Abstract
This study aimed to examine the interplay between epicardial adipose tissue (EAT) features, macrophage polarization, and the cytokines Resistin and Apelin in the context of coronary artery disease (CAD). Using a case-control design with 21 CAD and 20 non-CAD individuals, the study collected demographic data, cardiovascular risk factors, and medical histories. Metabolic risk factors were assessed through laboratory tests, and CAD presence was confirmed by imaging studies. Detailed measurements of epicardial adipose characteristics were obtained through CT scans. Blood samples were analyzed for Resistin and Apelin levels, and tissue samples from EAT for macrophage polarization. The results revealed no significant differences in EAT volume and density between CAD and non-CAD groups, but the CAD group exhibited a significantly higher Calcium score. Apelin and Resistin mRNA expression levels in the right ventricular epicardial and atrioventricular fat tissue showed significantly lower Apelin and higher Resistin levels in CAD patients. CD206 expression levels in EAT were substantially lower in the CAD group, while CD11c expression levels were significantly higher. The CAD group exhibited a significantly higher CD11c/CD206 ratio in adipose tissue macrophages. This investigation highlights the significance of molecular characteristics of EAT in CAD development. While no significant differences were found in EAT volume and density, lower Apelin and higher Resistin mRNA expression in CAD patients’ right ventricular fat tissue were observed. Changes in macrophage polarization markers, lower CD206 and higher CD11c, along with a higher CD11c/CD206 ratio in the macrophages of CAD patients have been shown in two investigated regions of EAT.
Similar content being viewed by others
Introduction
Ischemic heart disease is the first cause of mortality worldwide1. According to World Health Organization reports, 17.9 million deaths worldwide in 2019 were attributable to cardiovascular diseases, 32% of the total deaths. Heart attack and stroke deaths accounted for 85% of this mortality. In 2019, non-communicable diseases led to 17 million premature deaths (before age 70), 38% of which were related to cardiovascular diseases2. CAD refers to a group of complex coronary vessel pathologies, all characterized by the hallmark presence of atherosclerosis. This condition can be presented with chest pain or other acute manifestations, known as acute coronary syndrome, or remain asymptomatic for a while. However, early diagnosis and prognosis of the disease can be crucial in preventing consequent irreversible adverse effects3.
EAT is a type of adipose tissue located within the pericardial space4. Recent investigations demonstrate that fatty acid breakdown and pro-inflammatory cytokine secretion rates are higher in EAT than in subcutaneous and omental adipose tissue5. Although EAT contains only 1% of the overall fat mass, studies have shown that its quantity is strongly related to different cardiac and metabolic risk factors, independent of intra-abdominal visceral adiposity6. The majority of EAT is located in atrioventricular and interventricular grooves of the heart asymmetrically, and the association of EAT with atherosclerosis is region-specific7. Previous studies have demonstrated that EAT thickness is significantly associated with CAD risk and is considerable compared to the normal population in CAD patients. Epicardial adipose tissue thickness is shown to be a valuable diagnostic marker for coronary artery disease involvement and severity8.
The macrophages in the epicardial adipose tissue are involved in atherosclerotic plaque formation. They can be divided into the M1/M2 phenotype. M1 macrophages, marked by CD11c positivity, produce inflammatory cytokines (e.g., TNF-α, IL-1β) through TLR and NFκB pathways, while M2 macrophages, identified by CD206 positivity, exhibit anti-inflammatory activity via PPARγ and IL-4-STAT6 pathways9,10,11,12,13,14. Polarization of M1 macrophages can lead to the progression and development of an atherosclerotic plaque, while reversion to M2 macrophages can improve plaque resolution through anti-inflammatory effects and prevent atherosclerosis15,16. In previous studies, the ratio of CD11c/CD206-positive cells, as a marker of macrophage polarization, is used as a prognostic factor of atherosclerosis plaque formation. It was demonstrated that coronary atherosclerosis is associated with the expression of pro- and anti-inflammatory adipocytokines and consequent macrophage activation in the epicardial adipose, which can lead to CAD17.
Among the various cytokines, serum Resistin is a potent biomarker elevated in patients with coronary artery disease and myocardial infarction12 and plays a significant role in insulin resistance, inflammation, and vascular dysfunction, linking metabolic signals to atherosclerosis and CAD. Elevated Resistin levels in EAT and serum have been associated with CAD and myocardial infarction18,19,20,21. Conversely, Apelin is involved in cardiovascular regulation and energy metabolism and is lower in CAD patients, highlighting its potential anti-ischemic properties. The contrasting roles of Resistin and Apelin underline their significance in CAD pathology and their potential as biomarkers for disease progression and therapeutic targets22,23.
In previous studies, parameters such as EAT density, calcium score, macrophage polarization, cytokine expression levels, and serum levels have not been comprehensively evaluated together. In addition, the location of EAT was not noted enough as a considerable factor. In this study, the inflammatory profile of EAT was investigated alongside imaging findings to propose a novel diagnostic marker for detecting the inflammatory profile of EAT and facilitating the early prevention of atherosclerosis.
