Abstract
Alkaline stress exacerbates ionic toxicity, leading to a decline in plant growth and yield. Lagenaria siceraria (Molina) Stndl., commonly known as squash, an annual plant belonging to the Cucurbitaceae family, is sensitive to alkalinity because of its impact on physiological growth and yield of plant. However, studies on the utilization of plant growth regulators to alleviate alkali stress in Lagenaria siceraria is scarce. In the present study, a pot experiment was conducted to evaluate the impact of foliar application of NAA (0, 50, 75, or 100 ppm) on the growth, yield, and biochemical parameters of L. siceraria under both normal and alkaline stress conditions (0 and 40 mM). The findings revealed that alkaline stress significantly diminished plant growth, biomass, and leaf and fruit counts, whereas NAA application amplified all growth and yield characteristics under both stressful and normal conditions. Additionally, compared with salt stress alone, alkaline stress markedly decreased the levels of photosynthetic pigments; however, 75% NAA application resulted in 43% increase in Chl a, 53% increase in Chl b, and 66% increase in carotenoids. Moreover, there was a notable increase in the primary and secondary metabolites of plants treated with NAA, including a 27% increase in total soluble proteins (TSP), a 38% increase in total free amino acids (TFA), and a 28% and 27% increase in total phenolic and flavonoid contents, respectively, in comparison with the plants subjected only to stress. To further explore the impact of NAA on biochemical parameters of L. siceraria, antioxidant enzymes like catalase (CAT) and peroxidase (POD) activities were assessed. The results indicated that alkali stress increased enzyme activities, which were further increased under the influence of foliar-applied NAA as compared to the plants exposed solely to stress. These findings underscore the beneficial role of exogenous NAA application in mitigating the adverse effects of alkali stress on L. siceraria.
Similar content being viewed by others
Introduction
Salinity-alkalinity stress represents a significant abiotic stressor that hinders plant growth and agricultural productivity on a global scale 1. In particular, plants exposed to saline-alkaline stress encounter disruptions in their metabolic activities due to increased osmotic pressure and the accumulation of reactive oxygen species (ROS) 2,3. Consequently, plants respond by upregulating a cascade of antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD), as well as increasing the activities of enzymes that eliminate ROS and maintain the structural integrity of cellular membranes by decreasing in lipid peroxidation rates 4,5.
Elevated levels of alkaline salts in the plant growth medium decrease the potassium (K+) concentration and increase sodium (Na+) uptake and accumulation, resulting in the efflux of K+ ions and the promotion of K+ leakage from plant cells. Furthermore, under alkaline stress conditions, the Na+ concentration exceeds the K+ concentration, leading to poor nutrient absorption and disruption of the Na+/K+ balance. A relatively high pH also causes aberrant root morphology, resulting in leaf chlorosis and necrotic lesions, all of which impede plant growth and development, ultimately reducing crop yield 6. Although saline-alkaline stresses seldom occur together, their mechanisms are intricately interwind. Salinity stress is primarily caused by the accumulation of neutral salts, exacerbated by high pH levels exceeding 8.5, which compromise root vigor, photosynthesis, and cell membrane integrity 7. Alkaline stress also effects plant metabolism at the physiological and biochemical levels, inducing variations in phytohormone levels and altering gene expression networks 5.
Several studies regarding geographical and significant temporal variations in phytohormones in response to different abiotic stress conditions have been reported in literature. Among the phytohormones, cytokinins (CK), salicylic acid (SA), auxins, ethylene (ET), gibberellins (GA), abscisic acid (ABA), jasmonates (JA), and brassinosteroids (BR) play important roles in abiotic stress signaling pathways. In general, ABA modulates osmotic stress, SA boosts important enzymes of the antioxidant system, and IAA and CK support the plant in sustaining development in high-pH growth media 1,8.
Naphthalene acetic acid (NAA) has been demonstrated to increase root development in various plant species, including maize, citrus, rice, and malus, under adverse environmental conditions 9,10. Studies have indicated that the auxin gradient induced by stress plays a pivotal role in regulating a wide array of physiological and biochemical processes, such as the synthesis of aquaporins, control of the stomatal aperture, positioning of lateral roots, and accumulation of crucial osmolytes such as soluble sugars and proline. Auxin has been shown to increase plant stress tolerance by effectively scavenging reactive oxygen species (ROS), reducing the accumulation of Na+ ions, and safeguarding photosystem II (PSII) against stress-induced damage 6.
