Abstract
Tendon and ligament disorders, such as tendinopathy, cause pain and limit levels of activities of daily living. Thus, devising methods to heal them is crucial. Although treatment with autologous platelet rich plasma (PRP) is reportedly useful against tendon injury, PRP requires blood sampling and its quality varies. Here we show that platelet-like cells (ASCL-PLCs) derived from a heterologous human adipose-derived mesenchymal stem cell line (ASCL) promote significant tendon repair in a collagenase-induced injury model in rat Achilles tendons. Single administration of human ASCL-PLCs to rat Achilles tendon after 2 weeks of collagenase treatment significantly increased tendon strength and improved semi-quantitative histological evaluation scores in 4 weeks relative to PBS-treated controls. Moreover, xeno-graft reactions were not evident in ASCL-PLC-administered rats. In vitro, ASCL-PLC treatment significantly upregulated Col1a1, Lox and Mkx gene expression in NIH3T3 fibroblasts and activated ERK signaling. Overall, ASCL-PLCs could serve as a useful tool to repair injured tendons and treat tendinopathy. This approach eliminates the need for blood sampling, ensures consistent quality, supports xeno-transplantation, and increases injured tendon strength.
Similar content being viewed by others
Introduction
Tendon disorders such as tendinopathy due to overuse are a common problem in athletes and workers. Tendon disorders account for 36–50% of all sports-related injuries and almost half of all occupation-related disabilities in the United States; thus, managing them is crucial1,2. The pathophysiology of tendon disorders due to overuse is thought to be due to accumulation of tendon fiber damage and the limited ability of damaged tendons to repair themselves3. Strategies to activate tendon repair could hasten recovery from tendinopathy and other injuries.
Tendons are highly organized connective tissues composed of collagen molecules. Overall, tendons play a role in effective load transfer between muscle and bone4 and become damaged if subjected to excessive mechanical stress. The main components of tendons are primarily tenocytes, which are tendon-specific cells, and extracellular matrix collagen (COL) type I proteins4, although tendons also contain various types of extracellular matrix proteins (namely, COL types III, V, and XI, elastin, proteoglycans (e.g., decorin, lubricin, versican)), glycoproteins (such as cartilage oligomeric matrix protein (COMP) and tenascin C), and matrix metalloproteinases (MMPs)4. Type I collagen is most prevalent in mature ligaments and tendons and known for its superior strain resistance. On the other hand, type III collagen is more abundant in developing and healing tissues and is characterized by lower tensile strength5.
Tendon tissue injury progresses through a three-stage healing process: inflammation, matrix production and cell proliferation, and remodeling and maturation6. During this process, a disorganized matrix of randomly arranged collagen fibers, mainly COL type III, is first produced. Subsequently, during remodeling, COL type III and COL type I gradually replace each other, creating a stronger tissue structure7. Therefore, stimulating COL type III expression followed by replacement with COL type I would likely promote repair of injured tendons.
Currently, platelet-rich plasma (PRP), which is a platelet-enriched solution prepared from whole blood by centrifugation, is often used to treat tendon disorders8. PRP reportedly contains and releases a wide range of growth factors, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and insulin-like growth factor (IGF), all likely functioning in tendon repair9,10. However, PRP is purified by drawing blood from each patient and cannot be mass-produced; therefore, its quality varies depending on a patient’s condition. Consequently, uniformly high quality PRP has not been mass-produced for use in clinical research. Also, numerous methods are used to prepare PRP, making comparative analysis of different preparations challenging11.
Interestingly, human adipose-derived mesenchymal stem cell lines (ASCLs) have been shown to differentiate into megakaryocytes that when subjected to platelet differentiation conditions give rise to platelet-like cells (ASCL-PLCs)12. Abundant ASCL-PLCs can be purified from mass-cultured ASCLs without the need for gene transfer12, raising the possibility that ASCL-PLCs could serve as an alternative to PRP that does not require blood collection, is donor-independent, and is of uniform quality. On the other hand, it is unclear whether mass-purified ASCL-PLCs have tendon repair activity, can be cryopreserved, maintain their quality after freeze-thawing, or are useful for allogeneic transplantation.
