Abstract
The wear performance of partially oxidized NiCr and NiCrBSiFe coatings were investigated by varing load and speed. The partial oxidized powders were processed from the alloy powder using a flame spray process that involved spraying into distilled water. The partially oxidized powder was then plasma-sprayed onto MDN321 steel. The coatings were characterized for adhesive strength, microhardness, and density. The wear behavior was evaluated at disc speeds of 1, 2, and 3 m/s, with loads ranging from 10 to 50 N, over a 3000 m sliding distance. A significant difference in wear rates between the coating and substrate was observed. Operating at a sliding velocity of 1 m/s under a 10 N load, the substrate’s wear rate was found to be 3.56 times higher than that of the NiCrBSiFe coating, whereas for NiCr coating, it was 2.78 times higher. Wear rate coefficient performance shift takes place between the coatings at 12 N-m/s, product of applied load (C) and sliding velocity (V). In NiCrBSiFe coating, the wear mechanism observed at lower speeds and loads is micro-brittle and mechanism shifts to abrasive wear at higher speeds and loads. In the NiCr partially oxidized coating, the wear mechanism observed involves spallation of the coating at higher loads and adhesive wear at lower loads. Thermo gravimetric analysis of the coatings revealed a weight loss percentage of 1.42 for NiCrBSiFe and 14.09 for NiCr coatings. These findings highlight the NiCrBSiFe partially oxidized coating as being tenfold more stable at high temperatures compared to the NiCr partially oxidized coating.
Similar content being viewed by others
Introduction
In contemporary industrial and automotive sectors, there is a growing demand for wear-resistant coatings due to the increase in energy consumption attributed to wear1. Various methodologies such as laser melting2,3,4,5, friction stir processing6,7,8, heat treatment9,10, and oxidation techniques are being employed to enhance the efficacy of wear-resistant coatings while simultaneously reducing costs and upholding quality standards11. Among these techniques, thermal spray processes, even though traditional, have been extensively utilized in safeguarding turbine sections within aircraft engines12. However, recent advancements in thermal spray processes and the composition of feedstock materials have significantly improved, facilitating the attainment of requisite mechanical properties and wear resistance in coatings13.
The integration of hard particles into feedstock materials holds promise for augmenting the tribological properties of coatings. Addition of Al2O3 and CeO2 oxides to metallic Nickel during plasma spraying has demonstrated enhanced wear resistance owing to the synergistic reinforcement effects of oxides14. Studies by Feng Liu et al.15 have explained that the incorporation of metal oxides into NiCr alloys effectively reduces frictional coefficients and enhances wear resistance, particularly at temperatures exceeding 400 °C. Composite coatings have shown excellent wear characteristics in the temperature range of 400 to 1000 °C. Nevertheless, a significant challenge lies in the preparation of oxide powders via gas atomization, which proves to be costly, and achieving a homogeneous mixture of these powders remains a formidable task. In the present investigation, both the above limitations are overcoming with the partially oxidized coatings. Processing oxides directly from alloy powders ensures the homogeneity and less cost.
The process of producing partially oxidized powder from NiCr alloy powder involves utilizing a flame spray method to spray the alloy powder into distilled water. NiCr alloy powder is widely acknowledged as the primary material for bond coats in thermal spray processes16,17. The application of the partly oxidized powder onto MDN321 steel substrates is made possible by the use of a plasma spray technique that does not involve the application of a bond coat. This results in a reduction in the total costs associated with the coating process. Partially oxidized powders consists of hard oxides at the outer layer as well as ductile metallic areas in its center. Due to hard oxide layer, coatings will demonstrate a significant amount of resistance to wear and due to ductile metallic area, adhesion strength of the coating is improved. The oxides in concern generate a protective layer on the coating when it is subjected to sliding wear tests. The presence of this layer serves as an obstacle against deterioration and friction, which eventually leads to a reduction in the amount of energy that is used as a result of wear. Furthermore, in compared to oxide powders, it is predicted that partly oxidized powder would exhibit a higher level of toughness. The toughness of the core of the coating splats is preserved as a consequence of the oxidation of coated splotches that takes place mostly at the outer limits of the coating.
