Abstract
Foot-and-mouth disease (FMD) is a highly contagious viral disease of domesticated animals that causes major economic losses globally. In this meta-analysis, 29 studies were evaluated using a random-effects model to analyze the efficacy of FMD vaccines. The quantifying heterogeneity between the groups was low (tau2 = 0.000, tau = 0.000, and I2 = 0.0% [0.0%; 24.6%]). The meta-analysis revealed that the inactivated vaccine provides the best protection among different vaccine types, with the following ranking from highest to lowest efficacy: inactivated vaccine > mRNA vaccine > E. coli vaccine > plant vaccine > recombinant virus vaccine > phage vaccine > synthesize vaccine > DNA vaccine > negative control. The findings revealed that the inactivated vaccine provides the best protection among the different types of vaccines. Based on these findings, we recommend using inactivated vaccines as controls in the development of novel vaccines, as they achieved the highest efficacy among all evaluated vaccine types.
Similar content being viewed by others
Introduction
Foot-and-mouth disease (FMD), one of the most contagious animal diseases, occurs in most parts of Africa, Asia, the Middle East, and South America1. More than 100 species of wild, laboratory, or domesticated animals have been infected with the FMD virus (FMDV) naturally or experimentally2. In endemic areas alone, the production losses and vaccination costs caused by FMD amount to US$6.5 to US$21 billion annually3.
Meta-analysis can be used to compare differences in the effects of various FMD vaccines to identify the most effective vaccines4. Network meta-analysis (NMA) is superior to the traditional paired meta-analysis method, as it evaluates the efficacy of interventions within a single framework, improves accuracy, compares intervention pairs that have never been directly compared in experiments, and provides the level of interventions according to their effectiveness5.
FMD is highly contagious, which hinders the performance of virus challenge studies, leading to a relative lack of data on virus challenges. Therefore, the data of virus challenge reported in the literature is very valuable6. As a step toward filling this research gap, this meta-analysis compared the effects of different vaccines. This study used Bayesian network meta-analysis to compare the protective effects of different FMD vaccines and identify the most effective vaccine.
Materials and methods
Search strategy
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines. The literature retrieved in this meta-analysis was collected by searching databases from their inception until August 2024 and evaluated by three researchers (PW, ZQL, and JJ). “King of Medical Literature” software was used to search for and delete duplicate files. The National Library of Medicine (Medline via PubMed) and Embase databases were searched using the keywords (FMDV AND vaccine) OR (FMDV AND protect). The China National Knowledge Infrastructure and Wanfang Data databases were searched for studies on FMDV vaccines using the keywords “FMDV,” “protect,” and “vaccine” using King of Medical Literature software.
Inclusion and exclusion criteria
The following inclusion criteria were used: (1) the virus serotype must be type O. There are several reasons why FMDV serotype O is included. There is no cross protection between different serotypes of FMDV7. First, the FMDV serotype O serotype has the widest global distribution, covering multiple regions, including Asia, Africa, Europe, and the Americas, and as such, its harm to livestock far exceeds that of other serotypes8,9. Second, the structure of FMDV serotype O is the most unstable and easily degraded, and its vaccine immune effect is the worst10,11. Third, among FMD vaccines, O serum vaccine has enough data to meet the requirements of high-quality meta-analysis12,13,14. (2) the vaccine must be an FMD vaccine; and (3) the vaccine protective effects against FMDV must be evaluated and include challenge potency studies (direct potency studies, not only serology studies) with FMDV. The following exclusion criteria were used: (1) the carrier was not related to FMD; (2) the results did not provide the necessary basic data; and (3) the number of challenged animals was not recorded.
Data extraction and summation
Two researchers conducted a preliminary screening by reading the titles and abstracts of the retrieved studies before reading the full text. They then made their selection according to the inclusion and exclusion criteria. JJ and ZQL extracted the data, and if they disagreed about any aspect of the extraction, PW made the final decision. The data extracted included the first author’s name, publication date, and total number of animals in the trials.
