Abstract
The difference between hematocrit and serum albumin (HCT-ALB) demonstrates diagnostic significance in infectious diseases, yet the nonlinear relationship between HCT-ALB and hospital mortality in ICU patients with sepsis remains unexplored. This retrospective multicenter cohort study analyzed 7,546 ICU sepsis patients (mean age 66 ± 16 years) to elucidate the HCT-ALB-mortality relationship. Using Cox proportional hazards models with smooth curve fitting, we identified a U-shaped association: Threshold analysis revealed an inflection point at 6.1. Below this threshold, each unit HCT-ALB increase corresponded to reduced mortality risk (adjusted HR 0.986, 95%CI 0.972–0.999; P = 0.036). Conversely, values ≥ 6.1 predicted escalating risk (adjusted HR 1.048 per unit increase, 95%CI 1.037–1.060; P < 0.0001). Significant age interaction was observed (P for interaction < 0.05), with heightened mortality risk in elderly patients (≥ 65 years: HR 1.022, 95%CI 1.014–1.031). These findings establish HCT-ALB as a non-linear predictor of sepsis outcomes, emphasizing its critical threshold dynamics and age-dependent prognostic implications.
Similar content being viewed by others
Introduction
Sepsis is defined as a life-threatening condition characterized by systemic physiological and biochemical derangements, culminating in acute organ dysfunction and representing a leading cause of in-hospital mortality1,2,3,4. As one of the most prevalent diagnoses in intensive care units (ICUs), sepsis continues to account for substantial global ICU mortality despite decades of research elucidating its pathophysiology and therapeutic strategies5,6. While numerous severity scoring systems exist for critically ill patients (e.g., SOFA, APACHE II), their clinical utility is often limited by complex multi-parameter requirements7,8,9,10. This underscores the urgent need to identify pragmatic prognostic biomarkers that balance predictive accuracy with clinical feasibility for sepsis management.
In clinical practice, alterations in hematocrit (HCT) and serum albumin (ALB) levels are well-documented biomarkers in patients with systemic inflammatory conditions. Under physiological conditions, healthy individuals maintain stable HCT and ALB levels within reference ranges of 40–45% and 35–45 g/L, respectively11. As a whole-blood parameter, HCT quantifies the volumetric proportion of erythrocytes to plasma. It serves as both a prognostic marker in critical illness and a guide for fluid resuscitation strategies12. Accumulating evidence indicates that HCT independently predicts mortality risk in sepsis and septic shock populations13,14. A landmark retrospective cohort analysis demonstrated that patients with subnormal HCT levels (defined as < 42% for males and < 37% for females) exhibited a 58.9% increase in 30-day mortality compared to those with normal ranges (HR = 1.589, 95% CI: 1.009–2.979, P < 0.05)12. ALB, the predominant plasma protein, exerts critical physiological functions through maintaining colloid osmotic pressure and regulating endothelial permeability15. Substantial clinical data validate ALB as a robust predictor of mortality across critically ill cohorts16,17. Longitudinal studies reveal that dynamic ALB trajectories—including admission levels, nadir concentrations, and declining trends—constitute independent mortality predictors. Notably, a pronounced negative ALB trend correlates with a 70.6% reduction in survival probability (P < 0.001)16. Despite the high prevalence of hypoalbuminemia in ICU settings (reported in > 60% of sepsis cases) and its strong association with adverse outcomes18, ALB monitoring remains underutilized. Based on current evidence, routine serial ALB measurements should be considered an essential component of sepsis management to improve risk stratification and guide therapeutic decisions.
Recent studies highlight the importance of integrating biomarkers to capture complex pathophysiological processes in sepsis. For instance, while HCT reflects hemoconcentration due to fluid loss and ALB indicates capillary leakage, their combined difference (HCT-ALB) may better represent the net effect of these opposing mechanisms19. While prior studies have explored HCT-ALB as a diagnostic biomarker for infection (AUC 0.87 vs. 0.60 in non-infectious conditions)20 and its association with mortality in specific populations (e.g., elderly sepsis patients)21, the nonlinear relationship between HCT-ALB and outcomes in sepsis remains uncharacterized. Our study extends this by analyzing a large multicenter cohort to reveal a U-shaped relationship between HCT-ALB and mortality in sepsis, a novel finding that highlights the dual risks of extreme hemoconcentration and hypoalbuminemia. This pattern aligns with nonlinear risk profiles observed in other critical illnesses, such as the J-shaped association between hematocrit-to-albumin ratio (HAR) and acute kidney injury in acute pancreatitis22.