In this study, we aimed to reveal the association of coronary artery disease with epicardial adipose tissue features. Also, we tried to identify the macrophages’ polarization and activation with CD11c and CD206 markers and analyzed the serum Resistinan and Apelin concentration as inflammatory cytokines of the epicardial tissue in the Left Atrio-Ventricular (AV) groove and Right Ventricular (RV) free wall to investigate novel diagnostic, prognostic and therapeutic targets.
Materials and methods
Study population
Case and control group definition
In this study, 21 adult patients between 18 and 70 years old with coronary artery disease, who underwent coronary angiography with moderate to severe coronary artery stenosis and became candidates for CABG surgery, were studied as a case group. Non-CAD patients with normal coronary arteries in angiography who underwent cardiac surgery for non-coronary etiology were considered as a control group.
Exclusion criteria
Patients who had a history of heart surgery or valve replacement in the last 6 months, infective endocarditis, rheumatic heart valve involvements, heart failure, obesity with BMI greater than 30 Kg/m2, liver failure with transaminase more than 40 unit/L, renal failure with creatinine more than 1.1 mg/dL were excluded from the sample population of the study.
Patients’ information registration
For each patient, demographic features, including age, gender, and race are obtained. A complete history and physical examination were conducted for each patient. Cardiovascular risk factors containing diabetes, smoking, and family history were determined. All information was recorded. The patient’s medical and drug history were recorded, including aspirin, statins, ACE inhibitors and ARBs, beta-blockers, calcium channel blockers, and nitrates consumption.
Laboratory tests
Considerable risk factors for metabolic and cardiovascular disease among the laboratory tests were investigated by Auto-Chemistry Analyzer (BANA-803 company: BioLAB- Canada) and Fasting Blood Sugar (FBS, mg/dL), Low-Density Lipoprotein Cholesterol (LDL-C, mg/dL), High-Density Lipoprotein Cholesterol (HDL-C, mg/dL), Triglycerides (TG, mg/dL), and C-Reactive Protein (CRP, mg/L) have compared between the two groups.
Imaging study
Echocardiography
The echocardiographic information in the patients’ electronic medical records were obtained, and Echocardiography findings, including LVEF and valvular status, were recorded.
Angiography
The angiography findings were used to confirm the stenosis of coronary arteries. The results were used to divide the patients into two groups; CAD and non-CAD patients. Also, the severity of stenosis and the number of vessel involvements were considered.
Cardiac imaging to determine the thickness, volume and density of epicardial adipose tissue
Coronary CTA for measurement of the size and thickness of epicardial adipose tissue and calcium scoring were conducted using a dual-source 256-slice CT scanner (SOMATOM Definition Flash, Siemens Healthcare, Germany). Prior to administering contrast, a non-contrast cardiac CT scan was performed from the tracheal carina to the diaphragm.
The thickness of epicardial fat was estimated separately in different parts of the heart structure, including the Left atrioventricular groove, Right atrioventricular groove, Anterior interventricular groove in the four-chamber view and Inferior interventricular groove, Superior interventricular groove and right ventricle free wall in short axis view.
Images for calcium scoring were reconstructed with a slice thickness of 2.5–3 mm and a slice interval of 1.25–1.5 mm, using a tube voltage of 120 kVp. The total calcium score was determined with Vitrea2 software, following the Agatston method based on Hounsfield Units, which were automatically detected and color-marked by the software. The total calcium score was calculated by summing all identified lesions24,25.
Blood sample Preparation
About 5 ml of peripheral blood sample was collected from all the patients in the clot activator tubes and centrifuged at 800 × g for 40 min and isolated serum was used for detection of serum level of Apelin (orb1762782) as anti-inflammatory adipokine and Resistin (orb775090) as pro-inflammatory adipokine through the Enzyme-Linked Immunosorbent Assay (ELISA).
Tissue sampling method
In this study, samples containing 5 mm3 EAT were harvested during surgery, from the right ventricle and the atrioventricular groove regions. The samples for immunofluorescence assay were immediately placed in a 10% formaldehyde solution and fixed. The tissue samples for RNA extraction immediately after isolation were kept in vial containing DMEM culture medium and then was removed from the culture medium and stored at -70° C for further evaluations.
RNA extraction of total RNA from EAT
Total RNA was extracted from the EAT using extraction kit (NORGEN, Canada), as previously described26, the purity of the extracted RNAs was measured with a Nanodrop spectrophotometer (ThermoFisher Scientific, USA) and absorbance ratio (260/280 nm) was evaluated. RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, USA) was used to reverse transcription of 1 mg of RNA into cDNA and SYBR Green Master Mix (Takara Bio, Japan) was used for quantitative real-time Polymerase Chain Reaction (qPCR) which was performed in a Rotor-Gene real-time thermocycler (QIAGEN, Germany). The expression levels of Apelin and Resistin genes were normalized to the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and relative gene expressions were calculated with the 2ˆ−ΔCt method. The sequences of primes were as follows (QIAGEN, Germany): human Resistin (5’-AGAGGCGCCTGCAGGATGAAAG- 3’/5’-TGGAGGTGACGCTCTGGCAC-3’), human Apelin (5’-AAGGCACCATCCGATACCTG-3’/5’-ATGGGACCCTTGTGGGAGA-3’) and human GAPDH (5’-CGCCAGCCGAGCCACATC-3’/5’-GCCAGCATCGCCCCACTTGA-3’).