Statistical analysis
R 4.3.0 language can be used to conduct Bayesian meta-analysis using JAGS 4.3.0 software. In R4.3.0, the “meta,” “grid,” and “net meta” libraries were used. The occurrence of zero events leading to bias was caused by the small number of animals involved. When the number of events was zero, the total number of events increased by 0.01. When all cases had events, 0.01 was subtracted from the total number of events. The random-effects model was used for meta-analysis to calculate the risk ratio along with a 95% confidence interval for dichotomous results15. The calculation of the random-effects model was actively performed using R software16. Where applicable, the results from the individual trials estimated were presented in a forest plot. The different FMD vaccine groups were compared in R4.3.0. Tau2 was used to quantify heterogeneity.
Results
Study identification
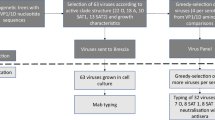
Figure 1 presents the flowchart of the article selection process. Of the 944 articles screened, 76 were relevant for full-text review. These articles contained a total of 29 studies that underwent meta-analysis.
Study characteristics
The selected articles included 29 studies focusing on FMD using the FMDV strain17. All studies evaluated the protective effects of FMD vaccines and performed virus challenge studies (Table 1).
Meta-analysis
The network of FMD vaccines is presented in Fig. 2. The results indicated that the number of comparisons made with blank groups, inactivated vaccines, recombinant virus vaccines, and Escherichia coli vaccines was the largest. All groups were directly compared with the blank control group.
Furthermore, the quantifying heterogeneity between the groups was low (tau2 = 0.000, tau = 0.000, and I2 = 0.0% [0.0%; 24.6%]) (Fig. 3).
A funnel plot was developed for visual investigation of possible small-study effects. Overall, the plot resembled a funnel chart. Funnel plot analysis revealed that the bias between the groups was within an acceptable range (Fig. 4). The result of heterogeneity testing was small, with tau2 = 0.000.
The results of the meta-analysis indicated that FMD vaccines produced through different methods provide different degrees of protection, with the following ranking from highest to lowest efficacy: inactivated vaccine > mRNA vaccine > E. coli vaccine > plant vaccine > recombinant virus vaccine > phage vaccine > synthesize vaccine > DNA vaccine > negative control (Table 2).
Figure 5 shows that research has primarily focused on inactivated, phage, and peptide vaccines and that relatively little research has investigated plant-derived vaccines. Among all vaccines, inactivated FMD vaccines are the most extensively used.
Discussion
Currently, the primary method of preventing animals from being infected with FMD virus is through vaccination. With the continuous progress of biotechnology, new vaccines are constantly being developed and undergoing continuous research and further development. The performance of FMD vaccines is inconsistent, and the identification of the most effective vaccine remains controversial. This study compared the data on all vaccines with known efficacy, particularly vaccines that have not undergone direct comparison, to determine their value and guide their follow-up in vaccine development. The inactivated vaccine (0.728) has the highest score, indicating its effectiveness and reliability. It is a traditional vaccine type, in which the virus is chemically inactivated but retains its ability to induce an immune response. Its high score suggests widespread use, proven efficacy, and safety in controlling FMD outbreaks44. However, it may require cold chain storage and multiple doses for sustained immunity45. mRNA vaccines (0.6774) represent a modern approach, leveraging genetic technology to stimulate immune responses. Although mRNA vaccines scored slightly less than inactivated vaccines, they offer advantages such as rapid development and scalability. Their lower score might reflect challenges in stability, delivery, or limited long-term data in FMD applications46. E. coli vaccines (0.6559) use E. coli as a vector to express FMD antigens. Their scores indicate moderate effectiveness, likely due to their cost-effectiveness and ease of production47,48. However, they may face limitations in antigen presentation or immune response robustness compared with other methods. DNA vaccines (0.2142) have the lowest score, indicating limited success in FMD applications. Although they offer simplicity in design, they often struggle with low immunogenicity, delivery efficiency, and regulatory hurdles. Inactivated vaccines remain the most effective option for FMD, while mRNA and E. coli vaccines show promise as modern alternatives49,50. Plant and recombinant virus vaccines offer innovative approaches but face practical challenges51,52. Phage, synthesized, and DNA vaccines are currently less effective, highlighting the need for further research and development53,54.