Methods
Data source
This retrospective observational study utilized data from the eICU Collaborative Research Database (eICU-CRD)23, an international repository containing de-identified electronic medical records of over 200,000 ICU patients from 208 U.S. hospitals (2014–2015)23. Access requires completion of the PhysioNet Review Board’s data use agreement certification process and compliance with the Health Insurance Portability and Accountability Act (HIPAA) Safe Harbor provisions for protected health information. Data analysis was conducted through the Collaborative Institutional Training Initiative (CITI) program-certified researchers (record ID: 59205891, 21-Oct-2023). The study received institutional review board exemption as it involved no patient intervention and utilized HIPAA-compliant de-identified data validated by Privacert (Cambridge, MA). All procedures adhered to Declaration of Helsinki principles and relevant regulatory standards.
Study population
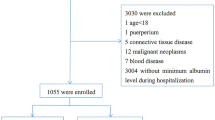
The study initially included 23,136 patients diagnosed with sepsis upon ICU admission. After applying Sepsis-3 diagnostic criteria (confirmed infection with SOFA score ≥ 2)1, 13,024 adult patients (≥ 18 years) were identified from the eICU-CRD database. Exclusion criteria comprised: (1) missing ICU outcome data, (2) unavailable HCT measurements, (3) absent ALB records, and (4) biologically implausibleHCT-ALB values, which were excluded to mitigate potential data entry errors and unit conversion inconsistencies inherent to multicenter retrospective databases. Figure 1 illustrates the participant selection flowchart.
Outcomes and variables
The primary outcome was hospital mortality. All participant data were extracted from the eICU-CRD at the first record after ICU admission. Baseline characteristics included: age (year), sex, BMI (BMI were grouped into underweight (BMI < 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥ 30.0 kg/m2))24, ethnicity, and hospital length of stay (days). Physiological parameters comprised temperature (°C), respiratory rate (bpm), heart rate (HR, /min) and mean blood pressure (MBP, mmHg). Laboratory indices encompassed HCT (%), ALB (g/L), WBC (109/L), creatinine (mg/dL), BUN (mg/dL), and lactate level (mmol/L) were collected. Comorbidities included acute immunodeficiency syndrome (AIDS), metastatic cancer, congestive heart Failure (CHF), acute myocardial infarction (AMI), pneumonia, and arrhythmia. And site of infection, ventilation use, intubated use, dialysis use, and vasopressor use were included in this study. Disease severity was assessed using SOFA score, APACHE IV score, Acute Physiology Score III, and Glasgow Coma Scale (GCS) score.
Statistical analysis
All statistical analyses were performed using EmpowerStats (X&Y Solutions Inc., Boston, MA) and R software version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria)25. To evaluate the association between HCT-ALB and hospital mortality, participants were stratified into tertiles based on baseline HCT-ALB values. Continuous variables with normal distributions are presented as mean ± standard deviation, while skewed variables are summarized as median (interquartile range).Categorical variables are expressed as frequency or percentages. Between-group comparisons were conducted using: (1)one-way ANOVA for normally distributed continuous variables, (2)Kruskal–Wallis H test for non-normally distributed continuous variables, (3)and chi-square test for categorical variables, as appropriate.
To identify independent predictors of hospital mortality, we performed Cox proportional hazards regression analyses in sequential stages. First, univariate Cox regression was conducted for all candidate variables. Variables demonstrating statistical significance (P < 0.05) or clinical relevance were subsequently entered into multivariable models. Three models were constructed: (1) Crude model: No covariate adjustment, (2) Model I: Adjusted for demographic variables (sex and age), (3) Model II: Fully adjusted for age (years), sex, BMI (kg/m2), SOFA score, ventilation use, dialysis use, site of infection, temperature (°C), heart rate(HR, /min), MBP (mmHg), WBC (109/L), vasopressor use, AIDS, metastatic cancer, diabetes, CHF, AMI, pneumonia, arrhythmia. Results are reported as hazard ratios (HR) with 95% confidence intervals (CI). Covariate selection followed established epidemiological principles, prioritizing variables with established biological plausibility and clinical significance in sepsis outcomes26.