Measurement of serum level of biomarkers
The serum level of Resistin and Apelin was detected by the enzyme-linked immunosorbent assay (ELISA) technique using commercially available kits and according to the instructions of the kit (Biorbyt, Germany). The marker concentrations were detected with sensitivity of 8.25 pg/mL for Apelin and 0.078 ng/mL for Resistin kits.
Expression of CD11c and CD 206 markers
In this study, CD11c as an inflammatory marker and CD206 as an anti-inflammatory marker is measured in the AV groove and RV-free wall by immunofluorescence assay. Immunofluorescence was done on adipose tissue sections as previously describe27. Primary antibodies used were anti-human CD11b (Abcam, ab8878) and anti-human CD206 (Abcam, ab313398). To determine unspecific fluorescent signal, negative controls with no primary antibodies were prepared and no unspecific signal was seen. Immunofluorescence was quantified using fluorescence microscope with magnification of 400x (Olympus, Japan). A photo of each tissue section was taken and analyzed for quantitative analysis using Image J software.
Ethical considerations
Before conducting the study, necessary information about the research plan and the confidentiality of personal information was presented to the patient. Then, a written informed consent form was obtained from the patients. Sampling and conducting experiments were kept anonymous and confidential. Also, Ethical approval was obtained from the Ethics Committees of Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran (IR.IUMS.FMD.REC.1399.496), and all stages of the experiments were under the supervision of the Ethics Committees. In addition to this, all methods were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
Statistical analysis involved summarizing categorical (nominal) variables using frequency and percentage distributions, whereas continuous variables were described using mean and standard deviation (SD) for normally distributed data, or median and interquartile range (IQR) for non-normally distributed data. Normality of the data was assessed using the Kolmogorov-Smirnov test. Group comparisons between two independent samples were performed using either the independent Student’s t-test for normally distributed variables or the non-parametric Mann-Whitney U test for variables that did not follow a normal distribution. Correlation analyses were conducted using Pearson’s correlation coefficient for normally distributed variables and Spearman’s rank correlation coefficient for non-normally distributed variables. All statistical analyses and graphical visualizations, including charts and plots, were carried out using GraphPad Prism version 10. A p-value of less than 0.05 was considered statistically significant.
Results
Demographic features
A total of 21 patients with CAD and 20 non-CAD patients who underwent CABG surgery were included in the study. The average age of patients in the CAD and non-CAD was 61.2 and 53.2 years, respectively.
Indication for surgery in the case group included 18 patients (85.7%) who had effort angina, 1 patient (4.8%) who had NSTEMI, and 2 patients (9.6%) who had unstable angina. As expected, the age of patients in the CAD group was significantly higher than the non-CAD group (P = 0.030). In the CAD group, 81% of the patients were male; in the non-CAD group, it was 45%. Portion of males and females was significantly different among the groups (Table 1).
Risk factor distribution between groups
Cardiovascular risk factors in patients of the two groups are compared in Table 1. There was no difference between the cardiovascular risk factors in the two study groups.
Drug history of the case and control groups
Drug history of the patients, including Aspirin, Statins, Angiotensin-converting-enzyme inhibitors, Angiotensin receptor blockers, Beta-Blocker, Calcium channel blockers and Nitrates, were investigated and compared between two groups which Aspirin, Statins and Nitrates were consumed more in the CAD group (Table 1).
Laboratory and paraclinical findings
Laboratory findings, including FBS, LDL-C, HDL-C, TG and CRP, are compared between the two groups. Findings demonstrated that there is not any significant difference in the lab test results between the two groups (Table 1).
Imaging study’s findings
The echocardiographic findings demonstrated that the left ventricular Ejection Fraction (LVEF) of patients in the CAD group was 50%, and in the non-CAD group was 52% average which was similar (P value = 0.6). Angiography results showed that in CAD patients, a total of 17 patients (81%) involved three vessels disease (3VD), 1 patient (8.4%) involved two vessels disease (2VD), and 3 patients (14.3%) involved single vessel disease (SVD) in angiography (Table 2).
Comparison of EAT thickness, density, volume and calcium score in studied patients
The thickness of EAT
EAT thickness in the parts mentioned above in the studied patients in CAD and non-CAD groups was not significantly different (Table 2).
Calcium score
The average calcium score of patients in the CAD group was 757.2, and in the non-CAD group, it was 53.98. as expected, the CAD group was significantly higher than the non-CAD group. (P < 0.0001) (Table 2; Fig. 1).
Volume and density of epicardial fat tissue
In this study, the volume and density of epicardial fat tissue (fat tissue volume and fat tissue density) were determined and recorded in two CAD and non-CAD groups, which can be observed in Table 2; Figs. 1. The volume and density of epicardial fat in the patients of this study in CAD and non-CAD groups were not significantly different.