FMD is highly contagious, which hinders the performance of virus challenge studies, leading to a relative lack of data on virus challenges. As a step toward filling this research gap, this meta-analysis compared the effects of different vaccines. Under Bayesian mediation analysis, the inference is straightforward and exact, which makes it appealing for studies with small samples55, and this is one of the most widely used methods56,57. The advantages of meta-analysis include the provision of a comprehensive retrieval strategy and qualification criteria for retrieval research. Nevertheless, the use of meta-analysis in this study posed several limitations. The possibility of publishing studies with undesired results is low, which could lead to higher vaccine protection data58. First, FMDV is highly infectious, which limits the number of animals that can be used for experiments. Accordingly, having insufficient data, such as in studies related to new vaccines, increases the deviation of the results. Second, there may be bias in the funnel plot used for the visual (and fully subjective) investigation of possible small-study effects59. Third, a source of heterogeneity may be related to the dose of the vaccine; some vaccines are dose-dependent, only exerting fully protective effects at high doses60.
Only FMDV serotype O was included for several reasons. Although there are some data on FMDV serotype A, more research is needed to meet the requirements of meta-analysis61,62. For these reasons, it has the most important value for the study of the FMD vaccine. NMA can contribute to resolving controversies, reducing the reliance on laboratory animals, avoiding duplication of work, and guiding future research directions. NMA helps researchers study important and previously unanswerable questions, which has contributed to the rapid increase in the number of studies using NMA in the biomedical literature4. To our knowledge, our study is the first to use NMA to investigate FMD vaccines, making our study design innovative and our findings significant.
Conclusions
In this meta-analysis, 29 studies were evaluated to analyze the efficacy of FMD vaccines. The findings revealed that the inactivated vaccine provides the best protection among the different types of vaccines. Based on these findings, we recommend using inactivated vaccines as controls in the development of novel vaccines, as they achieved the highest efficacy among all evaluated vaccine types.
Data availability
All data generated or analysed during this study are included in this published article.
References
Hammond, J. M., Maulidi, B. & Henning, N. Targeted FMD vaccines for Eastern Africa: The AgResults foot and mouth disease vaccine challenge project. Viruses 13, 1830 (2021).
Weaver, G. V., Domenech, J., Thiermann, A. R. & Karesh, W. B. Foot and mouth disease: A look from the wild side. J. Wildl. Dis. 49, 759–785 (2013).
Stenfeldt, C. et al. Virulence beneath the fleece; A tale of foot-and-mouth disease virus pathogenesis in sheep. PLoS ONE 14, e0227061 (2019).
Watt, J. & Del Giovane, C. Network meta-analysis. Methods Mol. Biol. 2345, 187–201 (2022).
Seitidis, G., Nikolakopoulos, S., Hennessy, E. A., Tanner-Smith, E. E. & Mavridis, D. Network meta-analysis techniques for synthesizing prevention science evidence. Prev. Sci. 23, 415–424 (2022).
Sanghi, D. K. & Tiwle, R. A detail comprehensive review on vaccines. Int. J. Res. Dev. Pharmacy Life Sci. 3, 887–895 (2014).
Momtaz, S., Towheed, S. T., Ali, R., Ullah, H. & Hossain, M. A. Molecular epidemiology of foot-and-mouth disease virus serotypes circulating in Bangladesh. Microbiol. Res. J. Int. 158, 82–94 (2012).
An, Q. et al. Global foot-and-mouth disease risk assessment based on multiple spatial analysis and ecological niche model. Vet. Q. 45, 1–11 (2025).
Verma, A. K., Kumar, A., Mahima, & Sahzad,. Epidemiology and diagnosis of foot-and-mouth disease: A review. Indian J. Animal Sci. 82, 543–551 (2012).
