Abstract
Glioblastoma (GBM) is the most common and deadly malignant tumor of the nervous system. RIMS1, a member of the RAS gene superfamily, plays a critical role in signaling pathways regulating cell growth and differentiation. However, the prognostic value of RIMS1, particularly its relationship with immune cell infiltration in gliomas, has not been fully explored. RIMS1 expression in glioblastoma cells and tissues was assessed using bioinformatics platforms. The association between RIMS1 expression levels and overall survival was analyzed using Kaplan-Meier analysis and Cox regression model. To evaluate the proliferative and migratory capacity of GBM cells, we conducted CCK-8, colony formation, transwell, and scratch assays. With data from The Cancer Genome Atlas (TCGA), we investigated the correlation between RIMS1 expression and immune cell infiltration levels and assessed the prognostic impact of RIMS1 on GBM patient survival, focusing on its potential involvement in immune pathways. Lower RIMS1 expression was associated with poorer overall survival and was linked to patient age, gender, and tumor grade. Importantly, RIMS1 expression showed a significant correlation with immune cell infiltration levels, suggesting that RIMS1 influences glioblastoma survival, at least in part, through immune-related mechanisms. In glioblastoma patients, elevated RIMS1 expression may serve as an independent prognostic biomarker, potentially through its impact on immune cell infiltration.
Similar content being viewed by others
Introduction
The overall survival of GBM patients is short-lived, with no current cure, while neurological dysfunction and decreased quality of life due to the tumor are devastating to patients and their families1,2,3. Current treatments mainly include surgical resection, radiotherapy, chemotherapy, gene-targeted therapy and tumor treating fields (TT Fields)4,5,6, but their therapeutic effects are limited.
The clinical use of immunotherapy has faced limitations due to conflicts with current mainstream tumor chemotherapy regimens. With the continuous discovery of various immune regulatory loci, we have gathered more evidence on drug effectiveness, and many researchers have begun focusing their studies on immunotherapeutic regimens for tumors7. Many immune pathways are involved in the process of tumor cell apoptosis, and an effective immune response can improve clinical tumor treatment8. Immunotherapeutic regimens are characterized by a low impact on cellular activity and significant improvement in patient prognosis9. However, in the process of growth and proliferation, some tumor cells develop immune tolerance, effectively avoiding recognition and clearance by the immune system and promoting tumor development and deterioration10.
Ras proteins are membrane-bound GTP/GDP-binding proteins with molecular weight of approximately 21 kDa, localized on the inner side of the cell membrane, and play an important role in transmitting signals for cell growth and differentiation11, belonging to the group of guanosine triphosphate (GTP)-binding proteins (a kind of coupling factor for cellular information transmission), which regulate the transmission of information through the interconversion of GTP and guanosine diphosphate (GDP). Mutations in RAS proteins are involved in the development of a variety of tumors including breast, lung, colorectal, and pancreatic cancers12,13,14. Targeting clinically important RAS mutations (e.g., G12V, G13D, and A146T) has been problematic due to the lack of reactive amino acid residues or hydrophobic pockets in the proteins15. The protein encoded by RIMS1 is a member of the RAS gene superfamily that regulates synaptic action at synaptic vesicles and is also involved in the regulation of voltage-gated calcium channels during neurotransmitter and insulin release16. Mutations in RIMS1 are thought to be associated with cognitive function and have been linked to the cause of cone retinal dystrophy17. There are multiple transcript variants of this gene encoding different isoforms. Compared to other organ systems, RIMS1 has a high expression in brain tissue18. Recent studies have demonstrated that reduced levels of RIMS1 are involved in the progression of a variety of craniocerebral-related diseases. For example, a study by Wen Zhen et al.19 found that silencing of RIMS1 expression led to the proliferation of M2 macrophages, which indirectly promoted the onset and progression of LGG, and a study by Byron Andrews et al.18 found that RIMS1 expression was markedly elevated in the brains of neurodegenerative and senescent mammals. Furthermore, high expression of RIMS1 in gastric cancer correlates negatively with patient survival19. Yang et al.20 demonstrated that RIMS1 expression was downregulated in adamantinomatous craniopharyngiomas. Moreover, Zhang et al.21 reported the prognostic values of RIMS1 expression in pilocytic astrocytomas, and Zhang et al.22 utilized a bioinformatics approach to identify RIMS1 as a potential immunotherapeutic target in diffuse gliomas. However, it is unclear whether RIMS1 expression levels are associated with immune cell infiltration in tumors and OS in GBM patients.