Comparison of (A) Calcium score, (B) Fat tissue volume, and (C) Fat tissue density in CAD and non-CAD patients. (D) Right AV adipose tissue groove thickness, (E) Left AV groove adipose tissue thickness, (F) RV free wall adipose tissue thickness, (G) Inferior interventricular groove adipose tissue thickness, (H) Anterior interventricular groove adipose tissue thickness and (I) Superior interventricular groove adipose tissue thickness between the two CAD and non-CAD patients.
Measurement of serum Apelin and resistin levels
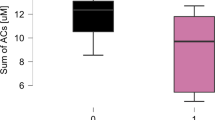
Serum concentrations of Apelin and Resistin were assessed using an ELISA kit, revealing levels of 1.023 ± 0.25 ng/mL and 0.783 ± 0.166 in the non-CAD and coronary artery disease CAD groups, respectively, for Apelin. Similarly, Resistin levels were determined to be 6.28 ± 1.67 ng/mL and 8.10 ± 2.00 ng/mL in the non-CAD and CAD groups, respectively (Table 3; Fig. 2).
Evaluation of Apelin and resistin mRNA expression levels
The relative mRNA expression levels of two adipokines, Apelin and Resistin, were compared in the RV free wall and AV groove regions of the two study groups.
The relative expression level of Apelin mRNA in patients with CAD in both RV free wall and AV groove regions was significantly lower than in patients without CAD (P < 0.0001) (Table 3; Fig. 3). Also, in patients with CAD, the relative expression level of Apelin mRNA was compared between two regions of RV free wall and AV groove regions. The obtained results showed that Apelin level in RV free wall was significantly higher than AV groove regions (P < 0.0001).
The relative expression level of Resistin was also compared in two study groups and it was significantly higher in patients with CAD in both RV free wall and AV groove than non- CAD group (P < 0.0001) (Fig. 3). Also, comparison of Resistin level in patients with CAD in two regions showed no significantly different between RV free wall and AV groove (Fig. 3).
Also it should be considered that in the CAD group, the expression of both Apelin and Resistin were notably higher in RV groove compared to AV groove (Fig. 3).
Comparison of the relative Apelin expression in (A) right ventricular epicardial fat and (B) atrioventricular epicardial fat in two CAD and non-CAD groups. (C) Comparison of the relative Apelin expression in atrioventricular epicardial fat and right ventricle in CAD patients. Comparison of the relative Resistin expression in (D) right ventricular epicardial and (E) atrioventricular epicardial fat in two CAD and non-CAD groups (F) Comparison of the relative Resistin expression in atrioventricular epicardial fat and right ventricle in CAD patients.
Correlation of EAT thickness with Apelin and resistin level
A correlation analysis examining Apelin and Resistin serum levels with the right ventricle and atrioventricular epicardial fat demonstrated a significant association. Elevated Resistin levels correlated with both regions of epicardial fat thickness (p-value 0.044 for the right ventricle and 0.01 for the atrioventricular region). Conversely, higher levels of Apelin were associated with lower epicardial fat thickness in both regions (p-value less than 0.0001 for both regions). Notably, this association was found to be stronger in Apelin compared to Resistin, as indicated by the R correlation score in statistical analyses (Fig. 4).
Macrophage polarization markers; expression of CD11 and CD 206 markers
In the epicardial fat tissue, the expression levels of CD206 and CD11c were compared between the CAD and non-CAD groups in both the right ventricle and atrioventricular epicardial fat, as outlined in Table 4. The findings reveal a substantial decrease in CD206 expression in both the right ventricle and atrioventricular epicardial fat within the CAD groups compared to the non-CAD group (Table 4; Fig. 5). Conversely, CD11c expression in both regions demonstrates a noteworthy elevation in the CAD groups compared to the non-CAD group (Table 4; Fig. 6).
It is noteworthy that the CD11c/CD206 ratio, serving as a representative indicator of M1/M2 macrophage polarization, is higher in the CAD groups compared to the non-CAD groups in both the right ventricle and atrioventricular regions (Fig. 7).
Discussion
The present study delved into the examination of EAT volume and density in both CAD and non-CAD groups. Interestingly, no significant differences were observed in these parameters. However, the Calcium score in the CAD group exhibited a notable increase. Furthermore, the mRNA expression levels of Apelin and Resistin in the right ventricular epicardial and atrioventricular fat tissue demonstrated a marked decrease in Apelin levels and an elevation in Resistin levels among CAD patients. Examining CD206 expression levels in EAT across the two regions, a considerable decrease was noted in the CAD group compared to the control group. Conversely, CD11c expression levels exhibited a significant increase in the CAD group. Moreover, the CAD group displayed a significantly higher ratio of CD11c/CD206 in adipose tissue macrophages within the two regions.