Pacheco, J. M. & Mason, P. W. Evaluation of infectivity and transmission of different Asian foot-and-mouth disease viruses in swine. J. Vet. Sci. 11, 133–142 (2010).
Juan, et al. Evaluation of infectivity, virulence and transmission of FDMV field strains of serotypes O and A isolated in 2010 from outbreaks in the Republic of Korea. PLoS ONE 11, 1–21 (2016).
Tsai, C. P., Pan, C. H., Liu, M. Y., Lin, Y. L. & Yang, P. C. Molecular epidemiological studies on foot-and-mouth disease type O Taiwan viruses from the 1997 epidemic. Vet. Microbiol. 74, 207–216 (2000).
Tang, H., Liu, X. S., Fang, Y. Z., Pan, L. & Zhang, Y. G. Geometrical study on FMDV genome based on Z-curve. J. Anim. Vet. Adv. 11, 2630–2640 (2012).
Abubakar, M., Arshed, M. J., Ali, Q. & Hussain, M. Spatial trend of Foot and Mouth Disease virus (FMDV) serotypes in cattle and buffaloes, Pakistan. Virol. Sin. 27, 320–323 (2012).
Kanters, S. Fixed- and random-effects models. Methods Mol. Biol. 2345, 41–65 (2022).
Nestler, S. & Erdfelder, E. Random effects multinomial processing tree models: A maximum likelihood approach. Psychometrika 88, 809–829 (2023).
Hajam, I. A. et al. Co-administration of flagellin augments immune responses to inactivated foot-and-mouth disease virus (FMDV) antigen. Res. Vet. Sci. 95, 936–941 (2013).
Lee, G. et al. Vaccine strain of O/ME-SA/Ind-2001e of foot-and-mouth disease virus provides high immunogenicity and broad antigenic coverage. Antiviral Res. 182, 104920 (2020).
Hema, M., Chandran, D., Nagendrakumar, S. B., Madhanmohan, M. & Srinivasan, V. A. Construction of an infectious cDNA clone of foot-and-mouth disease virus type O 1 BFS 1860 and its use in the preparation of candidate vaccine. J. Biosci. 34, 45–58 (2009).
Lu, B. et al. A ferritin-based nanoparticle displaying a neutralizing epitope for foot-and-mouth disease virus (FMDV) confers partial protection in guinea pigs. BMC Vet. Res. 20, 301 (2024).
Shao, J., Liu, W., Gao, S., Chang, H. & Guo, H. A recombinant multi-epitope trivalent vaccine for foot-and-mouth disease virus serotype O in pigs. Virology 596, 110103 (2024).
Shi, X. et al. Development and efficacy evaluation of a novel nano-emulsion adjuvant for a foot-and-mouth disease virus-like particles vaccine based on squalane. Nanomaterials (Basel) 12, 3934 (2022).
Rangel, G. et al. Chimeric RHDV virus-like particles displaying foot-and-mouth disease virus epitopes elicit neutralizing antibodies and confer partial protection in pigs. Vaccines (Basel) 9, 470 (2021).
Jo, H. et al. The HSP70-fused foot-and-mouth disease epitope elicits cellular and humoral immunity and drives broad-spectrum protective efficacy. NPJ Vaccines 6, 42 (2021).
Yang, Y. et al. Enhanced immunogenicity of foot and mouth disease DNA vaccine delivered by PLGA nanoparticles combined with cytokine adjuvants. Res. Vet. Sci. 136, 89–96 (2021).
Cañas-Arranz, R. et al. A single dose of dendrimer B(2)T peptide vaccine partially protects pigs against foot-and-mouth disease virus infection. Vaccines (Basel) 8, 19 (2020).
Xu, H. et al. Immunogenicity of T7 bacteriophage nanoparticles displaying G-H loop of foot-and-mouth disease virus (FMDV). Vet. Microbiol. 205, 46–52 (2017).
Li, H. et al. Novel chimeric foot-and-mouth disease virus-like particles harboring serotype O VP1 protect guinea pigs against challenge. Vet. Microbiol. 183, 92–96 (2016).