To assess potential nonlinear associations between HCT-ALB and hospital mortality, we employed a multi-stage analytical approach. First, Cox proportional hazards regression with restricted cubic splines (RCS) was used to visualize dose-response relationships through smooth curve fitting. Threshold effects were subsequently quantified using piecewise linear regression models, with optimal inflection points determined via grid search algorithms. The superiority of nonlinear models over linear assumptions was statistically validated through log-likelihood ratio tests comparing one-segment versus two-segment specifications27,28,29. Sensitivity analyses included: (1) treating HCT-ALB as tertile-based categorical variables with trend tests, and (2) excluding extreme HCT-ALB outliers.
Subgroup analyses were conducted using stratified Cox models, with interaction effects evaluated through likelihood ratio tests comparing models with versus without interaction terms. All models adjusted for clinically relevant covariates identified in univariate screening (P < 0.05). To evaluate the predictive performance of biomarkers, receiver operating characteristic (ROC) curve analysis was performed to calculate the area under the curve (AUC) and identify the best threshold for HCT-ALB, HCT, and ALB in predicting sepsis mortality. Comparative analyses evaluated incremental value beyond standalone HCT-ALB, HCT and ALB measurements using DeLong’s method. Eventually, a P-value < 0.05 was considered statistically significant.
Ethics approval and consent to participate
Data was extracted from the eICU-CRD23 in accordance with the data usage agreement (our record ID: 59205891, 21-Oct-2023) by the PhysioNet review committee. The database used is released under the HIPAA safe harbor provision. This study involved a retrospective analysis using an anonymous database for research purposes and did not require ethical approval from the local ethics committee.
Results
Baseline characteristics
The final analytic cohort comprised 7,546 sepsis patients from the eICU-CRD database, with a mean age of 66 ± 16 years and 47% (n = 3,581) female representation. Table 1 stratifies baseline characteristics across HCT-ALB tertiles, including demographics, laboratory profiles, severity of illness, site of infection, comorbidities, vasopressor use, intubated use, ventilation, and dialysis. Compared to lower tertiles, patients in the highest HCT-ALB tertile exhibited significantly elevated HCT (35.04 ± 6.04% vs. 26.13 ± 4.93%) and reduced ALB levels (20.58 ± 5.18 vs. 27.97 ± 5.29 g/L). Significant inter-tertile differences (P < 0.05) were observed in: sex, ethnicity, temperature, heart rate, mean BP, laboratory results, severity of illness (excluding SOFA score), site of infection, pneumonia status, intubated use, ventilation use, and dialysis use.
Hospital mortality
The overall hospital mortality rate was 21.96% (1,657/7,546), with mortality rates increasing across HCT-ALB tertiles: 19.61% (487/2,483) in the lowest tertile (-20.0 to 3.0), 18.80% (478/2,542) in the middle tertile (3.1–9.3), and 27.45% (692/2,521) in the highest tertile (9.4–38.8) (Table 1).
Besides, non-survivors exhibited significantly higher HCT-ALB levels compared to survivors (7.68 ± 8.59 vs. 5.93 ± 7.17, P < 0.001) (Supplementary Table 1).
Unadjusted association between baseline variables and hospital mortality
Univariate analysis demonstrated a dose-dependent association between HCT-ALB and mortality, with each 1-unit increase corresponding to a 2.5% higher unadjusted mortality risk (HR = 1.025, 95% CI: 1.019–1.032; P < 0.00001). Compared to the lowest HCT-ALB tertile (-20.0 to 3.0), the highest tertile (9.4–38.8) exhibited a 40.5% increased mortality risk (HR = 1.405, 95% CI: 1.251–1.577; P < 0.00001). Otherwise, in the dataset, HRs for hospital mortality were statistically significant for age, African American, temperature, heart rate, mean BP, WBC, site of infection (renal/UTI (including bladder), and cutaneous/soft tissue), comorbidities (metastatic cancer, and diabetes), vasopressor or ventilation use, and severity of illness (Table 2).