EAT stands out due to its distinctive anatomical position, situated closely to the heart. Its transcriptome and secretome exhibit significant distinctions compared to other adipose tissues28. The physiological and pathological characteristics of EAT are contingent upon its specific location. EAT demonstrates a dual nature; it can serve as a robust protective barrier for the myocardium through its dynamic, brown fat-like thermogenic activity. Simultaneously, it can pose risks by releasing pro/anti-inflammatory cytokines through paracrine or endocrine pathways, potentially causing harm29. Epicardial fat is a source of various adipokines. Through the paracrine secretion of both pro- and anti-inflammatory cytokines, epicardial adipose tissue may contribute to adiposity-related inflammation and atherosclerosis30,31,32. Resistin, a cytokine from adipocytes and monocytes, plays a key role in regulating insulin action, energy balance, and glucose-lipid homeostasis. It is strongly associated with insulin resistance and its complications, linking metabolic signals, inflammation, and vascular dysfunction33,34,35,36. On the other hand, Apelin, an endogenous 77 amino acid peptide, exhibits widespread distribution in tissues, implying involvement in various physiological processes such as blood pressure regulation, fluid hemostasis, stress response, cardiac function, angiogenesis, and energy metabolism37. Moreover, it plays a role in pathological conditions like heart failure, obesity, and diabetes38.
Several studies highlighted the role of Resistin in EAT. Nikoleta Karampetsou studied 32 CAD patients undergoing CABG surgery, finding significantly elevated levels of Resistin, MMP-9, CCL-5, and follistatin in diseased EAT segments compared to non-diseased segments, aligning with our findings18. Christou et al. revealed increased mRNA expression of Resistin in EAT associated with left ventricular diastolic dysfunction, while adiponectin was linked to left ventricular systolic dysfunction19. Maciej Rachwali et al. showed that patients with a history of MI had significantly higher Resistin levels in EAT, independent of other risk factors20. Silvia Langheim et al. demonstrated that acute coronary syndrome is associated with increased Resistin expression and secretion in EAT, contributing to enhanced endothelial permeability. These studies collectively emphasize the role of Resistin in localized and systemic inflammation in CAD22.
In the present study, Resisitin serum level was higher in CAD patients, while Apelin serum level was significantly lower in CAD patients. Similarly, studies have shown that plasma Resistin levels are linked to inflammation markers and can predict coronary atherosclerosis, while high Resistin and adiponectin levels are associated with increased mortality, particularly in MI patients. Conversely, higher Apelin levels are correlated with better coronary collateral development. Additionally, epicardial adipose tissue volume is significantly higher in CAD patients, making it a distinguishing feature among at-risk groups21,39. In a study by Mohamed Hassan et al., the CAD patients exhibited a significantly higher total EAT volume compared to the control group. In their study, serum Resistin showed a significant correlation with EAT volume, which is similar to our findings. It can be concluded that the volume of peri-coronary epicardial adipose tissue is significantly associated with the extent and severity of coronary atherosclerosis, potentially serving as a determinant of plaque vulnerability40. Of course, the findings of increased EAT volume were opposed to our study and we did not observe a significant change in the EAT volume in comparison of CAD and non-CAD patients.
In the present study, we excluded patients with a BMI > 30 kg/m², as general obesity is an independent risk factor for CVD and is often linked to a pro-inflammatory profile that could confound results41,42. Our goal was to isolate the specific role of EAT in CVD-related inflammation, avoiding the influence of systemic inflammation seen in general obesity. This allowed us to focus solely on the contribution of pericardial fat to inflammation and its direct impact on cardiovascular health, without overlap from broader obesity effects. This approach allows for a more accurate assessment of the localized effects of EAT in the context of cardiovascular risk. In addition, in this study, we observed the lack of significant differences in the metabolic profile between CAD and non-CAD groups, which can be attributed to several factors. A notable proportion of patients with valvular heart disease are also affected by metabolic syndrome, a condition that predisposes individuals to CAD. This shared metabolic risk likely reduces the disparity between the two groups43,44,45,46. Furthermore, many patients in both groups were receiving medical treatment for metabolic conditions, such as diabetes, hypertension, or dyslipidemia. These interventions may have effectively controlled metabolic abnormalities, minimizing observable differences at the time of evaluation.
Several studies highlight the relationship between EAT and metabolic and cardiovascular risk factors. Tzung-Dau Wang et al., in a study of 148 patients, showed that EAT thickness in the left atrioventricular groove correlated significantly with all components of metabolic syndrome—blood pressure, lipids, and glucose47. Another study of CAD patients found thicker EAT in individuals with diabetes and hypercholesterolemia and introduced the adiponectin/Resistin index (ADIPO-IRAR) as an independent predictor of EAT thickness48. Koshelskaya et al. further revealed elevated EAT deposition and lower adiponectin levels in type 2 diabetes patients, contributing to higher cardiovascular risk. Notably, EAT thickness in the left atrioventricular groove, rather than total EAT volume, remained strongly correlated with coronary atherosclerosis and metabolic syndrome after adjusting for other risk factors, with plasma Resistin levels showing a unique association49.
The specific location of EAT can also be considered as a notable parameter in CAD disease. It was demonstrated that although both the total volume of EAT and the thickness of EAT in the left atrioventricular groove were initially found to be associated with coronary atherosclerosis, after adjusting for conventional risk factors such as body mass index, waist circumference, and intra-abdominal visceral fat, only the thickness of EAT in the left atrioventricular groove remained significantly correlated with coronary atherosclerosis in a dose-dependent manner. Furthermore, the thickness of EAT in the left atrioventricular groove was uniquely correlated with metabolic syndrome as well as with plasma levels of Resistin47,50.