Dong, Y. M., Zhang, G. G., Huang, X. J., Chen, L. & Chen, H. T. Promising MS2 mediated virus-like particle vaccine against foot-and-mouth disease. Antiviral Res. 117, 39–43 (2015).
Wang, X. H., Jin, Y. Z. & Fan, X. B. Construction and Immunogenicity of DNA Vaccine Plasmid Expressing VP1 and VP4 Genes of Foot-and-Mouth Disease Virus. Chin. J. Biol. 23, 168–171+175 (2010).
Ren, Z. J. et al. Orally delivered foot-and-mouth disease virus capsid protomer vaccine displayed on T4 bacteriophage surface: 100% protection from potency challenge in mice. Vaccine 26, 1471–1481 (2008).
Yang, C. et al. Induction of protective immunity in swine by recombinant bamboo mosaic virus expressing foot-and-mouth disease virus epitopes. BMC Biotechnol. 7, 62 (2007).
Li, P., Yong-Guang, Z., Yong-Lu, W., Bao-Qin, W. & Qing-Ge, X. Protective immune response of guinea pigs against challenge with foot and mouth disease virus by immunization with foliar extracts from transgenic tomato plants expressing the FMDV structural protein VP1. Wei sheng wu xue bao Acta microbiologica Sinica 46 (2006).
Song, H. H. et al. A novel mucosal vaccine against foot-and-mouth disease virus induces protection in mice and swine. Biotechnol. Lett. 27, 1669–1674 (2005).
Li, G. J. et al. Comparison of immune responses against foot-and-mouth disease virus induced by fusion proteins using the swine IgG heavy chain constant region or beta-galactosidase as a carrier of immunogenic epitopes. Virology 328, 274–281 (2004).
Wang, J. H. et al. Induction of immunity in swine by purified recombinant VP1 of foot-and-mouth disease virus. Vaccine 21, 3721–3729 (2003).
Wu, L. et al. Expression of foot-and-mouth disease virus epitopes in tobacco by a tobacco mosaic virus-based vector. Vaccine 21, 4390–4398 (2003).
Qian, P., Li, X. M., Jin, M. L., Peng, G. Q. & Chen, H. C. An approach to a FMD vaccine based on genetic engineered attenuated pseudorabies virus: One experiment using VP1 gene alone generates an antibody responds on FMD and pseudorabies in swine. Vaccine 22, 2129–2136 (2004).
Carrillo, C. et al. Induction of a virus-specific antibody response to foot and mouth disease virus using the structural protein VP1 expressed in transgenic potato plants. Viral Immunol. 14, 49–57 (2001).
Chan, E. W. et al. An immunoglobulin G based chimeric protein induced foot-and-mouth disease specific immune response in swine. Vaccine 19, 538–546 (2000).
Wigdorovitz, A. et al. Induction of a protective antibody response to foot and mouth disease virus in mice following oral or parenteral immunization with alfalfa transgenic plants expressing the viral structural protein VP1. Virology 255, 347–353 (1999).
Carrillo, C., Wigdorovitz, A., Oliveros, J. C., Zamorano, P. I. & Borca, M. V. Protective immune response to foot-and-mouth disease virus with VP1 expressed in transgenic plants. J. Virol. 72, 1688–1690 (1998).
Jing-Yun, M. A., Feng, C., Yong-Chang, C., Qing-Feng, Z. & Ying-Zuo, B. I. Prokaryotic expression of VP1 gene of foot-and-mouth disease virus and detection of expression product immunogenicity. Chin. J. Vet. Sci. Technol. 34, 17–20 (2004).
Wu, P. et al. Layered double hydroxide nanoparticles as an adjuvant for inactivated foot-and-mouth disease vaccine in pigs. BMC Vet. Res. 16, 474 (2020).
Ranjan, R., Biswal, J. K., Sahoo, P. K., Tripathy, J. P. & Singh, R. P. Diagnostic application of formalin fixed archived tissues for detection of foot-and-mouth disease. J. Virol. Methods 318, 1–6 (2023).