Summer squash [Lagenaria siceraria (Molina) Stndl], a member of the Cucurbitaceae family, is widely distributed in the Indo-Pak region and is commonly consumed as a vegetable worldwide. Research efforts have resulted in the identification of various bioactive compounds in L. siceraria, including phenolic compounds such as chlorogenic acid, benzoic acid, quercetin, luteolin, and kaempferol, as well as p-coumaric acid and sinapic acid. Number of biochemical and physiological changes have been linked to abiotic stresses, such as alkaline stress, yet limited information is available in literatures regarding alkaline tolerance in squash. Additionally, there is a little data to support the evidence of the protective effects of growth hormones in enhancing crop yield under stressful conditions. The effect of exogenously applied NAA on squash cultivation under alkaline stress conditions is not well understood form the literature. This study addresses a significant gap in the research by exploring the potential of foliar applied NAA to mitigate alkaline stress in squash. Moreover, this study highlights the novel effect of NAA in the growth performace, biochemical and physiological resilience under alkaline stress, an areae with limited prior reported studies. The findings of the current study suggest that "the application of naphthalene acetic acid foliarly may improve the growth and yield of squash under alkaline stress."
Materials and methods
The research study took place in the wire house facility at the prestigious Institute of Molecular Biology and Biotechnology (IMBB) at the University of Lahore, Pakistan. This study aimed to investigate the impact of foliar NAA application on squash plants subjected to alkaline stress. All the experimental procedures were carried out at the state-of-the-art General Botany Research Laboratory at IMBB, University of Lahore.
Procurement of the plant material and experimental setup
The seeds of squash were collected from the local seed market and sown in clay pots containing 7 kg of soil in each pot. Five seeds were sown in each pot and let to germinate under natural conditions until the two-leaf stage. One week after seed germination, the pots were divided into two sets: one was considered as control, while the other set of pots was irrigated with 40 mM NaHCO3 causing alkine stress to the plants at an interval of 10 days for 4 times throughout the experimental period ensuring the sustained presence of stress (Fig. 1).
Exogenous application of NAA
After two weeks of application of stress at the appearance of visible stress signs, the plants were treated with 0, 25, 50 or 75 mg/L NAA. A surfactant (Tween-20) was added to the solution of NAA at a ratio of 5% per liter. The plants were sprayed NAA thrice at an intervals of 5 days, while the control plants were sprayed with distilled water. The plants were harvested one month after the onset of fruit, making sure that the roots and shoots were undamaged, and the growth parameters were recorded. The plants were grown for total 60 days from sowing to harvesting
Growth performance and yield evaluation
The plants were harvested after the onset fruiting stage, and the fresh biomass of the plants were measured on a weight scale. Root and shoot samples from each treatment were taken and dried in a laboratory oven at 65°C to measure the dry biomass of the plants.
The root and shoot lengths of the harvested plants were recorded with a measuring tape. Fruits were separated from the petiole, and the number of fruits was recorded and their weight was also measured. Leaves from each plant in each treatment group were collected, and the number of leaves per pot was recorded. The volume displacement method was used to measure the fruit volume. The volume was calculated via the following formula:
Analysis of stress induced physiological changes
The plant leave extract was prepared by taking 0.5 g of fresh leaf from four replications of each treatment in 10 ml of phosphate buffer solution by storing it in a freezer for further analysis.
Analysis of photosynthetic pigments
Photosynthetic pigments, including chlorophyll a, chlorophyll b, total chlorophyll and carotenoids, were determined by the methods proposed by Arnon (1949) 11. For tgese analysis, fresh leaves (0.5 g) from each pot of each treatment were chopped and immersed in 80% acetone. The tubes were incubated at room temerature overnight, and the absorbance was measured with a spectrophotometer at three different wavelengths of 480, 645 and 663 nm, separately taking acetone (80%) alone as a blank.