Relationship between HCT-ALB and hospital mortality
This study evaluated the relationship between HCT-ALB and hospital mortality using Cox proportional hazards models across three sequential adjustment tiers (Table 3) The unadjusted model demonstrated a significant positive association per 1-unit HCT-ALB increase (HR = 1.025, 95% CI:1.019–1.032; P < 0.00001), with the highest tertile (9.4–38.8) exhibiting 40.5% greater mortality risk versus the lowest tertile (-20.0-3.0) (HR = 1.405, 95% CI:1.251–1.577; P < 0.00001). After adjusting for demographic factors (Model I: age, sex), the association persisted (per-unit HR = 1.026, 95% CI:1.020–1.033; P < 0.00001), with tertile 3 showing 42.3% elevated risk (HR = 1.423, 95% CI:1.267–1.598; P < 0.00001). The fully adjusted model (Model II) incorporating clinical covariates (BMI, SOFA, vital signs, laboratory values, interventions, comorbidities) maintained significance, though marginally attenuated (per-unit HR = 1.022, 95% CI:1.015–1.029 vs. Model I HR = 1.026; both P < 0.00001), with tertile 3 risk increasing by 34.3% (HR = 1.343, 95% CI:1.184–1.522; P < 0.00001). Sensitivity analyses treating HCT-ALB as tertiles confirmed consistent dose-response relationships across all models (P < 0.05). These findings establish HCT-ALB as an independent mortality predictor in sepsis.
Identification of nonlinear relationship
We assessed potential nonlinear associations between HCT-ALB and hospital mortality in sepsis through smoothing spline fitting analysis. The multivariable-adjusted model revealed a U-shaped dose-response relationship (Fig. 2), with an the inflection point at HCT-ALB = 6.1 (Table 4). Log-likelihood ratio testing confirmed distinct threshold effects between low (< 6.1) and high (≥ 6.1) ranges (χ²=23.6, P < 0.001). Below the threshold, each 1-unit HCT-ALB increase was associated with 1.4% mortality reduction (HR = 0.986, 95%CI:0.972–0.999; P = 0.0356). Conversely, above the threshold, every 1-unit increment corresponded to 4.8% mortality increase (HR = 1.048, 95%CI:1.037–1.060; P < 0.0001).
Smoothing spline fitting curve. Associations between the HCT-ALB and Hospital Mortality in ICU patients with sepsis. A threshold, nonlinear association between HCT-ALB and Hospital Mortality was found in a Cox proportional hazards regression model. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. All adjusted for Age; Sex; BMI; SOFA Score; Ventilation; Dialysis; Site of Infection; Temperature; Heart Rate; Mean BP; WBC; Vasopressor Use, AIDS; Metastatic Cancer; Diabetes; CHF; AMI; Pneumonia; Arrhythmia.
Subgroup analysis and interaction test
To identify potential effect modifiers, we conducted subgroup analyses evaluating interactions between HCT-ALB and key demographic/clinical variables (age, sex, BMI, ventilation use, diabetes status, CHF status, pneumonia status, and arrhythmia) on mortality risk (Table 5). Significant effect modification was observed only for age (interaction P < 0.05), with stratified analysis revealing heightened HCT-ALB-associated mortality risk in elderly sepsis patients (≥ 65 years: HR = 1.022 per unit increase, 95%CI = 1.014–1.031; P < 0.0001).
Predictive value of HCT-ALB in hospital mortality
We performed ROC analysis to evaluate the predictive capacity of HCT-ALB, HCT, and ALB for hospital mortality. The AUC of HCT-ALB was 0.5611 (95%CI:0.5448, 0.5775), demonstrating intermediate discrimination that exceeded HCT(AUC = 0.5335, 0.5173–0.5498) but underperformed ALB (AUC = 0.6182, 0.6027–0.6337) (Supplementary Fig. 1 and Supplementary Table 2). The optimal HCT-ALB threshold (11.45) provided 32.5% sensitivity and 78.8% specificity for mortality prediction (Supplementary Table 2). A direct statistical comparison using the DeLong test revealed that the AUC of HCT-ALB (0.5611) was not significantly superior to HCT alone (0.5335, P = 0.12) but was inferior to ALB (0.6182, P < 0.001) (Supplementary Table 3).