In this research, we verified the expression level of RIMS1 in GBM cells with the use of experimental and bioinformatics methods. In addition, our study proved the association between RIMS1 expression level and prognosis of GBM patients. Finally, we found a significant relevance between low RIMS1 expression and immune infiltration.
Materials and methods
Cell culture, cell transfection and quantitative real-time PCR
The U251 and U87 cells were provided by Dr. Jun Yang (First Affiliated Hospital of Nanchang University). After obtaining these two cell types, we commissioned Shanghai Yi He Applied Biotechnology Co., Ltd. to conduct professional STR cell authentication, and the results confirmed the accuracy of the cells. Human glioblastoma cell lines U87 and U251 cells were cultured using DMEM high glucose medium (DMEM, Solarbio) in under 5% CO2 at 37°C. RNA was extracted from both cell lines using TRIzol reagent. The following primers were utilized: RIMS1 Forward (F): 5′- GTACCATCCAGAGAAGCACAGAA-3′, Reverse (R): 5′-CCAGTCGCACTCCAGGAAATATT-3′. We used β-actin as the internal parameter, β-actin Forward (F): 5′-CACCCAGCACAATGAAGATCAAG-3′, Reverse (R):5′-TCATAGTCCGCCTAGAAGCATTT-3′. Total RNA was extracted and reverse transcribed to obtain cDNA. Quantitative real-time PCR (qRT-PCR) was performed using a Bio-Rad real-time fluorescence PCR instrument with TransStart Top Green qPCR SuperMix (Yeasen Biotechnology, Shanghai, China). siRNA sense (5’-3’): CAAGAUGCGAGACGAGUUA (dT), antisense (5’-3’): UAACUCGUCUCGCAUCUUG(dT). RIMS1-siRNA was used to silence RIMS1 expression. Cells were seeded in 6-well plates and transfected with Lipofectamine 3000 Transfection Reagent and siRNA once they reached approximately 60% confluence, following the manufacturer’s instructions. Forty-eight hours later, cells were passaged and subjected to analysis. The Lipofectamine 3000, all siRNAs and primers were purchased from Tsingke Biotechnology Co., Ltd. (Peking China).
Screening of GBM differentially expressed genes
We obtained GBM-related genes from GEO databases (GSE15824 and GSE16011) to identify differentially expressed genes (DEGs) between GBM and non-tumor tissues.
Expression of RIMS1 and acquisition of related data
We extracted data on human RIMS1 RNA and protein expression through the HPA (https://www.proteinatlas.org/) online database. Data on RIMS1 mRNA expression in tumor, paraneoplastic tissues and normal samples were also extracted from the TCGA (https://cancergenome.nih.gov) and GTEx (https://gtexportal.org/) databases.
The study was statistically analyzed using R (v 4.3.2) software. RIMS1 gene expression data from GBM patients were plotted using the “ggplot2” software package. The median of the expression data was determined as the threshold value. Intergroup variability was determined by Wilcoxon rank sum test.
Estimated resources for tumor immunity
We reanalyzed RIMS1 expression data using the Tumor Immunity Estimation Resource (TIMER) (https://timer.cistrome.org/) online database, which includes tumor-infiltrating immune cells from 32 cancer types. Additionally, we performed a pan-cancer analysis of immune cell infiltration. We verified the association between RIMS1 expression and the level of immune infiltration in GBM patients using the “TIMER-Gene” module. Different algorithms in the “Immune-Gene” module were also used to analyze the correlation between RIMS1 expression and immune infiltration in TCGA.
Prognostic analysis of patients with RIMS1 and GBM
Survival analysis of TCGA-GBM expression data and clinical data was performed using the R packages “survminer” and “survival”. The prognosis of GBM patients was examined using Kaplan-Meier (K-M) analysis with forest. The predictive accuracy of RIMS1 at 1–5 years was assessed using a time-dependent ROC curve (Time ROC) method.