In this study, we cryopreserved ASCL-PLCs, thawed them and then evaluated efficacy of thawed preparations in a rat Achilles tendon collagenase-induced damage model. We demonstrate that cryopreserved and thawed ASCL-PLCs release growth factors such as VEGF, bFGF and BMP2. Moreover, use of ASCL-PLC significantly increased mechanical strength of collagenase-treated tendons and significantly improved the histological score in semiquantitative tissue assessment in rats. Moreover, we observed no adverse heterogeneous reactions, such as immune cell infiltration. These findings suggest that ASCL-PLCs may be a useful tool to treat tendon disorders.
Results
Cryopreserved ASCL-PLCs maintain growth factor-releasing activity after thawing
ASCL-PLCs were prepared by stimulating ASCLs in platelet-inducing conditions, as described12. Conventional PRP was also prepared as described13. PRP and ASCL-PLCs were cryopreserved, and then both preparations were thawed and stimulated 15 min with CaCl2 to promote platelet activation and subsequent growth factor release12,14. Levels of VEGF, bFGF and BMP2, all of which function in tendon repair, were then measured in both samples (Fig. S1). ASCL-PLCs and PRP released equivalent levels of VEGF, while ASCL-PLCs released significantly higher levels of bFGF than did PRP (p = 0.020) (Fig. S1). BMP2 release by PRP was below the detection limit but evident in ASCL-PLCs (Fig. S1).
ASCL-PLC treatment increases maximum strength of collagenase-treated rat Achilles tendons
To model tendon damage, we used in vivo collagenase treatment of rat Achilles tendons (Fig. S2). To do so, we injected rat Achilles tendons percutaneously with collagenase and then 2 weeks later similarly injected ASCL-PLC or PBS at injury sites. We then measured tendon maximum strength after 2 or 4 weeks and found that it was significantly higher in the ASCL-PLC relative to the control group at 4 weeks after ASCL-PLC or PBS treatment (p = 0.034) (Fig. 1).
Biomechanical strength of Achilles tendons increases after ASCL-PLC treatment. Analysis of maximum tendon strength in ASCL-PLC-treated versus control rats at 2 and 4 weeks after treatment. Note that at 4 weeks, tendons from ASCL-PLC-treated animals showed significantly higher maximum strength. Data represent the mean ultimate load ± SD (2w n = 3, 4w n = 6, *P < 0.05, parametric, Student’s t-test, paired, using Excel).
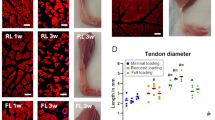
We next analyzed histological parameters of tendons semi-quantitatively (Fig. 2). As above, ASCL-PLCs or PBS was administered 2 weeks after collagenase treatment of rat Achilles tendons, and tissue sections were prepared from those tendons at 1, 2, and 4 weeks thereafter and subjected to Hematoxylin and Eosin (HE) as well as Alcian Blue staining (Fig. 2a). Based on histological analysis, no xeno-graft reaction, such as immune cell infiltration, was evident in ASCL-PLC-treated Achilles tendons (Fig. 2a). We then evaluated five histological parameters—namely, cell morphology, collagen arrangement, cellularity, vascularity, and ground substance—and scored them semi-quantitatively to calculate the Bonar score (Figs. 2b and S3a–e). At 4 weeks post-treatment, ASCL-PLC treatment significantly improved the Bonar score based on those parameters relative to PBS treatment (p = 0.046).
ASCL-PLC treatment stimulates repair of collagenase-treated Achilles tendons. (a) HE (upper) or Alcian Blue (lower) staining of Rat Achilles tendons injected with ASCL-PLC or PBS analyzed at indicated time points post-treatment. Bar, 100 μm. (b) Semi-quantitative evaluation based on the Bonar score, defined as the sum of the scores of five parameters evaluated in HE and Alcian Blue staining (namely, cell morphology, collagen arrangement, cellularity, vascularity, and ground substance). Each parameter was quantified using a 0–3 scale: 0 (normal), 1 (slightly abnormal), 2 (moderately abnormal) and 3 (maximally abnormal). At 4 weeks post-treatment, ASCL-PLC treated animals showed significant improvement in the Bonar score relative to PBS-treated controls. Data represent mean score ± SD (n = 6 each, *P < 0.05, parametric, Student’s t-test, paired, using Excel).