Partially oxidized powder can be synthesized from any alloy powder containing elements sensitive to oxidation18, possessing the necessary ductility to form a bond with the substrate19,20,21, and exhibiting suitable dilatometer properties22,23. This method is particularly significant in industries where maintaining high wear resistance is essential for preserving precision between mating components. Within the paper printing sector, engraver rollers play a vital role in transferring ink onto paper. These rollers, which are typically manufactured out of stainless steel, have a bond coat that is composed of materials such as nickel (Ni), Ni-chromium, or Ni-aluminum. Additionally, they have a topcoat that is composed of pure oxide powders such as chromium oxide, silicon oxide, or titanium oxide, which are sprayed using air plasma spray technology. Ensuring optimal wear resistance and hardness in these rollers is critical for prolonging their operational lifespan, especially when operating at high speeds24. Thermo gravimetric analysis of NiCr and NiCrBSiFe demonstrates, NiCrBSiFe coating is better suitable on boiler tube steel to withstand high temperature conditions25.
In this research, a methodology is employed to create partially oxidized coatings on MDN321 steel through a hybrid approach involving flame spray and plasma spray techniques. The study evaluates the wear and frictional behaviour of the NiCr and NiCrBSiFe coatings using a wear testing machine, with varying speed and load. Scanning Electron Microscopy (SEM) along with Energy Dispersive Spectroscopy (EDS) and X-Ray Diffraction (XRD) techniques are carried out to analyse the microstructure and phases of the NiCr and NiCrBSiFe coatings. Additionally, the wear rate coefficient of both the coatings and MDN321 steel is determined using the PV factor theory.
Experimentation
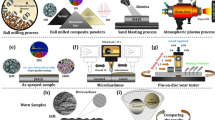
The previous article on partial oxidation of powders explained the process of preparing feedstock powder and depositing partially oxidized powders on MDN321 substrate. The feedstock, comprising partially oxidized powder, was produced via a flame spray method utilizing Ni-20Cr, NiCrBSiFe alloy powder26. Illustrated in Fig. 1, this involved feeding the alloy powder into a flame spray gun, directing it onto distilled water, and maintaining specific parameters27. High temperature alloy powder exits from the flame spray gun underwent partial oxidation upon contacting the water surface. Subsequent steps included collecting, drying, and sizing the partially oxidized powder through ball milling and sieving. Plasma spraying, employing partially oxidized powder, occurred using METCO USA 3 MB equipment. Process parameters of flame spray and plasma spray are listed in Table 1. MDN321 steel, with applications in various high-temperature settings, served as the substrate, prepared with alumina grid blasting and characterized by its chemical composition as per our previous article28. The resultant coating achieved an average thickness of 250 μm on the MDN321 steel plate through the plasma spray process. The elemental composition of the substrate and alloy powders is given in Table 2.
In accordance with ASTM C633-13, a pullout test was performed to determine bond strength. Using DP460 epoxy glue, coated samples (25 × 25 × 5 mm) were adhered to uncoated counter blocks (ϕ25 mm × 60 mm) on their broader faces. The specimens were pulled apart using a Shimadzu AG-X Plus hydraulic tensile testing machine, which measured the bond strength of the coating by applying a strain rate of 0.016 mm/min. An inverted optical microscope (Zeiss Axiovert 200 MAT) with image analyzer software was used to measure porosity along the coating’s cross-section in accordance with ASTM B276. An Omni-tech Vickers tester (MVH-S-AUTO) was used to measure the micro-hardness throughout the coating’s cross-section. Five readings were taken from the interface with a 300-gram load and a 10-second dwell duration, separated by 50 μm. A pycnometer was used to measure the coating density in accordance with ASTM C-135-96.
Sliding wear tests were conducted in accordance with ASTM G-99 standards, employing a high-temperature pin-on-disk tribometer (model: Ducom Instruments Pvt. Ltd., Bangalore, India-20LE-PHM 400-CHM 600). During these tests, the test material was in conformal contact with a rotating Al2O3 disk. The coated MDN321 plate underwent cutting to dimensions of 12 × 12 × 5 mm specifically for these tests. Test parameters selected were varying sliding speed 1, 2, and 3 m/s and applied loads 10, 20, 30, 40 and 50 N loads for 3000 m sliding distance. Flame spray and Plasma spray process parameters are mentioned in an earlier studies29,30,31.
Process of preparation of partially oxidized NiCr powder and deposition of the coating26.