Protein-protein interaction (PPI) network analysis of RIMS1
The STRING database (https://cn.string-db.org) was used to collect and integrate potential protein interaction mechanisms with RIMS1 for PPI network analysis.
Acquisition of data for RIMS1-related genes
We assembled data on the top 100 RIMS1-related genes and analyzed the expression association between RIMS1 and the top 3 genes from GEPIA2 (https://gepia2.cancer-pku.cn/), which contains 162 GBM samples and 207 non-tumor samples, TCGA and GTEx project RNA sequence data on a web server.
Functional enrichment analysis of RIMS1
We performed Gene Ontology (GO) functional and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of genes that are closely related to RIMS1, and genes with interactions with RIMS1 were accessed through the STRING database (https://cn.string-db.org/). The related and interacting genes of RIMS1 were analyzed by GO and KEGG pathway enrichment, and the critical threshold was set to p-value < 0.01. The results were presented in the form of bubble plots by “ggplot2”.
CCK-8 test
Cell proliferation activity was measured using the Cell Counting Kit-8 (CCK-8) (Shanghai Shenger Technology) according to the reagent manufacturer’s instructions. Cell inoculation was performed in 96-well plates, where 700 cells were inoculated per well. To detect the relative cell proliferation rate, Add CCK-8 reagent to the experimental wells and control wells respectively, and incubate in the cell chamber for 4 h, The measurement was repeated 2 h later. Absorbance at 450 nm was measured using a microplate reader (Thermo Scientific Multiskan FC), which quantified the cell viability. The increase in absorbance correlates with the number of viable cells, reflecting the cell proliferation activity.
Wound healing assay
Cells were separately inoculated in 6-well plates for experimental and control groups, and the wounds were gently scraped using a 200 µl pipette tip when fusion reached 90–100%. After cleaning the residual cells, DMEM high sugar medium without FBS was added and incubated in a cell chamber. 24 h later, the wounds were videotaped using a microscope. Three replicates of each of the 2 cell lines were performed. Cell migration rate was assessed using the ratio of the wound area to the original wound area.
Transwell cell migration assay
We used Matrigel (Corning, USA) without reduction in growth factors for the invasion assays. Cells were added to transwell chambers (Corning, USA) pre-coated with matrix gel solution in 24-well plates for incubation. The chambers were removed and immersed in 4% paraformaldehyde after 48 h. The residual cells were washed away using PBS and stained with 0.1% crystal violet (Solarbio, China).
Clone formation assay
Cells (400 cells/well) were inoculated into six-well plates and cultured in a cell chamber for 10 days. The 6-well plates were removed and rinsed slowly with PBS, and the 6-well plates were completely immersed face down in 4% paraformaldehyde (Solarbio; China), and the cells were stained with 5% crystal violet (Solarbio, China).
Statistical analysis
All experiments related to this study were performed with at least 3 replications. Unpaired data were analyzed using the t-test, and multiple comparisons were analyzed by ANOVA. Statistical analysis and graphing were performed using R language (4.3.2) and GraphPad Prism version 8.00 software.
Results
Expression analysis of DEGs in GBMs
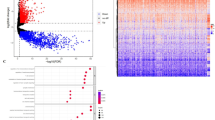
We obtained 167 GBM tissue samples and 10 non-tumor brain samples to identify DEGs through two GBM gene expression profiles, GSE15824 and GSE16011, in the GEO database. We analyzed the DEGs between GBM and non-tumor tissues and performed volcano plots using the R package (ggplot2). Subsequently, the top 25 genes with |logFC| > = 1 in GSE15824 and GSE16011 were filtered by |logFC|, and heatmaps were generated using the R package (ggplot2). The top 25 differentially expressed genes in GSE15824 and GSE16011 with p-value < 0.05 and |Log FC|> = 2 were identified as 471 DEGs, and the top 25 differentially expressed genes with |LogFC| ≥ 1 and |LogFC|≤-1 were shown in volcano plots (Fig. 1A). The heatmap showed the gene expression levels in both datasets (Fig. 1B). Subsequently, Venn diagrams were plotted using the R package (ggvenn package) and showed the intersection of DEGs in these two datasets. The results showed that a total of 90 DEGs were identified in GBM tissues in both datasets compared to non-tumor tissues (Fig. 1C).