Discussion
This multicenter retrospective study, utilizing the large-scale eICU-CRD database, demonstrated a U-shaped association between HCT-ALB values and hospital mortality in sepsis patients. Both elevated and reduced HCT-ALB levels were associated with increased mortality risk, with the highest risk observed at extremes (lowest and highest tertiles). This relationship persisted after multivariable adjustment for age, sex, BMI, SOFA score, ventilation use, dialysis use, site of infection, temperature, HR, MBP, WBC, vasopressor use, AIDS, metastatic cancer, diabetes, CHF, AMI, pneumonia, arrhythmia. Stratified analyses and interaction testing revealed significant effect modification by age (P for interaction < 0.05), with stronger associations observed in elderly patients (≥ 65 years). To our knowledge, this represents the first documentation of a nonlinear relationship between HCT-ALB and mortality in critically ill sepsis populations.
The identification of reliable biomarkers for sepsis diagnosis, therapeutic monitoring, and prognosis remains a critical need in clinical practice. Despite advances, sepsis persists as the leading cause of infection-related mortality in ICUs30, underscoring the urgency for early prognostic stratification. Hypoalbuminemia, a prevalent finding in critical illness31,32, has been consistently associated with poor sepsis outcomes15. A notable finding in our study is that all HCT-ALB tertiles exhibited hypoalbuminemia (Tertile 1:27.97 g/L, Tertile 2:23.69 g/L, Tertile 3:20.58 g/L), with levels consistently below the normal range (35–45 g/L)11. Especially, the highest HCT-ALB tertile exhibited progressively lower albumin levels, aligning with recent multicenter evidence from 5,894 critically ill adults, where admission hypoalbuminemia independently predicted 30-day mortality (adjusted HR = 1.89, 95%CI:1.45–2.47)33.
Emerging evidence highlights the prognostic value of hematological parameters in sepsis. Whole blood viscosity, erythrocyte aggregation, and deformability have been implicated as mortality risk factors in sepsis and septic shock34. Furthermore, HCT demonstrates U-shaped associations with mortality across diverse populations, including those with heart failure, ischemic heart disease, and end-stage renal disease35,36,37,38,39. A recent study of 2,057 sepsis patients revealed that subnormal HCT levels (male ≤ 42%, female ≤ 37%) at ICU admission independently predicted 30-day mortality (HR = 1.58, 95%CI:1.21–2.06)12, aligning with prior sepsis cohorts14,40. Our study extends these observations by demonstrating a U-shaped relationship between HCT-ALB and hospital mortality—a novel finding in sepsis research. Patients in the middle HCT-ALB tertile exhibited the lowest mortality (18.8%), compared to the highest (27.45%) and lowest tertiles (19.61%; P < 0.001). This pattern mirrors HCT-associated mortality curves reported in cardiovascular diseases35, with elevated HCT-ALB values reflecting dual pathophysiology: hemoconcentration and hypoalbuminemia. Specifically, the highest tertile showed marked HCT elevation (35.04% vs. 26.13% in tertile 1) and ALB reduction (20.58 vs. 27.97 g/L; P < 0.001), consistent with sepsis-related capillary leakage and hemodilution patterns21.A retrospective analysis utilizing two large databases determined the optimal HCT-ALB cutoff for ICU mortality through ROC curve Youden Index evaluation, identifying 6.721, as the critical threshold—a value closely aligned with our study’s findings. Threshold analysis in our research revealed that ICU sepsis patients exhibited the lowest in-hospital mortality rate at an HCT-ALB level of 6.1. Deviation from this critical value (either above or below 6.1) consistently demonstrated an upward trend in mortality risk. This discovery provides novel evidence to inform clinical management strategies for sepsis. Our findings align with emerging evidence that multi-biomarker approaches improve risk stratification in critical care21. Specifically, the U-shaped relationship between HCT-ALB and mortality suggests that both extremes—severe hemoconcentration (e.g., dehydration) and profound hypoalbuminemia (e.g., capillary leakage)—confer elevated risk. This dual-risk profile contrasts with linear associations reported for HCT or ALB alone (References12,16), highlighting the unique prognostic value of their combined difference. Importantly, our threshold analysis (inflection point at 6.1) provides actionable cutoffs for clinical decision-making, a contribution not addressed in prior studies22.
Notably, Wang et al.21 reported that elevated HCT-ALB levels correlate with 1.41-fold and 1.27-fold increased risks of ICU and hospital mortality, respectively, in elderly sepsis patients. Consistent with these findings, our study identified a statistically significant interaction between HCT-ALB and age (P for interaction < 0.05), with adjusted hospital mortality risk rising by 1.022-fold (95% CI: 1.014–1.031; P < 0.0001) per unit HCT-ALB increase in patients aged ≥ 65 years old.