Results and discussion
Microstructure and phase analysis
It is crucial to study the microstructure and phase changes in the powders and coatings before analyzing their wear characteristics; hence, this section focuses on these aspects. XRD analysis and scanning electron microscopy (SEM) images portraying the evolution of the material’s structure from its as-received state to partially oxidized powder and finally to the as-sprayed coating are depicted in Figs. 2 and 3 for NiCr and NiCrBSiFe respectively. During the partial oxidation process facilitated by flame spray, NiCr undergo oxidation, forming phases such as NiO, CrO, and NiCr2O4. Upon deposition of the NiCr coating using plasma spray (Fig. 2A), Cr2O3 emerges at elevated temperatures alongside NiO and NiCr2O4 phases (Fig. 2c), anticipated to manifest commendable tribological properties under high-temperature conditions15,32. The X-ray diffraction pattern of the as-sprayed coating reveals Ni and Cr2O3 as the principal phases, NiO and Cr2Ni3 appearing as minor phases33. Notably, the Scherrer formula indicates an inverse relationship between particle size and full-width half maxima, wherein the partial oxidized powder exhibits significantly reduced full-width half maxima compared to the as-received powder at the highest intensity peak, affirming an increase in powder particle size due to partial oxidation.
The XRD pattern of the NiCrBSiFe coating (Fig. 2B), reveals the presence of multiple phases, labeled 1 to 9, corresponding to various compounds. The phases 1 to 5 (highlighted in bold) are identified as oxide phases, which are formed due to the partial oxidation of the powder feedstock during processing. This partial oxidation plays a significant role in enhancing the wear resistance of the coating, as the resulting oxide phases contribute to improved surface durability and mechanical properties under tribological conditions.
In Fig. 3, it’s evident that the particle size of the partially oxidized powder is larger compared to the as-received powder29,31. However, brittle nature of oxidized particles causes them to break during planetary ball milling, while un-oxidized particles melt during plasma deposition, forming melted splats. Figure 4 indicating points 1 and 2 as partially melted and melted powder particle, respectively. EDS analysis (Fig. 4) reveals a higher oxygen content on partially oxidized coating surface, suggesting the presence of Ni and Cr oxides. Notably, point 2 representing melted splats, displays lower oxygen content compared to partially melted splats (Point 1). Analysis of the microstructure and phase composition revealed that the as-sprayed coating exhibited a significant presence of oxygen distributed uniformly throughout its thickness. During the partial oxidation process, Ni and Cr underwent oxidation, resulting in the formation of oxide phases. In Fig. 5, points 1 and 2 denote unmelted particles and melted splats on the coating surface. Unmelted particles exhibit a lower percentage of oxygen compared to melted particles because they did not undergo oxidation with the air during the coating process. The elemental composition was obtained via EDS at a selected point on the surface. The actual weight percentages of boron and carbon in the NiCrBSiFe coating are 3.1% and 0.6%, respectively (Table 2). The observed increase in boron and carbon percentages is likely due to their lower densities during the coating deposition process. Due to the significant density differences with other elements, boron and carbon may have floated above the higher-density elements during deposition. Additionally, EDS was conducted at a specific point, it resulted in higher percentage of boron and carbon.
Charaterization of the coatings
The adhesion strength of the coating is assessed following the ASTM C633-13 standard, utilizing the pull-off method (Table 3). This test is conducted in tension mode, employing a Universal Testing Machine (model: AG-X plus, Shimadzu hydraulic tensile test machine, Japan) with a strain rate set at 0.016 mm/s. Cylindrical samples of MDN321 steel (with dimensions ϕ25 mm × 60 mm) are prepared, and the coating is deposited using the plasma-sprayed process. Uncoated counter blocks with similar dimensions are attached on the coating side using DP460 Scotch-weld structural epoxy adhesive. Following a 24-hour curing period at room temperature (RT), the joints undergo the adhesion test. The adhesion strength of the partially oxidized coatings, namely NiCr and NiCrBSiFe, is determined to be 10.91 and 12.89 MPa, respectively. Comparable adhesion strength values have been reported for NiCr bond coat followed by a ceramic layer topcoat13,19,34.
Micro-hardness assessment across the coating’s cross-section is conducted using the Omni-tech Vickers tester (model: MVH-S-AUTO), applying 300 g load and with dwell time of 10s. The average micro-hardness values for NiCr and NiCrBSiFe coatings are determined to be 327 ± 8 HV and 810 ± 20 HV, respectively. It’s noteworthy that a significant increase in micro-hardness is observed on the MDN321 steel adjacent to the coating (refer to Fig. 6). This elevation may be attributed to the hardening impact that was created on the MDN321 steel by alumina grid blasting before to the coating process.35 Additionally, the quick impact of molten feedstock during the plasma spray method was also responsible for this elevation. It is possible that the presence of oxidized and partly oxidized splats is responsible for the significant variance in micro-hardness that occurs over the whole thickness of the coating. Hidalgo et al.36 and J.M. Miguel et al.37 reported lower microhardness values for NiCrBSiFe and NiCrBSi coatings, respectively, when prepared using the plasma-spray method. In contrast, the higher hardness observed in our study is likely due to partial oxidation occurring during the coating process. Point analysis at the cross-section of NiCr and NiCrBSiFe coating were discussed in the other article26.