Identification of differentially expressed genes (DEGs) in glioblastoma (GBM). (A) Volcano plots show up-regulated (red) and down-regulated (blue) DEGs in GSE15824 and GSE16011 datasets (log2 FC > 2, p-value < 0.05). (B) Heatmap displaying the top 50 DEGs significantly expressed between GBM and normal tissues. (C) Venn diagram illustrating the intersection of up-regulated and down-regulated genes in both datasets.
Analysis of RIMS1 expression data in GBM
The mRNA and protein of RIMS1 were mainly expressed in brain tissues (Fig. 2A). By analyzing the samples in the HPA database, it was shown that the mRNA of RIMS1 was mainly expressed in the cerebellum, cerebral cortex, basal ganglia, midbrain, and hypothalamus (Fig. 2B). RIMS1 protein was mainly expressed in the cerebral cortex, caudate nucleus, hippocampus, and soft tissues (Fig. 2C).
RNA and protein expression profiles of RIMS1 in human organs and tissues based on the Human Protein Atlas (HPA) database. (A) Overview of RIMS1 mRNA and protein expression in various human organs and tissues. (B) Detailed RIMS1 mRNA expression in different organs and tissues. (C) Detailed RIMS1 protein expression in different organs and tissues. (D) Expression of RIMS1 across 33 cancers and normal tissues. (E) Expression of RIMS1 in 33 cancers compared to adjacent normal tissues. (F) RIMS1 expression levels in tumors vs. normal tissues in TCGA-GBM data. (G) RIMS1 expression in gliomas of different grades, genders, and ages in the TCGA database.
By combining the pan-cancer types of 33 cancer data contained in the GEO and TCGA databases, the expression levels of mRNA in 33 of the cancers were mapped using the R (easyTCGA package) language package, and the results showed that there was a significant decrease in the expression of RIMS1 in the 24 cancers mentioned above, including GBM (Fig. 2D). By comparing with the normal tissues next to the cancer, the expression of RIMS1 in GBM and LGG was found to be significantly different (Fig. 2E), in which the expression in GBM was significantly decreased (P < 0.001) (Fig. 2F). Subsequently, changes in RIMS1 expression levels in gliomas of different ages, WHO grades, and gender were verified using the TCGA database (Fig. 2G), which revealed significant variability in RIMS1 expression levels across age, WHO grades, and gender.
Diagnostic and survival value of RIMS1 expression in GBM
In order to research the relevance of RIMS1 expression to the prognosis of GBM patients, we classified patients into high and low expression groups based on RIMS1 expression levels. As shown in Fig. 3, the K-M survival curves showed that low RIMS1 expression in GBM patients was associated with reduced patient survival time (Fig. 3A), exhibiting a graph of time-dependent AUC changes from 1 to 5 years (Fig. 3B). The clinical data of patients with grade 2–4 gliomas in TCGA were statistically organized, and a forest plot based on age, gender, WHO grade, IDH status, whether 1p19q was missing, and RIMS1 expression was performed (Fig. 3C), and the information on the clinical parameters of patients with GBM in TCGA was also used to construct a column-line diagram (Fig. 3D), which was used to calculate the prognosis of each patient by summing up each prognostic criteria were summed to calculate a total score for each patient, which was used to predict the survival of patients at 1, 2, and 5 years, respectively, with patients with higher total scores exhibiting poorer clinical outcomes. The Risk score of RIMS1 was also plotted by dichotomizing the survival outcomes (Fig. 3E).
Clinical significance of RIMS1 expression in GBM. (A) Kaplan-Meier survival analysis of RIMS1 in TCGA. (B) 1–5 year time-dependent AUC changes. (C) Prognostic columns for GBM patients at 1, 2, and 5 years. (D) Forest plot of baseline data for glioma patients in TCGA. (E) Distribution of risk scores for GBM patients in TCGA.
