, P = 0.648), atrial fibrillation (12.4% vs. 10.6%, P = 0.240), valvular heart disease (22.4% vs. 21.7%, P = 0.750), previous MI (6.5% vs. 6.8%, P = 0.860), hypertension (67.3% vs. 66.4%, P = 0.708), diabetes (40% vs. 35.8%, P = 0.056), COPD (21.2% vs. 21.8%, P = 0.788), renal insufficiency (32.2% vs. 28.6%, P = 0.084), and stroke (21.8% vs. 20.9%, P = 0.628).
Regarding clinical conditions at admission, there were no significant differences between the matched groups in LVEF (50.26 ± 12.34% vs. 50.78 ± 12.18%, P = 0.343) or NTproBNP levels (5396.87 ± 8763.20 pg/ml vs. 4747.07 ± 8467.84 pg/ml, P = 0.088). There were also no significant differences in the proportion of NSTEMI and STEMI patients (58.3% vs. 60.5%, P = 0.323) or Killip class (P = 0.223).
In terms of treatment, there were no significant differences between the matched groups in the proportion of patients undergoing PCI (74.5% vs. 75%, P = 0.799), use of antiplatelet drugs (98.3% vs. 98.5%, P = 0.859), or use of statins (98.3% vs. 98.3%, P = 1.000).
In summary, after PSM, the pulmonary infection and non-pulmonary infection groups were well-balanced in most baseline characteristics, reducing the impact of potential confounders and making the subsequent analysis more reliable.
Outcomes in propensity score matching analysis
Table 4 presents the impact of pulmonary infection on outcomes in the propensity score matching (PSM) analysis.
In the analysis without matching for antibiotic use, pulmonary infection significantly increased the risk of adverse outcomes in both NSTEMI (non-ST-segment elevation myocardial infarction) and STEMI (ST-segment elevation myocardial infarction) patients. Specifically, for NSTEMI patients, pulmonary infection increased the risk of adverse outcomes by 1.41 times (OR = 1.41, 95% CI: 1.12–1.79, P = 0.004); for STEMI patients, the risk increased by 1.48 times (OR = 1.48, 95% CI: 1.13–1.95, P = 0.0051); and in the overall population, the risk increased by 1.44 times (OR = 1.44, 95% CI: 1.21–1.72, P < 0.0001).
In the analysis with matching for antibiotic use, the impact of pulmonary infection on adverse outcomes was reduced and no longer significant. For NSTEMI patients, pulmonary infection was not significantly associated with the risk of adverse outcomes (OR = 0.91, 95% CI: 0.57–1.45, P = 0.691); for STEMI patients, there was also no significant association (OR = 1.06, 95% CI: 0.80–1.41, P = 0.6925); and in the overall population, there was no significant association either (OR = 1.02, 95% CI: 0.80–1.29, P = 0.8966).
These results indicate that pulmonary infection significantly increases the risk of adverse outcomes in myocardial infarction patients when antibiotic use is not adjusted for. However, when considering antibiotic use, the impact of pulmonary infection on adverse outcomes is no longer significant, suggesting that antibiotic use may play a role in modulating the effect of pulmonary infection on the prognosis of myocardial infarction patients.
Kaplan-Meier survival analysis
Figure 2shows the Kaplan-Meier survival curves for patients with STEMI (A) and NSTEMI (B), stratified by the presence or absence of pulmonary infection. The curves depict the probability of being free from recurrent myocardial infarction over time for both groups.
Kaplan-Meier survival analysis showing the probability of being free from recurrent myocardial infarction in patients with STEMI and NSTEMI with and without pulmonary infection. (A) STEMI patients: red curve indicates those with pulmonary infection, blue curve indicates those without. Pulmonary infection significantly reduces the probability (P = 0.0000). (B) NSTEMI patients: red curve indicates those with pulmonary infection, blue curve indicates those without. Pulmonary infection significantly reduces the probability (P = 0.0000).