The coating’s density is determined via the water immersion method employing a Pycnometer following ASTM C-135-96 guidelines. To measure the density, the as-sprayed coating is mechanically removed from the samples. The calculated average densities for the NiCr and NiCrBSiFe as-sprayed coatings are 5.28 and 5.46 g/cc, respectively. The porosity of the coating was evaluated using image analysis software and was found to be less than 1.5% in both coatings.
Wear studies
Experimental trials were carried out utilizing a pin-on-disc tribometer to scrutinize the wear and frictional characteristics of both the coatings and MDN321 steel. Subsequently, the worn surfaces of the coating underwent examination using XRD and SEM-EDS to attain a thorough understanding of their properties and the phases formed. The applied normal load, the sliding velocity of the disc, and the high-temperature environment are the primary elements that influence the adhesive wear of both the coating and the MDN321 steel, as shown in Fig. 7.
The wear rate increases with the applied normal load in both the coatings and MDN321 steel, regardless of sliding velocity and temperature26. Conversely, the wear rate decreases at a constant sliding distance with increasing sliding velocities. This reduction occurs because the detachment of asperities from the coating decreases due to less contact time of the asperities38. The maximum wear rate of MDN321 steel (75.36 × 10− 4 mm3/m) is 1.9 and 2.4 times higher than the wear rate of NiCr (39.36 × 10− 4 mm3/m) and NiCrBSiFe (31.2 × 10− 4 mm3/m) coatings, respectively, at a sliding velocity of 1 m/s under a 50 N load (Fig. 8). Additionally, a notable discrepancy in wear rates between the coating and substrate was observed. In the NiCrBSiFe coating at a sliding velocity of 1 m/s under a 10 N load, the wear rate of the substrate is 3.56 times higher than that of the coating, whereas for the NiCr coating, it is 2.78 times higher.
The coefficient of friction demonstrates a decline with the escalation of both the normal load and sliding velocity in both coatings, as depicted in Fig. 8. An increase in the applied normal load leads to the generation of heat at the interface, thereby decreasing the frictional coefficient. Generated heat at the interface causes sharp peaks to deform plastically and act as lubricants, thus reducing coefficient of friction. Similar findings regarding the decrease in the coefficient of friction with increasing applied normal load have been reported by Chowdhury et al.39 and Chen et al.40. Furthermore, the frictional coefficient decreases at higher speeds due to the formation of an oxide layer at the interface resulting from the heat generated by friction41. This oxide layer contributes to the reduction in friction, thereby influencing the frictional behavior observed in the coatings.
In the NiCrBSiFe partially oxidized coating, the wear mechanism observed at a sliding speed of 1 m/s is predominantly micro-brittle. At lower speeds, the coating undergoes local plastic deformation and micro-cutting wear, resulting in crack formation under a load of 10 N and the development of flaking pits under a load of 50 N. Occurrence of cracks is attributed to the high shear forces experienced between oxidized and partially oxidized regions during sliding at 1 m/s under a 10 N load, as depicted in Fig. 9a. Although cracks may also be present at varying sliding speeds, they are promptly welded by the oxide layer formed from melted regions during sliding, leading to a decrease in the frictional coefficient. At a speed of 1 m/s and 50 N load, crack initiation and propagation occur due to lower speeds and higher loads, leading to flaking pit formation on the surface, as depicted in Fig. 9b. Conversely, at higher speeds, the wear mechanism shifts to abrasive wear. The increased speed generates more heat at the interface and provides less time for crack formation, thus favouring abrasive wear. At lower loads, abrasive particles are evident, as shown in Fig. 9c. In contrast, higher loads result in severe grooves on the worn surface, as depicted in Fig. 9d. Debris present at the interface contributes to an increase in the frictional coefficient at higher loads.
In the NiCr partially oxidized coating, the wear mechanism observed involves spallation of the coating at higher loads and adhesive wear at lower loads as shown in Fig. 10. Debris formation occurs at higher loads due to the coating’s lower cohesive strength. At lower loads and speeds, the surface remains smooth, with sharp peaks flattened, leading to adhesive wear due to the continuous wear test over a 3000 m sliding distance. Conversely, at higher loads and speeds, spallation becomes uncontrolled due to the reduced cohesive strength of the coating. The temperature generated from the rubbing action at high loads and speeds appears to promote spallation rather than adhesive wear. Huang et al. explored the use of a NiCr/Cr3C2–BaF2CaF2 composite coating, revealing a relationship between cohesive strength and coefficient of friction42.