Knockdown of RIMS1 promotes GBM progression in vitro
To investigate the role of RIMS1 in human gliomas, we transfected U87 and U251 cells with RIMS1-siRNA and empty vectors, and verified the transfection by qPCR experiments (Fig. 4A). Cell scratch assay showed that knockdown of RIMS1 markedly promoted the migration of U251 cells (Fig. 4B). In cell counting and CCK8 experiments, we found that knockdown of RIMS1 significantly promoted the proliferation of U87 and U251 cells (Fig. 4C). Colony formation assays showed that colony formation capacity was also significantly elevated in RIMS1-deficient U251 cell relative to controls, suggesting that RIMS1 deficiency leads to enhanced cell proliferation (Fig. 4D). In addition, the invasiveness of U87 and U251 cells was significantly increased after knockdown of RIMS1, as shown in the Transwell migration assay (Fig. 4E).
Knockdown of RIMS1 promotes migration and proliferation of glioblastoma cells in vitro. (A) Knockdown of RIMS1 in U87 MG and U251 MG cells verified by RT-qPCR. (B) Wound healing and CCK-8 assays showing faster migration and enhanced proliferation in RIMS1 knockdown cells. (C) CCK-8 assay results indicating increased proliferation in U87 and U251 cells with RIMS1 knockdown. (D) Colony formation assay showing increased colony-forming ability in U251 MG cells with RIMS1 knockdown. (E) Transwell assay assessing migration and invasion in U87 and U251 cells with and without RIMS1 knockdown.
Analysis of RIMS1 interactions and associated genes and genetic alterations
To investigate the association between RIMS1 and other proteins in the GBM, we mapped the protein-protein interaction (PPI) network using the STRING website (Fig. 5A). To analyze the genes that are closely linked to RIMS1 expression, we displayed the top 100 genes using the GEPIA2 tool. As displayed in the graph (Fig. 5B), RIMS1 expression was positively related to DLGAP3 (R = 0.89), ATP1A3 (R = 0.87) and SVOP (R = 0.85). Using the intersection of the two sets of predicted genes, eight common genes were generated; BSN, CACNA1B, RAB3A, RAB3C, RAPGEF4, STXBP1, UNC13A, and UNC13C (Fig. 5C), all of which are implicated in signaling between nerve cells. To further analyse the relationship between RIMS1 and most cancers, the three genes most positively correlated with RIMS1 expression were subjected to cancer-associated heat mapping, which showed that they were most closely associated with GBM and LGG (Fig. 5D).
Protein-protein interaction (PPI) networks of RIMS1-binding proteins created using the STRING tool. (A) Correlation of RIMS1 with three related genes (DLGAP3, ATP1A3, SVOP) from the top 100 RIMS1-related genes in the TCGA program via GEPIA2. (B) Cross-tabulation analysis of RIMS1 interactions and related genes. (C) Heatmap of three genes in different tumors. (D) GO and KEGG enrichment analyses of 143 RIMS1 interacting and related genes. (E) Heatmap showing the top 30 genes positively (F) and negatively (G) associated with RIMS1 in GBM.
To predict gene function, 143 genes related to RIMS1 were enriched and analyzed (Fig. 5E). Gene ontology (GO) enrichment analysis showed that the most enriched GO terms were “synaptic signaling”, “acute inflammatory response”, “vesicular lumen”, and “oxidative stress”, along with “oxidoreductase activity”. As shown in the KEGG pathway diagram (www.kegg.jp/kegg/kegg1.html), most of the genes are associated with processes such as “regulation of trans-synaptic signaling,” “presynaptic activity,” “synaptic membrane functions,” “synaptic vesicle recycling,” and “SNARE bundling.” We further visualized the top 30 genes positively and negatively correlated with RIMS1 in GBM using separate heatmaps (Fig. 5F and G).