ASCL-PLC treatment increased tendon-related gene expression in collagenase-treated Achilles tendons in vivo
ASCL-PLCs or PBS was administered 2 weeks after collagenase treatment of rat Achilles tendons, and the expression of tendon repair-related factors namely Col-1a1, Col-3a1, Scleraxis (hereafter Scx), and Tenascin C (hereafter Tnc) in Achilles tendons was analyzed by realtime PCR at 1, 2 and 4 weeks after treatment with ASCL-PLC or PBS (Fig. 3a–d). Col-3a1 was significantly higher in the PBS group at 1 week (p = 0.00000035) (Fig. 3b), while Col-1a1 and Scx were significantly higher in the ASCL-PLC group at 4 weeks post-treatment with ASCL-PLC or PBS (Col-1a1 p = 0.027, Scx p = 0.047) (Fig. 3a and c).
ASCL-PLC treatment upregulates tendon-related gene expression in collagenase-treated Achilles tendons. Shown is expression of indicated genes in ASCL-PLC or PBS groups at various time points post-treatment. Note that Col-3a1 expression was significantly higher in the PBS group at 1 week (b), while Col-1a1 and Scx were significantly higher in the ASCL-PLC group at 4 weeks post-treatment (a and c). Data represent mean Col-1a1 (a), Col-3a1 (b), Scx (c), and Tnc (d) expression relative to β-actin levels ± SD in ASCL-PLC- relative to PBS-treated cells (1w and 2w, n = 6 each; 4w n = 10 each; *P < 0.05, **P < 0.01, ***P < 0.001, Col-1a1 data at 4w was nonparametric and the Wilcoxon signed-rank sum test was performed in R. Data in all other cases was parametric and analyzed by Student’s t-test, paired, using Excel).
ASCL-PLCs stimulate tendon-related gene expression in fibroblastic NIH3T3 cells
Finally, we performed in vitro analysis to define signals transduced by ASCL-PLC treatment using fibroblastic NIH3T3 cells (Fig. 4). To do so, we treated NIH3T3 cells with ASCL-PLC for 24 h and then extracted total RNA. We observed that Col-1a1, lysyl oxidase (hereafter Lox) and Mohawk homeobox (hereafter Mkx) but not decorin (hereafter Dcn) transcript levels were significantly upregulated in ASCL-PLC- relative to PBS-treated samples (Col-1a1 p = 0.044, Lox p = 0.023 Mkx p = 0.011) (Fig. 4a–d).
ASCL-PLC treatment upregulates tendon-related gene expression in NIH3T3 cells and activates MAPK pathways. (a–d) Analysis of Col-1a1(a), Lox(b) and Mkx(c) and Dcn(d) transcript levels relative to β-actin transcripts in ASCL-PLC- and PBS-treated samples. Note Col-1a1, Lox, and Mkx expression is significantly higher in ASCL-PLC-treated cells, while Dcn expression is not. Shown are expression levels relative to that of β-actin ± SD in indicated groups (n = 6 each for ASCL-PLC- and PBS-treated cells, *P < 0.05, parametric, Student’s t-test, unpaired, using Excel). (e and f) Semi-quantitative Western blot analysis (e) of NIH3T3 cells cultured with and without ASCL-PLCs. (f) Quantitative analysis of data shown in (e) revealing significantly upregulated phosphorylation of ERK1/2 but not of p38 and JNK. Data represent mean pERK1/2/ERK1/2, pp38/p38 or pJNK/JNK in ASCL-PLC relative to PBS-treated NIH3T3 cells ± SD (n = 3 each, *P < 0.05, parametric, Student’s t-test, unpaired, using Excel).