Wear rate coefficient
The wear rate coefficient (k) is defined as the ratio of the wear rate (Q) to the product of the load (C) and sliding velocity (V). This relationship is known as the PV factor theory43. According to this hypothesis, the standard of the coating is assessed by the value of the wear rate coefficient. A smaller value of k denotes higher quality, with a range from 0 to 1. Figure 11 shows the gradient of the linear regression for the mean wear rate coefficient of the coating and MDN321 steel. The wear rate coefficient for the NiCr coating is lower (3.2869 × 10− 4 mm3/N-m), while it is higher (5.1944 × 10− 4 mm3/N-m) for MDN321 steel. This difference prove NiCr partially oxidized coating exibit better wear rate coefficient than NiCrBSiFe partially oxidized coating (3.6125 × 10− 4 mm3/N-m) as the load and speed increase further. However shifting the performance of these coatings start at 12 N-m/s. This break even point is more useful to select the process parameters of pin-on-disc tribometer. Thermogravimetric analysis (TGA) conducted up to 900 °C suggests that the NiCrBSiFe coating exhibits improved stability, as it is less affected by temperature (Fig. 12).
Thermo gravimetric analysis (TGA)
Partially oxidized NiCrBSiFe and NiCr coatings were subjected to testing using a Thermal Gravimetric Analysis-Fourier Transform Infrared (TGA-FTIR) apparatus. The samples were heated at a controlled rate of 10 °C per minute in the temperature range of room temperature to 900 °C. Throughout the heating process, the TGA-FTIR continuously monitored and recorded the changes in the weight of the samples in response to the increasing temperature. This allowed for precise analysis of the thermal stability and oxidation behaviour of each coating, providing insights into their performance under high-temperature conditions.
Thermo gravimetric analysis (TGA) of coatings is particularly valuable for applications in high-temperature environments such as boiler tubes, heat exchangers, steam engines, and gas turbine blades44,45. Figure 12 illustrates the impact of temperature on the weight loss of partially oxidized coated samples. Veeresh et al. also noted an increase in weight loss with temperature for a nickel-based metal matrix composite (NMMC) powder consisting of NiCrSiB (70% weight) + Cr3C2-NiCr (30% weight)46. Coatings from the samples were mechanically peeled off for analysis. The initial weights of the NiCrBSiFe and NiCr coatings were 10.428 mg and 7.804 mg, respectively. The percentage of weight loss for NiCrBSiFe and NiCr coatings was 1.42% and 14.09%, respectively. These results highlight the significantly greater stability of NiCrBSiFe partially oxidized coating at high temperatures compared to NiCr partially oxidized coating, by a factor of ten.
Conclusions
-
1.
Partially oxidized NiCr and NiCrBSiFe coatings have been effectively developed on MDN321 steel substrate through a combination of flame spray and plasma spray processes. These coatings, produced under specified spray parameters, exhibit a seemingly dense structure with an average thickness of 250 μm. The average density, hardness, and adhesion strength of the coatings are measured at 5.28 and 5.46 g/cc, 327 and 810 HV, and 10.91 and 12.89 MPa, respectively.
-
2.
A significant difference in wear rates between the coating and substrate was observed. In NiCrBSiFe coating, the wear mechanism observed at lower speeds and loads is micro-brittle and mechanism shifts to abrasive wear at higher speeds and loads. Operating at a sliding velocity of 1 m/s under a 10 N load, the substrate’s wear rate was found to be 3.56 times higher than that of the coating, whereas for NiCr coating, it was 2.78 times higher. In the NiCr partially oxidized coating, the wear mechanism observed involves spallation of the coating at higher loads and adhesive wear at lower loads.
-
3.
The coefficient of friction experiences a decline with an increase in both the normal load and sliding velocity across both coatings. Elevated normal loads generate heat at the interface, consequently reducing the frictional coefficient.
-
4.
NiCr partially oxidized coating demonstrates a superior wear rate coefficient (3.2869 × 10–4 mm3/N-m) compared to NiCrBSiFe partially oxidized coating (3.6125 × 10–4 m3/N-m). Further increases in load and speed induce a performance shift in these coatings, evident at 12 N-m/s. This critical point serves as a valuable guide in selecting process parameters for the pin-on-disc tribometer.
-
5.