Figure 2A demonstrates that for STEMI patients, those with pulmonary infection (red curve) had a significantly lower probability of being free from recurrent myocardial infarction compared to those without pulmonary infection (blue curve) (P < 0.0001). The survival probability for the pulmonary infection group remained lower than that of the non-infection group throughout the entire follow-up period.
Figure 2B shows that for NSTEMI patients, those with pulmonary infection (red curve) also had a significantly lower probability of being free from recurrent myocardial infarction compared to those without pulmonary infection (blue curve) (P < 0.0001). Similar to STEMI patients, the survival probability for the pulmonary infection group in NSTEMI patients remained lower than that of the non-infection group throughout the follow-up period.
These results indicate that pulmonary infection significantly increases the risk of recurrent myocardial infarction in both STEMI and NSTEMI patients, further supporting the adverse impact of pulmonary infection on the prognosis of myocardial infarction patients.
Impact of different antibiotics on recurrent myocardial infarction
Figure 3 shows the impact of different antibiotics on rehospitalization due to myocardial infarction, presenting the regression coefficient values for various antibiotics.
The data indicate significant differences in the impact of different antibiotics on the risk of rehospitalization in myocardial infarction patients. Notably, the use of quinolone antibiotics is associated with an increased risk of rehospitalization (coefficient = 0.02), whereas the use of cephalosporin antibiotics (coefficient=-0.04) and other antibiotics (coefficient=-0.03) is associated with a reduced risk.
Among specific antibiotic categories, the use of new beta-lactam antibiotics (coefficient = 0.16) and penicillin antibiotics (coefficient = 0.17) is significantly associated with an increased risk of rehospitalization. Conversely, the use of aminoglycoside antibiotics (coefficient=-0.35) and nitroimidazole antibiotics (such as metronidazole, coefficient=-1.18) significantly reduces the risk of rehospitalization.
The use of beta-lactam antibiotics (coefficient = 0.37) and peptide antibiotics (coefficient = 0.43) is also significantly associated with an increased risk of rehospitalization.
These results suggest that different types of antibiotics have varying effects on the risk of rehospitalization in myocardial infarction patients. This variation should be considered in clinical treatment to optimize patient management strategies.
Discussion
This study systematically evaluated the impact of pulmonary infection and antibiotic use on the risk of recurrent myocardial infarction (RMI) in patients with myocardial infarction (MI). We found that pulmonary infection significantly increased the risk of RMI, and antibiotic use played a crucial role in modulating this risk.
Firstly, the analysis of baseline characteristics revealed significant differences between the pulmonary infection group and the non-infection group in terms of age, BMI, medical history, and clinical conditions at admission. These differences could influence the risk of RMI, so we adjusted for these potential confounders in the multivariable analysis. The results showed that pulmonary infection significantly increased the risk of RMI in both NSTEMI and STEMI patients, consistent with previous studies11,12. Pulmonary infection may increase the risk of cardiovascular events by exacerbating systemic inflammation and cardiac load8,13,14,15.This inflammatory response contributes to endothelial dysfunction, which impairs vascular reactivity and increases vascular resistance16. Additionally, pulmonary infection induces myocardial stress through augmented cytokine release and changes in cardiac workload, further heightening the risk of recurrent myocardial infarction17.
Secondly, the propensity score matching (PSM) analysis further confirmed the impact of pulmonary infection on the risk of recurrent myocardial infarction (RMI). After matching, the baseline characteristics of the two groups were balanced, reducing the influence of potential confounders. The matching analysis showed that, without adjusting for antibiotic use, pulmonary infection significantly increased the risk of RMI. However, when adjusting for antibiotic use, the impact of pulmonary infection on RMI was no longer significant, suggesting that antibiotic use plays a crucial role in modulating this risk. This is consistent with studies that have shown antibiotics’ potential to reduce inflammation and improve cardiovascular outcomes. For instance, certain antibiotics, such as macrolides, have been found to decrease systemic inflammation, which can mitigate the negative cardiovascular effects associated with infection18. On the other hand, other studies have pointed out that some antibiotics, such as quinolones, may exacerbate cardiovascular risks19,20.