Borrego, B. et al. Delivery of synthetic RNA can enhance the immunogenicity of vaccines 1 against foot-and-mouth disease virus (FMDV) in mice. Vaccine 31, 4375–4381 (2013).
Kim, T. H. & Wijerathna, S. M. FMDV Multi-VP1e can induces protection against lethal FMDV challenge 1, 1–2 (2017).
Liu, C. et al. Soluble FMDV VP1 proteins fused with calreticulin expressed in Escherichia coli under the assist of trigger factor16 (Tf16) formed into high immunogenic polymers. Int. J. Biol. Macromol. Struct. Funct. Interact. 15, 1532–1540 (2020).
Cenikli, D. A comparison of production, efficacy, and safety of mRNA and conventional vaccines. Int. J. High School Res. 4, 1–22 (2022).
Lee, W. S. et al. Randomized trial of same- versus opposite-arm coadministration of inactivated influenza and SARS-CoV-2 mRNA vaccines. JCI Insight 10, 1–11 (2025).
Geisbert, T. W. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J. Infect. Dis. 204, S1075 (2011).
Thanavala, Y., Huang, Z. & Mason, H. S. Plant-derived vaccines: a look back at the highlights and a view to the challenges on the road ahead. Expert Rev. Vaccines 5(2), 249–260 (2006).
Chirico, F., Silva, J. A. T. D., Tsigaris, P. & Sharun, K. Safety & effectiveness of COVID-19 vaccines: A narrative review. Indian J. Med. Res. 155, 91–104 (2022).
Head, T., Rao, V. B. & Black, L. W. REVIEW Structure and assembly of bacteriophage. 1, 69–110 (1977).
Yuan, Y. & MacKinnon, D. P. Bayesian mediation analysis. Psychol. Methods 14, 301–322 (2009).
Yarnell, C. J., Granton, J. T. & Tomlinson, G. Bayesian analysis in critical care medicine. Am. J. Respir. Crit Care Med. 201, 396–398 (2020).
Kruschke, J. K. Bayesian analysis reporting guidelines. Nat. Hum. Behav. 5, 1282–1291 (2021).
Zybert, A., Tarczyński, K. & Sieczkowska, H. Meta-analysis of the effect of chilling on selected attributes of fresh pork. J. Food Process Preserv. 43, 1–9 (2019).
Philip, S. & Marston, L. How to read a funnel plot in a meta-analysis. BMJ (Clin. Res. Ed.) 351, 1–3 (2015).
Jiao, J. & Wu, P. A meta-analysis: the efficacy and effectiveness of polypeptide vaccines protect pigs from foot and mouth disease. Sci. Rep. 12, 21868 (2022).
Brehm, K. E., Kumar, N., Thulke, H. H. & Haas, B. High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine 26, 1681–1687 (2008).
Xie, Y., Chang, H., Li, Z. & Zhang, Y. Adenovirus-vectored capsid proteins of the serotype a foot-and-mouth disease virus protect guinea pigs against challenge. Front Microbiol. 11, 1449 (2020).
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. The review was not registered.
Funding
This research was funded by Xinjiang Uygur Autonomous Region Tianchi Yingcai and State Key Laboratory of Pathogen and Biosecurity (SKLPBS2457).
Author information
Authors and Affiliations
Contributions
Study concept and design: P.W. Data search: J.J. and Z.L. Design of data analysis plan: P.W. and J.J. Study screening, data extraction and quality assessment: J.J. and Z.L. Analysis and interpretation: P.W. and J.J. Drafting of manuscript: P.W., H.Y. Y.P., J.Y., W.Z. and P.W. wrote the main manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiao, J., Yang, H., Liang, Z. et al. A meta-analysis on the effectiveness of serotype O foot-and-mouth disease vaccines. Sci Rep 15, 15381 (2025). https://doi.org/10.1038/s41598-025-99518-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99518-3