Thermo gravimetric analysis of the coatings revealed a weight loss percentage of 1.42 for NiCrBSiFe and 14.09 for NiCr coatings. These findings highlight the NiCrBSiFe partially oxidized coating as being tenfold more stable at high temperatures compared to the NiCr partially oxidized coating. Thus, NiCrBSiFe coating is better suitable on boiler tube steel to withstand high temperature conditions.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Holmberg, K. & Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 5, 263–284. https://doi.org/10.1007/s40544-017-0183-5 (2017).
Song, H. et al. Laser melting deposition of K403 Superalloy: the influence of processing parameters on the microstructure and wear performance. J. Alloys Compd. 805, 551–564. https://doi.org/10.1016/j.jallcom.2019.07.102 (2019).
Zhao, Y., Wu, X., Du, H., Shangguan, X. & He, W. Influence of different laser re-melting paths on the microstructure of a spray coating: defect prevention and tribological properties. Surf. Coat. Technol. 367, 11–18. https://doi.org/10.1016/j.surfcoat.2019.01.118 (2019).
Li, X. et al. Design, preparation, microstructure and properties of novel wear-resistant stainless steel-base composites using laser melting deposition. Vacuum 165, 139–147. https://doi.org/10.1016/j.vacuum.2019.04.016 (2019).
King, D., Middendorf, J., Cissel, K., Key, T. & Carney, C. Selective laser melting for the Preparation of an ultra-high temperature ceramic coating. Ceram. Int. 45, 2466–2473. https://doi.org/10.1016/j.ceramint.2018.10.173 (2019).
Vaira Vignesh, R. & Padmanaban, R. Influence of friction stir processing parameters on the wear resistance of aluminium alloy AA5083, Mater. Today Proc. 5 7437–7446. (2018). https://doi.org/10.1016/j.matpr.2017.11.415
Medabalimi, S., Hebbale, A. M., Gudala, S., Rokkala, U. & Ramesh, M. R. International journal of refractory metals and hard materials studies on high temperature erosion behavior of HVOF-sprayed (Cr ₃ C ₂ -NiCr) Si and WC-Co / NiCrAlY composite coatings. Int. J. Refract. Met. Hard Mater. 127, 106970. https://doi.org/10.1016/j.ijrmhm.2024.106970 (2025).
Medabalimi, S. R. Effect of microwave hybrid heating on High-Temperature adhesive wear behavior of High-Velocity oxygen Fuel-Sprayed WC-CrC-Ni and WC-Co / NiCrFeSiB coatings. J. Mater. Eng. Perform. https://doi.org/10.1007/s11665-022-07756-7 (2022).
Chen, L. Y. et al. Improved hardness and wear resistance of plasma sprayed nanostructured NiCrBSi coating via short-time heat treatment. Surf. Coat. Technol. 350, 436–444. https://doi.org/10.1016/j.surfcoat.2018.07.037 (2018).
HAZRA, B., BARANWAL, P., BERA, S. & SHOW, B. K. Improvement in dry sliding wear resistance of Al-17Si-5Cu alloy after an enhanced heat treatment process. Trans. Nonferrous Met. Soc. China (English Ed. 28, 1705–1713. https://doi.org/10.1016/S1003-6326(18)64814-9 (2018).
Bunshah, R. F. & Rossnagel, S. M. Handbook of Hard Coatings (Deposition Techonologies, Properties and Applications, 2001).
Behera, N. & Medabalimi, S. Effect of Impact Angles and Temperatures on the Solid Particle Erosion Behavior of HVOF Sprayed WC-Co / NiCr / Mo and Cr 3 C 2 -CoNiCrAlY (Coatings, 2023).
Carpio, P. et al. Microstructure and mechanical properties of plasma spraying coatings from YSZ feedstocks comprising nano- and submicron-sized particles. Ceram. Int. 41, 4108–4117. https://doi.org/10.1016/j.ceramint.2014.11.106 (2015).
He, L., Tan, Y., Wang, X., Xu, T. & Hong, X. Microstructure and wear properties of Al2O3-CeO 2/Ni-base alloy composite coatings on aluminum alloys by plasma spray. Appl. Surf. Sci. 314, 760–767. https://doi.org/10.1016/j.apsusc.2014.07.047 (2014).
Feng, J. J., Liu, Y., Zhou, X., Zhang, W. & Cao Tribological properties of NiCr-ZrO2 (Y2O3)-SrSo4 composites at elevated temperatures. Ceram. Int. 42, 12981–12987 (2016).