The lack of a significant difference in the number of hypertensive patients between the two groups warrants further discussion. While there are 10 hypertensive patients in the CAD group compared to 5 in the non-CAD group, hypertension is known to be closely associated with inflammation. This raises the question of whether hypertension might influence the observed differences in Apelin and Resistin mRNA expression levels and the shift in macrophage polarization from M2 to M1. However, this remains uncertain and requires further investigation51,52.
Macrophages in adipose tissue exist as pro-inflammatory M1 and anti-inflammatory M2 phenotypes, each with distinct functions in inflammation and tissue homeostasis. Imbalances in the M1/M2 ratio are linked to obesity-related inflammation and metabolic disorders53. M1 macrophages are characterized by CD11c positivity and produce inflammatory cytokines (e.g., TNF-α, IL-1β) through TLR and NFκB pathways, while M2 macrophages, marked by CD206 positivity, exhibit anti-inflammatory activity via PPARγ and IL-4-STAT6 pathways9,10,11,12,13,54.
Hirata Y et al. demonstrated an increased M1/M2 macrophage ratio in EAT of CAD patients, correlating positively with CAD severity and pro-inflammatory cytokines, and negatively with anti-inflammatory cytokines17. Similarly, Adam R. Baker et al. found increased CD45 expression in EAT, indicating macrophage infiltration, while other studies linked M1 polarization and JNK-NF-κB activation in EAT macrophages to inflammatory signaling in CAD55,56. Wei CY et al., in a case-control study with 48 patients, showed that nitrate ester treatment reduced NF-κB activity, M1/M2 ratio, and pro-inflammatory cytokines while increasing anti-inflammatory cytokines in EAT, ameliorating CAD severity. These findings highlight the association between EAT macrophage polarization and CAD, suggesting potential therapeutic targets in adipose tissue inflammation57.
In addition, this study examined two regions of EAT, the RV free wall and the left AV groove selected for their anatomical and functional differences. Findings revealed a more pronounced inflammatory profile in the left AV groove, likely due to its proximity to coronary arteries, potentially contributing to atherosclerosis and plaque instability similar findings are mentioned in previous studies17,50,58,59. These regional differences highlight the unique roles of EAT in CAD pathogenesis and emphasize the need for targeted therapeutic strategies. The study also identified distinct molecular signatures that may be pivotal in understanding CAD mechanisms beyond structural assessments, offering insights for more effective patient care.
Limitations
We acknowledge that the limited sample size, dictated by the need to source samples from non-CAD patients undergoing non-CABG surgeries, prevented us from performing subgroup analyses, which may have provided additional insights into sex-specific differences in the studied parameters.
Suggestions and prospects
We suggest that future studies expand the sample size and diversity to validate findings and explore sex-specific differences in EAT features, macrophage polarization, and inflammatory mediators. Longitudinal studies could clarify the temporal and causal relationships and assess the therapeutic potential of targeting macrophage polarization in EAT. Advanced imaging techniques and broader biomarker panels should be employed to provide a more comprehensive understanding of EAT’s role in CAD. Additionally, investigating molecular pathways, the impact of comorbidities, and the interplay between EAT and neighboring cardiac tissues could uncover novel diagnostic, prognostic, and therapeutic strategies, while animal models may offer controlled insights into these mechanisms.
Conclusion
In summary, this study underscores the importance of molecular features within EAT in the development of CAD. Despite no notable disparities in epicardial fat volume and density, distinct patterns were identified, such as decreased Apelin and elevated Resistin mRNA expression in the right ventricular fat tissue of CAD patients. The shift in macrophage polarization from M2 to M1, as indicated by decreased CD206 and increased CD11c levels, is associated with an elevated risk of developing coronary artery disease. The inflammatory profile of EAT might be a key determinant of CAD progression. Despite the similar size of the EAT depots in both groups, the inflammatory milieu in the CAD group could lead to greater local tissue damage, endothelial dysfunction, and plaque formation.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CAD:
-
Coronary Artery Disease
- CABG:
-
Coronary Artery Bypass Graft
- BMI:
-
Body Mass Index
- FBS:
-
Fasting Blood Sugar
- LDL-C:
-
Low-Density Lipoprotein Cholesterol
- HDL-C:
-
High-Density Lipoprotein Cholesterol
- TG:
-
Triglycerides
- CRP:
-
C-Reactive Protein
- LVEF:
-
Left Ventricular Ejection Fraction
- CT:
-
Computed Tomography
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- RNA:
-
Ribonucleic Acid
- cDNA:
-
Complementary DNA
- qPCR:
-
Quantitative Real-Time Polymerase Chain Reaction
- CD11c:
-
Cluster of Differentiation 11c (M1 macrophage marker)
- CD206:
-
Cluster of Differentiation 206 (M2 macrophage marker)
- M1:
-
Pro-inflammatory macrophage phenotype
- M2:
-
Anti-inflammatory macrophage phenotype
- TNF-α:
-
Tumor Necrosis Factor-alpha
- IL-1β:
-
Interleukin-1 Beta
- PPARγ:
-
Peroxisome Proliferator-Activated Receptor Gamma
- IL-4:
-
Interleukin-4
- STAT6:
-
Signal Transducer and Activator of Transcription 6
- TLR:
-
Toll-Like Receptor
- NFκB:
-
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
- MMP-9:
-
Matrix Metalloproteinase-9
- CCL-5:
-
Chemokine (C-C Motif) Ligand 5
- RV:
-
Right Ventricle
- AV:
-
Atrioventricular
- 3VD:
-
Three-Vessel Disease
- 2VD:
-
Two-Vessel Disease
- SVD:
-
Single Vessel Disease
- IQR:
-
Interquartile Range
- SD:
-
Standard Deviation
- MI:
-
Myocardial Infarction
- CVD:
-
Cardiovascular Disease
References
Organization, W. H. The top 10 causes of death 2020 [Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
Organization, W. H. Cardiovascular diseases (CVDs). (2021).