We then prepared lysates from ASCL-PLC-treated NIH3T3 cells at various time points and performed western blotting to detect phosphorylation indicative of activation of various components of the MAPK pathway, including ERK1/2, p38 and JNK (Fig. 4e). Semi-quantitative analysis of western blotting revealed significantly upregulated phosphorylation of ERK1/2 but not of p38 and JNK in NIH3T3 cells cultured with ASCL-PLCs versus those treated without ASCL-PLCs (p = 0.038) (Fig. 4f). Increases in Col-1a1 expression promoted by ASCL-PLC treatment of NIH3T3 cells were partially but significantly inhibited by treatment with U0126, a MEK/ERK inhibitor (p = 0.016) (Fig. S4).
ASCL-PLCs are cleared from transplanted sites within 7 days of injection
To define their clearance time, ASCL-PLCs were injected into rat Achilles tendons, and then animals were sacrificed on days 1, 3, and 7 post-injection. We found that expression of human beta-actin, which served as an indicator of remaining human cells, was sharply decreased by day 3 and no longer detectable by day 7 post-injection (Fig. S5).
Discussion
Tendon disorders, such as lateral epicondylitis, reduce activities of daily living and limit participation in sports activity due to pain, and also result in reduced work capacity15. Steroid injections are often the treatment of choice, but frequent steroid injections reportedly decrease tendon strength16. Recently, PRP was reported to be useful in treating tendinopathy17, but there is as yet no consensus on its efficacy due to difficulty in comparing PRP prepared using different methods and differences in the number of leucocytes contained in PRP. By contrast, ASCL-PLCs can be differentiated in large quantity from differentiated ASCLs and their quality can be checked in advance. Although growth factor production varies among ASCL-PLC lines, the advantage of using ASCL-PLCs is that cell lots exhibiting high growth factor levels can be selected in advance and cryopreserved. However, it was not known whether thawing cryopreserved ASCL-PLC preparations would have an adverse effect on growth factor release or activity or whether side effects would emerge from allogeneic transplantation. In this study, ASCL-PLCs were shown to release biologically active growth factors, even after freeze-thawing, and to exhibit tissue repair capacity without iatrogenic reactions when administered to a rat tendon disorder model.
Following administration of ASCL-PLC to rats, we found that human DNA was detectable in tendons up to 3 days later but was below the detection limit by 7 days post-administration (Fig. S5). This observation suggests that ASCL-PLC activity at an early stage likely exerts local effects over a long period of time. In fact, it is reported that when PRP or mesenchymal stem cells are used in regenerative medicine approaches, treatment effects can appear at a later time, even though injected PRP or cells are no longer detectable18.
PRP efficacy reportedly depends on induction of growth factors such as FGF-219, EGF20, or VEGF21, and on its ability to activate signaling pathways such as ERK1/222, suggesting that PRP effects are not mediated by a single factor or signal but rather by a combination of various factors and signaling pathways. Given the similarities of ASCL-PLCs and PRP, ASCL-PLC treatment should have effects comparable to PRP. In fact, here, we found that VEGF and bFGF were produced from ASCL-PLCs, and MAPK pathways were activated by ASCL-PLCs. It is reported that bFGF released from PRP promotes angiogenic activities and regenerative properties23 and also activates ERK1/2 signaling24. bFGF reportedly plays a role in tendon tissue repair25,26, and thus, release of substantial amounts of bFGF is an advantage of ASCL-PLCs. Immunostaining did not reveal a clear difference in Col1 and Col3 expression following ASCL-PLC treatment (Fig. S6), an outcome that may be due to differential sensitivity in detection between PCR, which detects gene expression, and immunostaining of proteins. Thus, further analysis of mechanisms underlying ASCL-PLC effects in treatment of tendinopathy is needed. Moreover, we did not evaluate in vivo levels of VEGF, bFGF, and BMP2 produced by ASCL-PLCs, as implanted ASCL-PLCs rapidly disappear in vivo.