Kai, C. D. et al. Excellent wear resistance of plasma-sprayed amorphous Al2O3-Y3Al5O12 ceramic coating, surf. Coat. Technol. 326, 96–102. https://doi.org/10.1016/j.scriptamat.2017.03.027 (2017).
Kumar, V. & Ramesh, B. M. R. Thermal analysis of a plasma sprayed ceramic coated diesel engine piston, trans. Indian Inst. Met. 71, 319–326. https://doi.org/10.1007/s12666-017-1184-9 (2018).
Unocic, K. A. & Pint, B. A. Oxidation behavior of co-doped NiCrAl alloys in dry and wet air. Surf. Coat. Technol. 237, 8–15. https://doi.org/10.1016/j.surfcoat.2013.07.068 (2013).
Karaoglanli, A. C., Dikici, H. & Kucuk, Y. Effects of heat treatment on adhesion strength of thermal barrier coating systems. Eng. Fail. Anal. 32, 16–22. https://doi.org/10.1016/j.engfailanal.2013.02.029 (2013).
Reddy, N. C., Kumar, B. S. A., Ramesh, M. R. & Koppad, P. G. Microstructure and adhesion strength of Ni 3 Ti coating prepared by mechanical alloying and HVOF 1. Phys. Met. Metallogr. 119, 462–468. https://doi.org/10.1134/S0031918X18050113 (2018).
Ghadami, F. & Rouh Aghdam, A. S. Preparation of NiCrAlY/nano-CeO2 powder with the core-shell structure using high-velocity oxy-fuel spraying process. Mater. Chem. Phys. 243 https://doi.org/10.1016/j.matchemphys.2019.122551 (2020).
Taylor, T. A. & Walsh, P. N. Dilatometer studies of NiCrAlY coatings, surf. Coat. Technol. 188–189. https://doi.org/10.1016/j.surfcoat.2004.08.003 (2004).
Babasafari, Z. et al. Effect of silicon and partitioning temperature on the microstructure and mechanical properties of high-carbon steel in a quenching and partitioning heat treatment. J. Mater. Sci. 56, 15423–15440. https://doi.org/10.1007/s10853-021-06270-w (2021).
Badiger, P. V., Desai, V., Ramesh, M. R., Joladarashi, S. & Gourkar, H. Tribological behaviour of monolayer and multilayer Ti-based thin solid films deposited on alloy steel Tribological behaviour of monolayer and multilayer Ti-based thin solid fi lms deposited on alloy steel, (2019).
Ramesh, M. R., Medabalimi, S., Kumar, R. S., Prasad, C. D. & Sollapur, S. B. Cyclic oxidation and Hot-Corrosion behavior of HVOF-Sprayed NiCrAl coating on industrial boiler tube steels. Jom 76, 3172–3184. https://doi.org/10.1007/s11837-024-06526-1 (2024).
Medabalimi, S. R. & Kadoli, R. M. R. R. Developing partially oxidized NiCr coatings using the combined flame spray and plasma spray process for improved wear behaviour at high temperature. Wear 1–11. (2021).
Bolelli, G. et al. Tribology of NiCrAlY + Al2O3 composite coatings by plasma spraying with hybrid feeding of dry powder + suspension. Wear 344–345. https://doi.org/10.1016/j.wear.2015.10.014 (2015).
Ramesh, M. R., Prakash, S., Nath, S. K., Sapra, P. K. & Venkataraman, B. Solid particle erosion of HVOF sprayed WC-Co/NiCrFeSiB coatings. Wear 269, 197–205. https://doi.org/10.1016/j.wear.2010.03.019 (2010).
Medabalimi, S. R., Ramesh, M. R. & Kadoli, R. High-Temperature solid particle Erosion behavior of partially oxidized NiCrBSiFe/NiCr plasma spray coatings. J. Therm. Spray. Technol. 30, 1638–1652. https://doi.org/10.1007/s11666-021-01225-8 (2021).
Medabalimi, S. R., Ramesh, R., Kadoli & M R and High-temperature wear and frictional behavior of partially oxidized al with NiCr composite coating. Mater. Res. Express. 6, 1–13. https://doi.org/10.1088/2053-1591/ab5c34 (2019).
Rao, M. S., Ramesh, M. R. & Kadoli, R. Solid particle Erosion behavior of partially oxidized al with NiCr composite coating at elevated temperature. J. Mater. Eng. Perform. https://doi.org/10.1007/s11665-021-05668-6 (2021).
Reddy, N. C. et al. HVOF sprayed Ni3Ti and Ni3Ti+(Cr3C2 + 20NiCr) coatings: microstructure, microhardness and oxidation behaviour. J. Alloys Compd. 736, 236–245. https://doi.org/10.1016/j.jallcom.2017.11.131 (2018).