Association analysis of RIMS1 expression with infiltrating immune cells
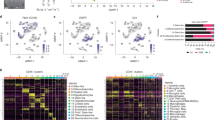
We first analyzed the associations between RIMS1 and the infiltration of 24 immune cells in 33 cancer types, and the results revealed that RIMS1 was strongly related to the infiltration of a variety of immune cells in 33 cancer types, including GBM (Fig. 6A), and then we analyzed the correlation between RIMS1 expression and the infiltration of immune cells in GBM. The results showed that RIMS1 was more related to the infiltration of multiple immune cells in 33 cancer types. (Fig. 6B). As shown in Fig. 6C, in GBM, RIMS1 expression was negatively correlated with the levels of monocytes, macrophages and CD8 T cells, but not with the levels of B lymphocytes in GBM. Furthermore, we investigated the association between the immune microenvironment and the level of RIMS1 expression. First, 698 patients in the TCGA dataset were categorized into two groups according to the level of RIMS1 expression. Subsequently, the differences in the expression of 24 immune cell subtypes between the two groups were evaluated. CD8 T cells, cytotoxic cells and other cells were increased in the low RIMS1 expression group (Fig. 6D). Meanwhile, we observed that 16 of the 24 immune cells mentioned above were negatively associated with RIMS1 expression (Fig. 6E). In addition, we investigated the association between the infiltration levels of different immune cells and RIMS1 expression in TCGA using TIMER2. The analysis showed a statistically negative correlation between RIMS1 expression and the estimated infiltration values of cancer-associated fibroblasts for GBM (Fig. 6F).
Immune infiltration analysis of RIMS1 in GBM and various cancer types. (A) Correlation between RIMS1 expression and 24 immune cell infiltration levels across 33 cancer types using Spearman’s method. (B) Correlation analysis of RIMS1 expression with 24 immune cell types based on the TCGA-GBM database, using the ssGSEA algorithm. (C) Relationship between RIMS1 expression and immune infiltration in GBM using the TIMER database. (D) Differences in levels of 24 immune cell subtypes in tumor samples with high and low RIMS1 expression. (E) Heatmap showing the correlation between RIMS1 expression and immune cell infiltration levels in fibroblast-associated cancers using different algorithms. (F) Scatterplot of the correlation between RIMS1 expression and immune cell infiltration levels in fibroblast-associated cancers.
Discussion
The treatment of GBM is complicated by tumor heterogeneity, altered immune checkpoints, and extensive immunosuppression in the hypoxic microenvironment23,24. RAS signaling plays a crucial role in driving normal physiological cell proliferation, and a dysregulated state of this signaling pathway is commonly found to be present during tumorigenesis25,26. Among these, mutations in the RAS protein itself have a profound impact during the development of many cancers27. Therefore, over the past decades, researchers have conducted numerous studies on RAS proteins and have gained approval of the KRAS G12C family of inhibitors sotorasib for use in a specific subgroup of patients with non-small cell lung cancer (NSCLC)28.RIMS1, as a member of the superfamily of RAS genes, plays an important role in neurotransmitter release through the regulation of voltage-gated calcium channels29. However, current studies correlating RIMS1 with GBM are lacking. In this study, we determined the clinical value of RIMS1 in GBM by bioinformatics methods and experimental validation.
Here, we analyzed the expression levels of RIMS1 in tumor and normal tissues using TCGA GBM data. The results showed that mRNA and protein expression of RIMS1 was significantly reduced in GBM patients, and GBM patients with high RIMS1 expression had good prognosis and prognostic value. Knockdown of RIMS1 significantly promoted the proliferative invasive function of the GBM cells. In this study, to understand the potential role of RIMS1 in GBM, we performed GO and KEGG analyses to look for RIMS1-related genes, and these results are consistent with the hypothesis that RIMS1 expression may be associated with RAS mutations.
Immune infiltration in GBM is a current hot topic30,31. It is beneficial to understand the immune infiltrating cells to develop immunotherapies for GBM. Brain tissue-specific immunology shapes the unique tumor microenvironment of GBM, in which multiple peripheral immune components are present, consisting of different kinds of monocytes and lymphocytes (TIME)32. However, their infiltration rate in gliomas is significantly lower than in other tumors. Tumor-infiltrating lymphocytes (TILs) are low in GBM, whereas the levels of CD4 + T cells, CD8 + T cells and Treg cells are high in GBM. In this study, we determined a significant association between RIMS1 expression and the level of immune infiltration in GBM. The level of RIMS1 expression was significantly and negatively correlated with the level of infiltration of B cells, regulatory T cells, monocytes, CD8 + T cells and macrophages in GBM. In addition, the relationship between RIMS1 and immune cells suggested that RIMS1 plays a crucial role in regulating the immune microenvironment of GBM and that RIMS1 expression affects the survival of GBM patients through immune cell infiltration. These findings demonstrated that RIMS1 may be a novel site for future tests of combination immunotherapies. However, we need to further investigate the RIMS1 function in the immunological microenvironment of tumors.