Bergmark, B. A., Mathenge, N., Merlini, P. A., Lawrence-Wright, M. B. & Giugliano, R. P. Acute coronary syndromes. Lancet 399 (10332), 1347–1358 (2022).
Sacks, H. S. & Fain, J. N. Human epicardial adipose tissue: a review. Am. Heart J. 153 (6), 907–917 (2007).
Mazurek, T. et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108 (20), 2460–2466 (2003).
Iacobellis, G. et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 88 (11), 5163–5168 (2003).
Abbara, S. et al. Mapping epicardial fat with multi-detector computed tomography to facilitate percutaneous transepicardial arrhythmia ablation. Eur. J. Radiol. 57 (3), 417–422 (2006).
Eroglu, S. et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 19 (3), 211–217 (2009).
Arkan, M. C. et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 11 (2), 191–198 (2005).
Saberi, M. et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell. Metab. 10 (5), 419–429 (2009).
Kang, K. et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell. Metab. 7 (6), 485–495 (2008).
Bouhlel, M. A. et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell. Metab. 6 (2), 137–143 (2007).
Bronte, V. & Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 5 (8), 641–654 (2005).
Ying, W., Cheruku, P. S., Bazer, F. W., Safe, S. H. & Zhou, B. Investigation of macrophage polarization using bone marrow derived macrophages. J. Visualized Experiments: JoVE 76, 50323. (2013).
Randolph, G. J. Mechanisms that regulate macrophage burden in atherosclerosis. Circ. Res. 114 (11), 1757–1771 (2014).
Peled, M. & Fisher, E. A. Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front. Immunol. 5, 579 (2014).
Hirata, Y. et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J. Am. Coll. Cardiol. 58 (3), 248–255 (2011).
Karampetsou, N. et al. Epicardial Adipocyte-derived TNF-α modulates local inflammation in patients with advanced coronary artery disease. Curr. Vasc Pharmacol. 20 (1), 87–93 (2022).
Christou, G. A. et al. Unraveling the role of resistin, retinol-binding protein 4 and adiponectin produced by epicardial adipose tissue in cardiac structure and function: evidence of a paracrine effect. Horm. (Athens). 22 (2), 321–330 (2023).
Rachwalik, M., Zyśko, D., Diakowska, D. & Kustrzycki, W. Increased content of resistin in epicardial adipose tissue of patients with advanced coronary atherosclerosis and history of myocardial infarction. Thorac. Cardiovasc. Surg. 62 (7), 554–560 (2014).
Akboga, M. K. et al. Relationship between plasma Apelin level and coronary collateral circulation. Atherosclerosis 235 (2), 289–294 (2014).
Langheim, S. et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am. J. Physiol. Heart Circ. Physiol. 298 (3), H746–H753 (2010).
Fraga-Silva, R. A. et al. Apelin-13 treatment enhances the stability of atherosclerotic plaques. Eur. J. Clin. Invest. ;48(3). (2018).
Almasi, A. et al. The value of coronary artery calcium score assessed by dual-source computed tomography coronary angiography for predicting presence and severity of coronary artery disease. Pol. J. Radiol. 79, 169–174 (2014).
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15 (4), 827–832 (1990).
Samiei, N. et al. Modulatory role of SIRT1 and resistin as therapeutic targets in patients with aortic valve stenosis. Arch. Med. Res. 50 (6), 333–341 (2019).
Gharibnaseri, M., Yahyavi, H. & Arabian, M. Antispasmodic activity of onion (Allium Cepa L.) Peel extract on rat ileum. Iran. J. Pharm. Res. 7 (2), e128585 (2022).
Leo, S., Tremoli, E., Ferroni, L. & Zavan, B. Role of epicardial adipose tissue secretome on cardiovascular diseases. Biomedicines 11 (6), 1653 (2023).
Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Reviews Cardiol. 19 (9), 593–606 (2022).
Iacobellis, G. & Barbaro, G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm. Metab. Res. 40 (7), 442–445 (2008).
Kremen, J. et al. Increased subcutaneous and epicardial adipose tissue production of Proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J. Clin. Endocrinol. Metab. 91 (11), 4620–4627 (2006).