Vorokh, A. S. Scherrer formula: Estimation of error in determining small nanoparticle size. Nanosyst Phys. Chem. Math. 9, 364–369. https://doi.org/10.17586/2220-8054-2018-9-3-364-369 (2018).
Irisawa, T. & Matsumoto, H. Thermal shock resistance and adhesion strength of plasma-sprayed alumina coating on cast iron. Thin Solid Films. 509, 141–144. https://doi.org/10.1016/j.tsf.2005.09.132 (2006).
Mathapati, M., Ramesh, M. R. & Doddamani, M. High temperature erosion behavior of plasma sprayed NiCrAlY/WC-Co/cenosphere coating, surf. Coat. Technol. 325, 98–106. https://doi.org/10.1016/j.surfcoat.2017.06.033 (2017).
Hidalgo, V. H., Varela, F. J. B., Menéndez, A. C. & Martínez, S. P. A comparative study of high-temperature erosion wear of plasma-sprayed NiCrBSiFe and WC-NiCrBSiFe coatings under simulated coal-fired boiler conditions. Tribol Int. 34, 161–169. https://doi.org/10.1016/S0301-679X(00)00146-8 (2001).
Miguel, J. M., Guilemany, J. M. & Vizcaino, S. Tribological study of NiCrBSi coating obtained by different processes. Tribol Int. 36, 181–187. https://doi.org/10.1016/S0301-679X(02)00144-5 (2003).
Liu, X. B. et al. Effects of temperature and normal load on tribological behavior of nickel-based high temperature self-lubricating wear-resistant composite coating. Compos. Part. B Eng. 53, 347–354. https://doi.org/10.1016/j.compositesb.2013.05.032 (2013).
Chowdhury, M. A., Khalil, M. K., Nuruzzaman, D. M. & Rahaman, M. L. The effect of sliding speed and normal load on friction and wear property of aluminum. Int. J. Mech. Mech. Eng. 11, 53–57 (2011).
Chen, W., Zhang, D. & Ai, X. Effect of load on the friction and wear characteristics of Si 3 N 4 -hBN ceramic composites sliding against steels. Ceram. Int. 43, 4379–4389. https://doi.org/10.1016/j.ceramint.2016.12.084 (2017).
Al-samarai, R. A., Haftirman, K. R., Ahmad, Y. & Al-Douri Effect of load and sliding speed on wear and friction of Aluminum - Silicon cast alloy. Int. J. Sci. Res. Publ. 2, 3–6 (2012).
Huang, C., Du, L. & Zhang, W. Preparation and characterization of atmospheric plasma-sprayed NiCr/Cr3C2-BaF2·CaF2 composite coating. Surf. Coat. Technol. 203, 3058–3065. https://doi.org/10.1016/j.surfcoat.2009.03.027 (2009).
Fernández, E. et al. Wear behaviour of laser clad NiCrBSi coating. Wear 259, 870–875. https://doi.org/10.1016/j.wear.2005.02.063 (2005).
Anand Babu, K., Jegadeeswaran, N., Nithin, H. S. & Kapilan, N. Studies on solid particle erosion by HVOF sprayed 25% (Cr3C2-25 (Ni20Cr)) + 75% NiCrAlY on Ti-31, Mater. Today Proc. 25 (2020). https://doi.org/10.1016/j.matpr.2020.10.431
Somasundaram, B., Kadoli, R., Ramesh, M. R. & Ramesh, C. S. High temperature corrosion behaviour of HVOF sprayed WC-CrC-Ni coatings. Int. J. Surf. Sci. Eng. 10, 400–413. https://doi.org/10.1504/IJSURFSE.2016.077542 (2016).
Chinnathaypgal, V. N., Samanta, S. K., Motagondanahalli, R. & Vijay, R. Evaluation of Wear Behaviour of Metal Injection Moulded (Nickel Based Metal Matrix Composite, 2018).
Author information
Authors and Affiliations
Contributions
S.M, U.R Conceptualization, Methodology & Formal analysis, Writing – original draft, S.G, N.B Conceptualization, Methodology, Formal analysis, R.M.R, M.D Review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Medabalimi, S., Rokkala, U., Gudala, S. et al. Wear and frictional behaviour of partially oxidized and plasma sprayed NiCr and NiCrBSiFe coatings. Sci Rep 15, 19717 (2025). https://doi.org/10.1038/s41598-025-99567-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99567-8