In our cohort, both the HCT and ALB levels across all groups remained below physiological norms. Under normal conditions, capillary endothelial gaps (6–7 nm) restrict erythrocyte permeation41, while ALB synthesis and catabolism remain balanced to preserve plasma oncotic pressure11. Sepsis fundamentally disrupts this equilibrium: accelerated ALB capillary leakage (up to 300%/hour)11,42,43 and systemic inflammation-induced endothelial dysfunction precipitate hypoproteinemia. This pathological cascade—compounded by hypermetabolism-driven ALB consumption, enteric protein loss, chronic hypoxia-mediated HCT elevation, and anemia—collectively alters the HCT-ALB ratio42.
Additionally, while non-survivors exhibited significantly higher HCT-ALB values than survivors (7.684 ± 8.585 vs. 5.931 ± 7.165, P < 0.001), the parameter demonstrated limited prognostic utility (AUC = 0.5611), underperforming isolated ALB measurements. This constrained predictive capacity likely stems from sepsis mortality’s multifactorial nature, involving age, comorbidities, multiorgan failure, and refractory shock44,45. Furthermore, therapeutic interventions like fluid resuscitation46 may dilute blood components, transiently reducing HCT and ALB levels, thereby confounding HCT-ALB interpretation. Nevertheless, the metric’s operational simplicity supports its potential role in early risk stratification within ICU settings.
The clinical rationale for using HCT-ALB lies in its dual reflection of hemoconcentration (elevated HCT due to fluid loss) and hypoalbuminemia (from capillary leakage or impaired synthesis), both central to sepsis pathophysiology. Unlike ratios such as serum to ascites albumin gradient (SAAG) or oxygenation index (PaO₂/FiO₂ ratio), which are interpretable only in specific contexts (e.g., ascites or respiratory failure), HCT-ALB aims to capture systemic derangements in sepsis. However, its interpretation requires caution: identical HCT-ALB values (e.g., HCT-ALB = 10) may arise from distinct combinations (HCT 45% - ALB 35 g/L vs. HCT 25% - ALB 15 g/L), each reflecting different clinical states. While our study identifies a U-shaped relationship between HCT-ALB and mortality, we acknowledge that this association does not imply causality. The observational nature of our data limits our ability to control for unmeasured confounders, such as dynamic changes in fluid balance or nutritional status during ICU stay. Additionally, the modest AUC of HCT-ALB (0.5611) underscores the need for integrating it with other clinical parameters (e.g., SOFA score, lactate levels) to enhance prognostic accuracy. Future prospective studies with serial biomarker measurements are warranted to validate these findings. Although HCT-ALB independently predicts mortality, it showed a nonsignificant trend toward superior discrimination compared to HCT alone (P = 0.12), it was significantly inferior to ALB (P < 0.001). This supports the role of HCT-ALB as a complementary tool rather than a replacement for established biomarkers. Future research should explore integrating HCT-ALB with dynamic parameters (e.g., fluid balance) to enhance predictive accuracy.
Strengths and limitations of the study
Several limitations should be noted. First, the retrospective design precludes causal inference, and residual confounding (e.g., unrecorded interventions like fluid resuscitation) may bias the observed associations. Second, HCT-ALB values were measured only at ICU admission, whereas dynamic changes during treatment may better reflect disease progression. It is inevitable that treatment measures, such as fluid resuscitation, could impact the detection of HCT-ALB levels and the survival rate in sepsis patients. Finally, we did not evaluate the nutritional and anemia status of the patients, which are important factors affecting HCT and ALB levels.
This study demonstrates three key strengths. First, we established a non-linear association between HCT-ALB and hospital mortality in sepsis through rigorous threshold analysis. Second, our analytical approach incorporated comprehensive adjustment for clinical confounders—exceeding the methodological rigor of prior investigations—which strengthens result validity. Furthermore, HCT-ALB represents a clinically accessible metric derived from routine laboratory parameters, enabling efficient risk stratification and early therapeutic decision-making. Importantly, the generalizable findings originate from a multicenter cohort of 7,546 patients, ensuring robust external validity.