Analysis of primary and secondary metabolites
The total free amino acid were determined spectrophotometrily following the protocol proposed by Hamilton and Slyke (1943), taking the absorbance at 570 nm. The Bradford method (Bradford, 1976) was used to determine the total soluble protein content in the plant leaves and the absorbance of the BSA solution was measured at 595 nm by using spectrophotometer (UV/V spectrophotometer, HALO SB-10). The total phenol content was determined according to the proposed protocol of Julkunen-Titto (1985) by taking the absorbance at 760 nm at spectrophotometer.
Analysis of antioxidant enzyme assays
Evaluation of antioxidant enzymes was performed by taking fresh leaves (500 mg) homogenized in 5 mL of phosphate buffer (50 mM, pH 7.8) at 4°C. For 15 minutes, this extract was centrifuged at 12,000 rpm. The supernatant of this centrifugate was collected in autoclaved Eppendorf tubes for the determination of peroxidase (POD) and catalase (CAT) activity 12.
Statistical analysis
The experimental data were analyzed via computer-based software (STATISTIX 8.1) ver. 10.1 using analysis of variance (ANOVA). Pairwise comparisons between the groups were performed via Tukey’s HSD test.
Results
Foliar application of NAA promoted growth indices in squash plants under alkaline stress
The impact of foliar- applied of NAA on growth and biomass of squash was studied under both normal and alkaline stress conditions. Alkalinity in the soil reduced the root and shoot lengths by 42.1% and 33.8%, respectively, compared to the control plants. Similarly, the fresh biomass of a plant comprising root and shoot fresh weights decreased by 53.4% and 29.3%, respectively, in saline-stressed plants. The foliar application of NAA solution accelerated the growth in both stressed and nonstressed plants. Foliar application of 75 mg/L NAA increased shoot length by 53% and 46%, root length by 51% and 4%, and fresh plant biomass by 68% and 62% in stressed and nonstressed plants, respectively. Furthermore, NAA foliar application also increased the number of leaves on squash plants under both conditions (Table 1). Notably, compared with control plants, alkali-treated plants exhibited a 43% reduction in leaf count. However, 75 mg/L NAA increased the leaf count by 65% under stressed conditions and by 47% under control conditions demonstrating the growth promoting effects of NAA.
Foliar application of NAA improved photosynthetic pigments in squash plants under alkaline stress
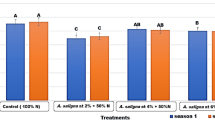
The photosynthetic pigments of squash plants including chlorophyll a, chlorophyll b, total chlorophyll and carotenoids were adversely reduced by 2% (Fig. 2a), 78% (Fig. 2b), 43% (Fig. 2c) and the 54% in alkaline-stressed plants as compared to control plants (Fig. 2d). However, an exogenously applied NAA solution increased the photosynthetic pigments, although more concentrated solution presented greater increase in pigments, as 75 mg/L NAA solution increased the chlorophyll a content by 54% and 43%, the chlorophyll b content by 53% and 87%, the total chlorophyll by 64% and 55%, and the carotenoid content by 45% and 66% in nonstressed and stressed plants, respectively (Fig. 2a–d). These findings underscore the role of NAA in enhancing photosynthetic efficiency under stress conditions.
Foliar application of NAA modulates the concentrations of plant metabolites in squash plants under alkaline stress
The effects of NAA application on plant primary metabolites such as total soluble proteins (TSPs) and total free amino acids (TFAs) revealed that alkaline stress led to an 8% decrease in both analysis in stressed squash plants. However, foliar application of NAA reversed this decline and a significant improvement was observed with increasing concentrations of NAA (0, 25, 50 and 75 mg/L) solution. The foliar application of 75 mg/L NAA resulted in an increase of 27% in total soluble proteins under both stressed and nonstressed conditions, while, the same concentration of NAA, exhibited an increases of total free amino acids by 38% and 46% noted under stressed and unstressed conditions, respectively (Fig. 3a, b).
The plant secondary metabolites were also adversely affected by alkaline stress and in turm modulated by the application of NAA. Compared with the control condition, alkaline stress increased the total phenolic and flavonoid contents by 12% and 45%, respectively. However, 75 mg/L NAA application modulate the phenolic content by 28% and 24% and the flavonoid content by 27% and 20% in nonstressed and stressed plants, respectively (Fig. 3c, d).