Khajali, Z. et al. Prevalence of cardiac dysfunction among adult patients with congenital heart disease: A Single-Center investigation. Iran. Heart J. 20 (3), 12–19 (2019).
Abate, N. et al. Resistin: An inflammatory cytokine. Role in cardiovascular diseases, diabetes and the metabolic syndrome. Curr. Pharm. Des. 20 (31), 4961–4969 (2014).
Jamaluddin, M. S., Weakley, S. M., Yao, Q. & Chen, C. Resistin: Functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 165 (3), 622–632 (2012).
Tripathi, D., Kant, S., Pandey, S. & Ehtesham, N. Z. Resistin in metabolism, inflammation, and disease. FEBS J. 287 (15), 3141–3149 (2020).
Su, K. et al. Relation of Circulating resistin to insulin resistance in type 2 diabetes and obesity: a systematic review and meta-analysis. Front. Physiol. 10, 1399 (2019).
Wysocka, M. B., Pietraszek-Gremplewicz, K. & Nowak, D. The role of Apelin in cardiovascular diseases, obesity and cancer. Front. Physiol. 9, 557 (2018).
Askin, L., Askin, H. S., Tanrıverdi, O., Ozyildiz, A. G. & Duman, H. Serum Apelin levels and cardiovascular diseases. North. Clin. Istanbul. 9 (3), 290–294 (2022).
Reilly, M. P. et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 111 (7), 932–939 (2005).
Hassan, M. et al. Segmental peri-coronary epicardial adipose tissue volume and coronary plaque characteristics. Eur. Heart J. Cardiovasc. Imaging. 17 (10), 1169–1177 (2016).
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: A scientific statement from the American heart association. Circulation 143 (21), e984–e1010 (2021).
Battineni, G. et al. Impact of obesity-induced inflammation on cardiovascular diseases (CVD). Int. J. Mol. Sci. 22 (9), 4798 (2021).
Sosnovik, D. E. & Elmariah, S. Metabolic dysfunction in aortic stenosis: A key piece of the pathophysiological puzzle. Circ. Cardiovasc. Imaging. 16 (10), e015977 (2023).
Katz, R. et al. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic study of atherosclerosis (MESA). Diabetes 58 (4), 813–819 (2009).
Moscarelli, M. et al. The influence of metabolic syndrome in heart valve intervention. A multi-centric study. J. Card Surg. 37 (12), 5063–5072 (2022).
Jakob, S. M. & Stanga, Z. Perioperative metabolic changes in patients undergoing cardiac surgery. Nutrition 26 (4), 349–353 (2010).
Wang, T. D. et al. Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J. Clin. Endocrinol. Metab. 94 (2), 662–669 (2009).
Baldasseroni, S. et al. Epicardial adipose tissue and insulin resistance in patients with coronary artery disease with or without left ventricular dysfunction. Monaldi Arch. Chest Dis. 80 (4), 170–176 (2013).
Koshelskaya, O. A. et al. [Metabolic, inflammatory and imaging biomarkers in evaluation of coronary atherosclerosis severity in patients with coronary artery disease and diabetes mellitus type 2]. Ter. Arkh. 93 (9), 1030–1036 (2021).
Wang, T. D. et al. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis 213 (1), 279–287 (2010).
Liakos, C. I. et al. Apelin and visfatin plasma levels in healthy individuals with high normal blood pressure. Am. J. Hypertens. 29 (5), 549–552 (2016).
Zhang, L., Curhan, G. C. & Forman, J. P. Plasma resistin levels associate with risk for hypertension among nondiabetic women. J. Am. Soc. Nephrol. 21 (7), 1185–1191 (2010).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8 (12), 958–969 (2008).
Ying, W., Cheruku, P. S., Bazer, F. W., Safe, S. H. & Zhou, B. Investigation of macrophage polarization using bone marrow derived macrophages. J. Vis. Exp. 2013(76).
Baker, A. R. et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 5, 1 (2006).
Huang, W. H. et al. KLF7 promotes macrophage activation by activating the NF-κB signaling pathway in epicardial adipose tissue in patients with coronary artery disease. Eur. Rev. Med. Pharmacol. Sci. 24 (12), 7002–7014 (2020).
Papotti, B. et al. Macrophage polarization markers in subcutaneous, pericardial, and epicardial adipose tissue are altered in patients with coronary heart disease. Front. Cardiovasc. Med. 10, 1055069 (2023).
Britton, K. A. et al. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the Framingham heart study. J. Am. Heart Assoc. 1 (6), e004200 (2012).
Fitzgibbons, T. P. & Czech, M. P. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J. Am. Heart Association 3(2):e000582 (2014).
Author information
Authors and Affiliations
Contributions
A.G.J. and B.M. contributed to data curation and drafting the original manuscript. A.T.B. and S.M.H. were involved in formal analysis. A.F. and H.P. worked on visualization. M.M. revised the draft, S.H. contributed to data curation, and M.A. was responsible for conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jolfayi, A.G., Beheshti, A.T., Hosseini, S.M. et al. Epicardial adipose tissue features as a biomarker and therapeutic target in coronary artery disease. Sci Rep 15, 14786 (2025). https://doi.org/10.1038/s41598-025-99600-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99600-w