Conclusion
This study demonstrates a U-shaped relationship between HCT-ALB and mortality in sepsis, a novel finding that extends prior work on linear associations. While HCT-ALB integrates hemoconcentration and hypoalbuminemia—key features of sepsis pathophysiology—its clinical interpretability is less direct than established ratios. Clinicians should use HCT-ALB cautiously, contextualizing it with volume status and serial ALB measurements.
Data availability
Data were fully available at https://eicu-crd.mit.edu/.
References
Singer, M. et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA 315 (8), 801–810 (2016).
Gotts, J. E. & Matthay, M. A. Sepsis: pathophysiology and clinical management. BMJ 353, i1585 (2016).
Torio, C. M. & Moore, B. J. National inpatient hospital costs: The most expensive conditions by payer, 2013. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD) (2006).
Liu, V. et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 312 (1), 90–92 (2014).
Maharaj, R., Mcguire, A. & Street, A. Association of annual intensive care unit sepsis caseload with hospital mortality from sepsis in the united Kingdom, 2010–2016. JAMA Netw. Open. 4 (6), e2115305 (2021).
Luo, M. et al. Association between hematocrit and the 30-day mortality of patients with sepsis: A retrospective analysis based on the large-scale clinical database MIMIC-IV. PloS One. 17 (3), e0265758 (2022).
Le Gall, J. R., Lemeshow, S. & Saulnier, F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270 (24), 2957–2963 (1993).
Knaus, W. A. et al. APACHE II: a severity of disease classification system. Crit. Care Med. 13 (10), 818–829 (1985).
Zimmerman, J. E. et al. Acute physiology and chronic health evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit. Care Med. 34 (5), 1297–1310 (2006).
Yuan, Z. N. et al. A nomogram for predicting hospital mortality of critical ill patients with sepsis and cancer: a retrospective cohort study based on MIMIC-IV and eICU-CRD. BMJ Open 13 (9), e072112 (2023).
Vercueil, A., Grocott, M. P. & Mythen, M. G. Physiology, pharmacology, and rationale for colloid administration for the maintenance of effective hemodynamic stability in critically ill patients. Transfus. Med. Rev. 19 (2), 93–109 (2005).
Luo, M. D. et al. Association between hematocrit and the 30-day mortality of patients with sepsis: A retrospective analysis based on the large-scale clinical database MIMIC-IV. PloS One 17(3) (2022).
Block, S. et al. Sepsis indicators in acute pancreatitis. Pancreas 2 (5), 499–505 (1987).
Juncal, V. R. et al. Clinical impact of sepsis at admission to the ICU of a private hospital in Salvador, Brazil. Jornal Brasileiro De Pneumologia. 37 (1), 85–92 (2011).
Takegawa, R. et al. Serum albumin as a risk factor for death in patients with prolonged sepsis: an observational study. J. Crit. Care. 51, 139–144 (2019).
Kendall, H., Abreu, E. & Cheng, A. L. Serum albumin trend is a predictor of mortality in ICU patients with Sepsis. Biol. Res. Nurs. 21 (3), 237–244 (2019).
Arnau-Barrés, I. et al. Serum albumin is a strong predictor of sepsis outcome in elderly patients. Eur. J. Clin. Microbiol. Infect. Dis. 38 (4), 743–746 (2019).
Infusino, I. & Panteghini, M. Serum albumin: accuracy and clinical use. Clin. Chim. Acta. 419, 15–18 (2013).
Sheng, S. et al. Association between hemoglobin and in-hospital mortality in critically ill patients with sepsis: evidence from two large databases. BMC Infect. Dis. 24 (1), 1450 (2024).
Dai, D. M. et al. Difference in hematocrit and plasma albumin levels as an additional biomarker in the diagnosis of infectious disease. Archives Med. Sci. 16 (3), 522–530 (2020).
Wang, Z. et al. The relationship between hematocrit and serum albumin levels difference and mortality in elderly sepsis patients in intensive care units-a retrospective study based on two large database. BMC Infect. Dis. 22 (1), 629 (2022).
Wu, W. et al. Association between hematocrit-to-albumin ratio and acute kidney injury in patients with acute pancreatitis: a retrospective cohort study. Sci. Rep. 14 (1), 27113 (2024).
Pollard, T. J. et al. The eICU collaborative research database, a freely available multi-center database for critical care research. Sci. Data. 5, 180178 (2018).