However, there are some limitations and drawbacks in this study. First, we identified the three genes most related to RIMS1 by bioinformatics and verified their roles in GBM development through bioinformatics, but we did not perform experimental validation of the relationship between these genes and RIMS1. Another drawback is that despite our experiments confirming the low expression of RIMS1 in GBM cells, the mechanisms that regulate GBM genesis and proliferation remain undefined. Additionally, while established glioma cell lines were used, future studies with patient-derived cell lines are needed to confirm the relevance and validity of these findings in more physiologically accurate models. Furthermore, more in vitro and in vivo studies are needed to confirm the efficacy of RIMS1 as a viable target in the GBM immune microenvironment and to develop GBM immunotherapies in the future.
Conclusion
We utilized TCGA, GEO database and online data analysis tools, combined with experimental validation, to provide an important insight into the significance of RIMS1 and presented RIMS1 as a more promising diagnostic marker and therapeutic target. Furthermore, we found that RIMS1 expression was negatively related to the immune infiltration of GBM, particularly with regard to immune cells such as monocytes, macrophages, and CD8 T cells, suggesting that RIMS1 may influence GBM progression through immune-related pathways. Therefore, further studies suggest the need for biological validation to support this conclusion.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Lucero, R. et al. Glioma-derived miRNA-containing extracellular vesicles induce angiogenesis by reprogramming brain endothelial cells. Cell. Rep. 30(7), 2065–2074e4 (2020).
Zheng, Y. et al. Spatial cellular architecture predicts prognosis in glioblastoma. Nat. Commun. 14(1), 4122 (2023).
Tan, A. C. et al. Management of glioblastoma: state of the Art and future directions. CA Cancer J. Clin. 70(4), 299–312 (2020).
Yeh, M. et al. MicroRNA-138 suppresses glioblastoma proliferation through downregulation of CD44. Sci. Rep. 11(1), 9219 (2021).
Szklener, K. et al. Enhancing glioblastoma treatment through the integration of tumor-treating fields. Front. Oncol. 13, 1274587 (2023).
Dono, A. et al. PTEN mutations predict benefit from tumor treating fields (TTFields) therapy in patients with recurrent glioblastoma. J. Neurooncol. 153(1), 153–160 (2021).
Dolton, G. et al. Targeting of multiple tumor-associated antigens by individual T cell receptors during successful cancer immunotherapy. Cell 186(16), 3333–3349e27 (2023).
Shin, M. H. et al. Recent advances in CAR-Based solid tumor immunotherapy. Cells 12, 12 (2023).
Zhang, Y. et al. Tumor metabolic crosstalk and immunotherapy. Clin. Transl Oncol. 26(4), 797–807 (2024).
Rui, R., Zhou, L. & He, S. Cancer immunotherapies: advances and bottlenecks. Front. Immunol. 14, 1212476 (2023).
Sran, S. & Bedrosian, T. A. RAS pathway: the new frontier of brain mosaicism in epilepsy. Neurobiol. Dis. 180, 106074 (2023).
Ray, P., Sarker, D. K. & Uddin, S. J. Bioinformatics and computational studies of chabamide F and chabamide G for breast cancer and their probable mechanisms of action. Sci. Rep. 14(1), 19893 (2024).
Li, K. et al. The HOXC10/NOD1/ERK axis drives osteolytic bone metastasis of pan-KRAS-mutant lung cancer. Bone Res. 12(1), 47 (2024).
Alig, A. et al. Impact of primary tumor sidedness and sex on prognosis and anti-epidermal growth factor receptor antibody efficacy in BRAF-mutant metastatic colorectal cancer: a pooled analysis of AIO studies FIRE-1, CIOX, FIRE-3, XELAVIRI, and VOLFI. ESMO Open. 9(9), 103677 (2024).