Foliar application of NAA improved the antioxidant defense system in squash plants under alkaline stress
The antioxidant activities of squash plants grown in alkaline soil were also investigated under the effect of foliar application of NAA. The data revealed that, alkaline stress boost the enzyme activities in squash plants with catalase (CAT) and peroxidase (POD) activities increasing by 26% and 40%, respectively. However, the application of 75 mg/L NAA amplified these defense mechanisms by increasing CAT activity by 42% and 32%, whereas POD activity by 51% and 34% in nonstressed and stressed plants, respectively. The results revealed the NAA efficacy in strengthening plant defense combating stress effectively (Fig. 4a, b).
These comprehensive analyses showed that application of NAA mitigate the worse effects of alkalinity and alsi enhances plant resilience, growth and metabolic functions under both stressed and non-stressed conditions
Discussion
Plant growth regulators (PGRs) are pivotal in regulating the life cycle of plants by orchestrating and overseeing numerous physiological processes that govern the plant growth and crop productivity. At lower concentrations, PGRs have the potential to enhance the physiological aspects of crops, contributing significantly in an increase crop yield 13.
Alkaline stress, an abiotic stress, typically induced by Na2CO3 and NaHCO3 which elevates soil pH levels inhibiting root growth, impair physiological functions, and disrupt cellular integrity. This stress disrupts ion homeostasis in roots, leading to the precipitation of Ca2+, Mg2+, and H2PO4−, while, the production of reactive oxygen species (ROS) is accelerated, which, interferes with metabolic pathways (Fan et al., 2021). High alkalinity caused by salts such as Na2CO3 and NaHCO3 pose significant harm to plant growth and nutrient absorption as evidenced in various crops 13,14.
In this study, foliar application of different concentrations of NAA (0, 50, 75, 100 ppm) showed significant improvement in growth parameters of squash plants under alkaline condition (Table 2). Alkalinity led to a decrease in plant length, fresh biomass, and leaf count, whereas the application of NAA ameliorated these reductions exhibiting its growth promoting effects. Similar reports have also been found for wheat, periwinkle, and tomato plants under alkaline stress conditions 15,16.
Alkaline stress also reduced the photosynthetic pigments, including chlorophyll a, b, total chlorophyll and carotenoids (Fig. 1). Photosynthetic pigments, key components of light reactions in photosynthesis, are sensitive to almost all types of stresses, including alkaline stress causing damage in the thylakoid membrane, the site where all different types of photosynthetic pigments accumulate and hence suppressing the pigment synthesis enzymes 17. Additionally, stress impairs the enzymes involved in carotenoid biosynthesis such as phytoene synthase causing reduction in carotenoids in fruits and vegetables 18,19. However the foliar application of NAA notably increased the pigment level restoring the photosynthetic effeicenct which initiates the synthesis of important signaling molecules to support plant growth and development 20. On the other hand, the findings of this study (Fig. 2) align with previous studies who showed that exogenous plant hormone like NAA alleviate abiotic stress by improving photosynthetic pigment retention 21,22.
The total free amino acid and total soluble protein, the primary metabolites of inorganic assimilation, decreased in squash plants under alkaline conditions, whereas exogenously applied NAA reversed these declines (Fig. 3a, b). Secondary metabolites (phenolics and flavonoids) increased under stress as a positive response (Fig. 3c, d). Foliar application of NAA enhanced these metabolites, corroborating findings in cotton under same stress conditions as studied in the current experiment 23.
The scavenging of reactive oxygen species (ROS) is carried out in plants via the antioxidant enzyme system 24. Antioxidant production in response to abiotic stress, such as salinity and water stress, is an effective strategy for combating oxidative stress 25. Plants generate antioxidants in response to stress, which operates as a protective mechanism against the detrimental effects of ROS. In response to stress-triggered ROS accumulation, plants activate antioxidant defense mechanisms to eliminate excess ROS from their cells 26.