Bhaskaran, K. et al. Association of BMI with Overall and cause-specific Mortality: a population-based Cohort Study of 3.6 Million Adults in the UK 6(12), 6944–953 (Lancet Diabetes & Endocrinology, 2018).
< EmpowerStats & Boston MA X&Y Solutions, Inc. Available at https://www.empowerstats.com.pdf
Jaddoe, V. W. et al. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ 348, g14 (2014).
Lin, L., Chen, C. Z. & Yu, X. D. The analysis of threshold effect using empower stats software. Zhonghua Liu Xing Bing Xue Za Zhi. 34 (11), 1139–1141 (2013).
Yu, X. D., Cao, L. L. & Yu, X. G. Elevated cord serum manganese level is associated with a neonatal high ponderal index. Environ. Res. 121, 79–83 (2013).
Yu, X. C. et al. Threshold effects of moderately excessive fluoride exposure on children’s health: A potential association between dental fluorosis and loss of excellent intelligence. Environ. Int. 118, 116–124 (2018).
Stanski, N. L. & Wong, H. R. Prognostic and predictive enrichment in sepsis. Nat. Rev. Nephrol. 16 (1), 20–31 (2020).
Erstad, B. L. Serum albumin levels: who needs them?? Ann. Pharmacother. 55 (6), 798–804 (2021).
Li, W., Li, N. & Li, S. [Relationship between postoperative immediate serum albumin level and postoperative acute kidney injury after major abdominal surgery in critically ill patients]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 33 (8), 955–961 (2021).
Jellinge, M. E. et al. Hypoalbuminemia is a strong predictor of 30-Day All-Cause mortality in acutely admitted medical patients: A prospective, observational, cohort study. PLoS One 9(8) (2014).
Totsimon, K. et al. The relationship between hemorheological parameters and mortality in critically ill patients with and without sepsis. Clin. Hemorheol. Microcirc. 65 (2), 119–129 (2017).
Boffetta, P. et al. A U-shaped relationship between haematocrit and mortality in a large prospective cohort study. Int. J. Epidemiol. 42 (2), 601–615 (2013).
Sharma, R. et al. Haemoglobin predicts survival in patients with chronic heart failure: a substudy of the ELITE II trial. Eur. Heart J. 25 (12), 1021–1028 (2004).
Mozaffarian, D., Nye, R. & Levy, W. C. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE). J. Am. Coll. Cardiol. 41 (11), 1933–1939 (2003).
Elwood, P. C. et al. Mortality and anaemia in women. Lancet 1 (7863), 891–894 (1974).
Harrison, K. A., Rossiter, C. E. & Tan, H. Family planning and maternal mortality in the third world. Lancet 1 (8495), 1441 (1986).
Chaturvedi, R. et al. The association of preoperative hematocrit with adverse events following exploratory laparotomy in septic patients: A retrospective analysis. J. Intensive Care Med. 37 (1), 46–51 (2022).
Guyton, A. C. & Hall, J. E. The Microcirculation and the Lymphatic System: Capillary Fluid Exchange, Interstitial Fluid, and Lymph Flow (Textbook of Medical Physiology, 2011).
Fleck, A. et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet 1 (8432), 781–784 (1985).
Nicholson, J. P., Wolmarans, M. R. & Park, G. R. The role of albumin in critical illness. Br. J. Anaesth. 85 (4), 599–610 (2000).
Rhee, C. et al. Prevalence, underlying causes, and preventability of Sepsis-Associated mortality in US acute care hospitals. JAMA Netw. Open. 2(2) (2019).
Vincent, J. L., Nelson, D. R. & Williams, M. D. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit. Care Med. 39 (5), 1050–1055 (2011).
Levy, M. M. et al. The surviving Sepsis campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 36 (2), 222–231 (2010).
Acknowledgements
The authors gratefully acknowledge Dr. Letian Xue for his professional support in data acquisition and collection.
Author information
Authors and Affiliations
Contributions
QL and WL: study design, data collection, data analysis, original draft writing and editing. SZ and XC: data collection and data analysis. PS: study design, data analysis, original draft editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Q., Lu, W., Zhou, S. et al. A U shaped association between the HCT-ALB and hospital mortality in patients with sepsis. Sci Rep 15, 14785 (2025). https://doi.org/10.1038/s41598-025-99459-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99459-x