Hofmann, M. H. et al. Expanding the reach of precision oncology by drugging all KRAS mutants. Cancer Discov. 12(4), 924–937 (2022).
Fan, F. et al. Dynamin deficiency causes insulin secretion failure and hyperglycemia. Proc. Natl. Acad. Sci. U S A 118, 32 (2021).
Martin-Gutierrez, M. P. et al. Dominant cone rod dystrophy, previously assigned to a missense variant in RIMS1, is fully explained by Co-Inheritance of a dominant allele of PROM1. Invest. Ophthalmol. Vis. Sci. 63(9), 14 (2022).
Andrews, B. et al. Multidimensional dynamics of the proteome in the neurodegenerative and aging mammalian brain. Mol. Cell. Proteom. 21(2), 100192 (2022).
Zhen, W. et al. Exploration and validation of m7G-related genes as signatures in the immune microenvironment and prognostic indicators in low-grade glioma. Am. J. Transl. Res. 15(6), 3882–3899 (2023).
Yang, J. et al. Gene expression profiles reveal key genes for early diagnosis and treatment of adamantinomatous craniopharyngioma. Cancer Gene Ther. 25(9–10), 227–239 (2018).
Zhang, S., Luo, Y., Sun, W., Tan, W. & Zeng, H. Prognostic values of core genes in pilocytic astrocytom. World Neurosurg. 176, e101–e108 (2023).
Zhang, Q. et al. Bioinformatics approach based on the Cancer-Immune cycle identifies potential immunotherapeutic targets in diffuse gliomas (2022).
Tajbakhsh, A. et al. The complex roles of efferocytosis in cancer development, metastasis, and treatment. Biomed. Pharmacother. 140, 111776 (2021).
Zhao, S. et al. Interaction, immune infiltration characteristics and prognostic modeling of efferocytosis-related subtypes in glioblastoma. BMC Med. Genomics. 16(1), 248 (2023).
Punekar, S. R. et al. The current state of the Art and future trends in RAS-targeted cancer therapies. Nat. Rev. Clin. Oncol. 19(10), 637–655 (2022).
Gimple, R. C. & Wang, X. Striking at the core of the oncogenic circuitry. Front. Oncol. 9, 965 (2019).
Hymowitz, S. G. & Malek, S. Targeting the MAPK pathway in RAS mutant cancers. Cold Spring Harb Perspect. Med., 8, 11 (2018).
Lim, T. et al. KRAS G12C in advanced NSCLC: prevalence, co-mutations, and testing. Lung Cancer. 184, 107293 (2023).
Snyder, K. et al. Gene knockout of RNA binding motif 5 in the brain alters RIMS2 protein homeostasis in the cerebellum and Hippocampus and exacerbates behavioral deficits after a TBI in mice. Exp. Neurol. 374, 114690 (2024).
Gieryng, A. et al. Immune microenvironment of gliomas. Lab. Invest. 97(5), 498–518 (2017).
Wainwright, D. A. et al. Targeting Tregs in malignant brain cancer: overcoming IDO. Front. Immunol. 4, 116 (2013).
Andrew, A. W. et al. Immunotherapeutic advances in glioma management: the rise of vaccine-based approaches. CNS Neurosci. Ther. 30(9), e70013 (2024).
Author information
Authors and Affiliations
Contributions
GBX, ZXL, EXT, and ZPX conceived the manuscript. WQX designed the experiments. GBX, ZXL, and EXT conducted the experiments. ZXL and ZPX analyzed the data. QMS, JLT, ZPX and WQX wrote the manuscript, with input and comments from all other authors. GBX, QMS, and JLT are responsible for revision work. All authors reviewed the manuscript. All the authors read and approved the submission and final publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the The First Affiliated Hospital, Jiangxi Medical College, Nanchang University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tao, E., Song, Q., Tang, J. et al. Prognostic biomarker RIMS1 and its association with immune infiltration in glioblastoma. Sci Rep 15, 15240 (2025). https://doi.org/10.1038/s41598-025-99499-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99499-3