Antioxidant enzyme activity including catalase (CAT) and peroxidase (POD) were clearly increased by alkaline stress (Table 2), as plant activated its defense systems to combat ROS accumulation, these results are in consistent with those of 27, who suggested that root cell damage, and consequently growth inhibition, in rice seedlings under alkaline stress is closely associated with ROS accumulation. Analysis of variance (ANOVA) also showed variations in morphological parameters (Table 3). Exogenous NAA application played its role in mitigating oxidative stress supporting plant defense mechanism. Mechanistically, NAA improved CAT and POD activities reducing oxidative damage under stress, moreover, the role of NAA in maintaining ionic homeostasis by improved ion transportation and reduced precipitation of Ca2+ and Mg2+ helps in plant growth and nutrient assimilation. These impacts may highlight the auxin-mediated homonal crosstalk to optimize signaling and metabolic programming 28,29.
Overall, the tested concentrations of NAA (0, 50, 75, and 100 ppm) applied exogenously improved the morphological, physiological and biochemical attributes of the squash plants grown in both the control and alkaline environments demonstrating its potential as a practical tool for managing crop productivity in stressed environments.
Conclusion
The current study demonstrates that foliar application of naphthalene acetic acid (NAA) effectively alleviates the adverse effects of alkaline stress on squash (Lagenaria siceraria), enhancing plant growth, yield, and biochemical attributes. Alkalinity-induced reductions in growth, photosynthetic pigments, and primary metabolites were significantly mitigated, with the foliar application of 75 mg/L NAA showing the most pronounced improvements. NAA not only boosted chlorophyll and carotenoid levels but also enhanced the synthesis of secondary metabolites like phenolics and flavonoids, essential for stress adaptation. Furthermore, it amplified the activities of antioxidant enzymes catalase (CAT) and peroxidase (POD), underscoring its role in fortifying plant defense mechanisms. These findings highlight the potential of NAA as a promising plant growth regulator to improve crop performance under both normal and stress-prone conditions, offering a practical approach to sustain agricultural productivity in alkaline soils. Further studies on molecular pathways and gene expression analysis will help to find out the precise mechanism underlying these responses
Data availability
All the data generated or analyzed during this study are included in this research article.
References
Yu, Z. et al. How plant hormones mediate salt stress responses. Trends Plant Sci. 25, 1117–1130 (2020).
Ahanger, M. A., Aziz, U., Alsahli, A. A., Alyemeni, M. N. & Ahmad, P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules 10, 42 (2020).
Luo, X. et al. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 229, 950–962 (2021).
Sun, J., He, L. & Li, T. Response of seedling growth and physiology of Sorghum bicolor (L.) Moench to saline-alkali stress. PLoS ONE 14, e220340 (2019).
Nisa, Z. U. et al. Strigolactone signaling gene from soybean GmMAX2a enhances the drought and salt-alkaline resistance in Arabidopsis via regulating transcriptional profiles of stress-related genes. Funct. Integr. Genomics 23(3), 216 (2023).
Mir, A. R., Siddiqui, H., Alam, P. & Hayat, S. Foliar spray of Auxin/IAA modulates photosynthesis, elemental composition, ROS localization and antioxidant machinery to promote growth of Brassica juncea. Physiol. Mol. Biol. Plants 26, 2503–2520 (2020).
An, M. et al. Application of compound material alleviates saline and alkaline stress in cotton leaves through regulation of the transcriptome. BMC Plant Biol. 20, 462 (2020).
Hyoung, S. et al. Cytokinin oxidase PpCKX1 plays regulatory roles in development and enhances dehydration and salt tolerance in Physcomitrella patens. Plant Cell Rep. 39, 419–430 (2020).
Wu, J. et al. Genome-wide comprehensive analysis of transcriptomes and small RNAs offers insights into the molecular mechanism of alkaline stress tolerance in a citrus rootstock. Hortic. Res. 6, 33 (2019).
Wen, T. et al. Changes in root architecture and endogenous hormone levels in two Malus root stocks under alkali stress. Sci. Hortic. 235, 198–204 (2018).
Arnon, D. I. Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 24(1) (1949) (Costa, A. G., Ribeiro, E., Braga, R. A., & Pinto, F. A. Measurement of volume of macaw palm fruit using traditional and the digital Moiré techniques. Rev. Bras. Engenharia Agríc. Ambient. 20, 152–157, 2016).
Chance B. & Maehly A. C. Assay of catalase and peroxidase, In (Colo Wick, S.P. & Kaplan, N.O. eds.) Methods in Enzymology. Vol. 76. (Academic Press, 1955).