Therefore, this study analyzed the impact of different antibiotics on the risk of RMI. The results showed that the use of quinolone antibiotics was associated with an increased risk of rehospitalization, while cephalosporin and nitroimidazole antibiotics were associated with a reduced risk. This finding is consistent with some studies, suggesting that different types of antibiotics may have varying effects on the cardiovascular system when treating pulmonary infections9,10. For example, quinolone antibiotics have been reported to be associated with QT interval prolongation and arrhythmias19,20, while cephalosporin antibiotics21,22, aminoglycoside antibiotics23, and nitroimidazole antibiotics24 have been less frequently associated with cardiovascular adverse events. Additionally, this study also found that beta-lactam antibiotics and penicillin antibiotics were associated with an increased risk of RMI, which is consistent with previous reports25,26.Given the varying effects of these antibiotics on MI patients, this study suggests that the type of antibiotic selected when managing MI patients with concurrent pulmonary infections should be carefully considered, as it may influence their cardiovascular outcomes.
Limitations and clinical significance
However, this study has several limitations. First, as a retrospective study, despite our efforts to adjust for potential confounding factors, we cannot completely rule out all possible confounders. Second, the data were sourced from a single center, which may introduce selection bias, and thus the generalizability of the results should be approached with caution. Finally, the information on antibiotic use was based on electronic medical records, which may be incomplete or inaccurate.
Despite these limitations, the study has important clinical implications. The results indicate that pulmonary infection significantly increases the risk of RMI in MI patients, and antibiotic use plays a crucial role in modulating this risk. This suggests that clinicians should closely monitor for pulmonary infections in MI patients and make informed antibiotic choices to minimize the risk of RMI and improve long-term patient outcomes.
Conclusion
This study systematically evaluated the impact of pulmonary infection and antibiotic use on the risk of RMI in MI patients. The findings demonstrate that pulmonary infection significantly increases the risk of RMI, and antibiotic use is crucial in modulating this risk. These insights provide clinicians with a new perspective on managing MI patients, emphasizing the need to consider antibiotic selection carefully when treating pulmonary infections to optimize treatment outcomes and improve patient prognosis.
Data availability
The datasets generated and analyzed during the current study are not publicly available due the database owner is reluctant to make them public but are available from the corresponding author upon reasonable request.
References
Byrne, R. A. et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur. Heart J. 44(38), 3720–3826 (2023).
Benjamin, E. J. et al. Heart disease and stroke statistics—2018 update: A report from the American heart association. Circulation 137(12) (2018).
Nichols, M., Townsend, N., Scarborough, P. & Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 35(42), 2950–2959 (2014).
Song, J. et al. Incidence, predictors, and prognostic impact of recurrent acute myocardial infarction in China. Heart 107(4), 313–318 (2021).
Younis, H. et al. Acute myocardial infarction–related hospitalizations in non-elderly patients with pneumonia: A population-based study. SN Compr. Clin. Med. 5(1) (2023).
Musher, D. M., Abers, M. S. & Corrales-Medina, V. F. Acute infection and myocardial infarction. N. Engl. J. Med. 380(2), 171–176 (2019).
de Boer, A. R. et al. Influenza infection and acute myocardial infarction. NEJM Evid. 3(7), a2300361 (2024).
Goedemans, L., Bax, J. J. & Delgado, V. Copd and acute myocardial infarction. Eur. Respir. Rev. 29(156), 190139 (2020).
Wong, A. Y. S., Chan, E. W., Anand, S., Worsley, A. J. & Wong, I. C. K. Managing cardiovascular risk of macrolides: Systematic review and meta-analysis. Drug Saf. 40(8), 663–677 (2017).
Al-Tawfiq, J. A. et al. Antibiotics in the pipeline: A literature review (2017–2020). Infection 50(3), 553–564 (2022).
Lin, Z. et al. Positive association between stress hyperglycemia ratio and pulmonary infection in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc. Diabetol. 22(1) (2023).