Zhang, H., Sun, X. & Dai, M. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Commun. 3(1), 100228. ISSN 2590-3462. https://doi.org/10.1016/j.xplc.2021.100228 (2022).
Zhang, H. et al. Effects of four types of sodium salt stress on plant growth and photosynthetic apparatus in sorghum leaves. J. Plant Interact. 13(1), 506–513. https://doi.org/10.1080/17429145.2018.1526978 (2018).
Farghaly, F. A., Nafady, N. A. & Abdel-Wahab, D. A. The efficiency of arbuscular mycorrhiza in increasing tolerance of Triticum aestivum L. to alkaline stress. BMC Plant Biol. 22(1), 1–17 (2022).
Pahare, P. & Das, J. N. Effect of alpha-naphthalene acetic acid [NAA] on growth, flowering and yield of Vinca rosea cv. Catharanthus caramel. Int. J. Curr. Microbiol. Appl. Sci. 9(6), 1961–1967. https://doi.org/10.20546/ijcmas.2020.906.242 (2020).
Billah, M. et al. Exploring regulatory roles of plant thylakoid-bound proteins involved in abiotic stress responses. J. Plant Growth Regul. https://doi.org/10.1007/s00344-023-11207-5 (2024).
Davies, B. H. Carotenoids. In Chemistry and Biochemistry of Plant Pigments (ed. Goodwin, T. W.) 38–165 (Academic Press, 1976).
Petropulos, S. A., Stafilov, T. & Stefova, M. Polyphenolic content and antioxidant activity of grape varieties. J. Food Sci. Technol. 52(10), 6061–6071 (2015).
Shaheen, S., Naseer, S., Ashraf, M. & Akram, N. A. Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J. Plant Interact. 8(1), 85–96 (2016).
Cho, M., Boo, H. & Kim, J. Physiological roles of plant growth regulators under abiotic stresses. J. Plant. Res. 121(2), 101–113. https://doi.org/10.1007/s10265-008-0152-7 (2008).
Yang, W., Lin, L. & Zhang, J. Effect of pre-treatment on grape drying quality. J. Food Eng. 96(3), 287–294. https://doi.org/10.1016/j.jfoodeng.2009.09.002) (2009).
Jeber, B. A. & Khaeim, H. M. Effect of foliar application of amino acids, organic acids, and naphthalene acetic acid on growth and yield traits of wheat. Plant Arch. 19(2), 824–826 (2019).
Fan, Y. et al. Cotton transcriptome analysis reveals novel biological pathways that eliminate reactive oxygen species (ROS) under sodium bicarbonate (NaHCO3) alkaline stress. Genomics 113(3), 1157–1169 (2021).
Huang, H., Ullah, F., Zhou, D. X., Yi, M. & Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10, 800. https://doi.org/10.3389/fpls.2019.00800 (2019).
Hasanuzzaman, M. et al. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 22(17), 9326. https://doi.org/10.3390/ijms22179326 (2021).
Muzaffar, A. et al. Abiotic stress: Interplay between ROS production and antioxidant machinery, signaling, and ROS homeostasis. OBM Genet. 6(4), 1–20 (2022).
Luo, P. et al. An overview of the mechanisms through which plants regulate ROS homeostasis under cadmium stress. Antioxidants 13(10), 1174 (2024).
Fujita, M. & Hasanuzzaman, M. Approaches to enhancing antioxidant defense in plants. Antioxidants 11(5), 925 (2022).
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2025R393) of King Saud University, Riyadh, Saudi Arabia.
Funding
Researchers supporting the project (RSP2025R393) at King Saud University. Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Uswa Ashiq Nadeem wrote the original draft and performed the experiments; Naila Ali and Anis Ali Shah performed the analysis; Zaib-un Nisa and reviewed the article; and Toqeer Abbas and Muhammad Arif also reviewed and drafted the article; Mansour K. Gatasheh and Shifa Shaffique revised the article and help in funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nadeem, U.A., Ali, N., Zaib-un Nisa et al. Naphthalene acetic acid induced morphological and biochemical alterations in Lagenaria siceraria under alkaline stress. Sci Rep 15, 15747 (2025). https://doi.org/10.1038/s41598-025-99455-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99455-1