Hwang, S. Y., Kim, S. H., Uhm, I. A., Shin, J. & Lim, Y. Prognostic implications for patients after myocardial infarction: An integrative literature review and in-depth interviews with patients and experts. Bmc Cardiovasc. Disord. 22(1) (2022).
Marriott, E., Singanayagam, A. & El-Awaisi, J. Inflammation as the nexus: Exploring the link between acute myocardial infarction and chronic obstructive pulmonary disease. Front. Cardiovasc. Med. 11 (2024).
Kumar, N. et al. Pseudomonas aeruginosa pulmonary infection results in S100A8/A9-dependent cardiac dysfunction. Plos Pathog. 19(8), e1011573 (2023).
Huang, J., Kuang, W. & Zhou, Z. Il-1 signaling pathway, an important target for inflammation surrounding in myocardial infarction. Inflammopharmacology (2024).
Cheng, D. et al. Inhibition of MPO (myeloperoxidase) attenuates endothelial dysfunction in mouse models of vascular inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 39(7), 1448–1457 (2019).
Wu, L. et al. SARS-cov-2 and cardiovascular complications: From molecular mechanisms to pharmaceutical management. Biochem. Pharmacol. 178, 114114 (2020).
Steel, H. C., Theron, A. J., Cockeran, R., Anderson, R. & Feldman, C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediat. Inflamm. 2012, 1–17 (2012).
Goldstein, E. J. C., Owens, R. C., Nolin, T. D. & Goldstein, E. Antimicrobial-associated Qt interval prolongation: Pointes of interest. Clin. Infect. Dis. 43(12), 1603–1611 (2006).
Lapi, F. et al. Fluoroquinolones and the risk of serious arrhythmia: A population-based study. Clin. Infect. Dis. 55(11), 1457–1465 (2012).
Chaudhry, S. B., Veve, M. P. & Wagner, J. L. Cephalosporins: A focus on side chains and β-lactam cross-reactivity. Pharmacy 7(3), 103 (2019).
Choi, J., Choi, J., Kim, M. & Rhie, S. Signal detection of adverse drug reactions of cephalosporins using data from a National pharmacovigilance database. Pharmaceuticals 14 (5), 425 (2021).
Wang, N., Luo, J., Deng, F., Huang, Y. & Zhou, H. Antibiotic combination therapy: A strategy to overcome bacterial resistance to aminoglycoside antibiotics. Front. Pharmacol. 13 (2022).
Li, B. Z. et al. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: Systematic review and network meta-analysis. Bmj 351, h4052 (2015).
Wilson, G. M. et al. Evaluating the clinical effectiveness of new beta-lactam/beta-lactamase inhibitor combination antibiotics: A systematic literature review and meta-analysis. Antimicrob. Stewardship Healthc. Epidemiol. 1(1) (2021).
Marantelli, S., Hand, R., Carapetis, J., Beaton, A. & Wyber, R. Severe adverse events following benzathine penicillin G injection for rheumatic heart disease prophylaxis: Cardiac compromise more likely than anaphylaxis. Heart Asia 11(2), e11191 (2019).
Author information
Authors and Affiliations
Contributions
Zc.L., Z.G. and L.P.: established the hypothesis, performed the statistical analysis, wrote the manuscript. X.H., L.Y., C.H., K.G., H.L., L.G., Y.L., L.P., and Jp.Z.: interpreted statistical analysis and conducted multivariate analysis. Zc.L.: data collection and participated follow-up. My.J.: initiated the study hypothesis, edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Xiangtan Central Hospital (Xiangtan, China, No.2023-02-001) and conformed to the principles outlined in the Declaration of Helsinki.The need for informed consent was waived by the ethics committee Review Board of Xiangtan Central Hospital, because of the retrospective nature of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Z., Gu, Z., Peng, L. et al. Impact of pulmonary infection and antibiotic use on recurrent myocardial infarction in patients with myocardial infarction. Sci Rep 15, 14954 (2025). https://doi.org/10.1038/s41598-025-99444-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99444-4