In general, PRP is prepared on an as-needed basis, and blood must be drawn from the patient for each procedure. Some PRPs are freeze-dried and stored27, but they still require a blood draw from a patient. The quality of the preparation depends on patients’ clinical characteristics28, and that quality cannot be checked in advance. On the other hand, ASCL-PLCs can be produced from ASCLs in large quantities and cryopreserved, making it possible to check quality before storage. Here, we show that ASCL-PLCs release growth factors after freezing and thawing, supporting the idea that ASCL-PLCs stored in large quantities could be used for allogeneic transplantation. PRP, which is autologously collected and utilized, has not be used in clinical application in cases of allogeneic transplantation. Platelet transfusion products have been used clinically as allogeneic transplantation products, and it has been suggested that PRP and ASCL-PLCs, which are platelet products, could also be used for allogeneic transplantation. In fact, in our analysis, ASCL-PLCs did not show any obvious signs of rejection, even in a xenogeneic context.
Limitations of our study include use of a cell line rather than primary tendon-derived cells for in vitro experiments. Also, we assessed a limited panel of genes, and for PCR assays we normalized gene expression to only one housekeeping gene. Finally, CSA, failure strain and stiffness were not analyzed, and we did not show direct comparison of ASCL-PLC with PRP other than via VEGF, bFGF, and BMP2 release in vitro.
Nonetheless, ASCL-PLCs represent a promising therapeutic option that can be mass-produced, quality-controlled, cryopreserved, and allogeneically-transplanted, and their use could promote tissue repair in cases of tendon damage.
Materials and methods
Study approval
This study was approved by the Keio University Institutional ethics committee (Approval No. 20210093), and all methods were performed in accordance with relevant guidelines and regulations. All patients provided written informed consent.
Preparation of ASCL-PLCs and analysis of their growth factor release
ASCL-PLCs were prepared as described12 and stored at -80℃. Briefly, maintenance of ASCs, subsequent generation of ASCLs, and ASCL differentiation into ASCL-PLCs were performed as described12. For both ASCs and ASCLs, we used cells from passages 4 to 10. In parallel, we collected 30 ml samples of peripheral blood from healthy adult human subjects (n = 3; Japanese males and females, aged 34–61 years) to obtain PRP. Blood was centrifuged to prepare PRP as described13 and PRP was stored at -80℃. After thawing the cryopreserved ASCL-PLC or PRP, no passage was performed before use.
The growth factor release assay was performed as described14. Briefly, both PRP and ASCL-PLC were thawed at room temperature and CaCl2 was added to both to a final concentration of 10 mM. Cells were incubated 15 min and then collected by centrifugation at 2500 rpm for 15 min at 25 °C. bFGF and VEGF-A concentrations in supernatants of both were determined using a Human FGF basic ELISA kit and a Human VEGF-A ELISA kit (both from Abcam, Cambridge, UK). BMP2 concentration was analyzed using a BMP2 ELISA kit (R&D systems, Minneapolis, Minnesota, USA).
Animals
All animal experiments complied with ARRIVE guidelines and were carried out in accordance with the National Institutes of Health guide for care and use of laboratory animals. Briefly, in vivo experiments were conducted using 3–10 rats per group. No animal died unexpectedly in this analysis. However, the protocols used required that in the event of unexpected deaths, animals would be excluded from analysis and additional experiments would be performed. Also, no animals were excluded due to poor conditions. All evaluations were conducted in a blinded manner in which evaluators were unaware of the group assignments until all assessments were completed.
Eight-week-old male Wistar rats, weighing 250–300 g, were purchased from Crea Japan (Tokyo, Japan). Rats were maintained under specific pathogen-free conditions in animal facilities certified by the Keio University Institutional Animal Care and Use Committee. Animals were housed up to 3 rats per cage and kept on a 12 h light/dark cycle. Water and food were available ad libitum. Animal protocols were approved by the Institutional Guidelines on Animal Experimentation at Keio University (Approval No.20210093).
Animal study design
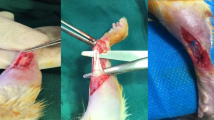
To induce tendinopathy, we administered 50 μl (700UI) of collagenase type 1, which has tendon degenerative effects, via percutaneous injection of both hindlimbs of 8-week-old rats under general isoflurane anesthesia induced by administering 4–5% isoflurane and maintained at 2–3% isoflurane. Rats exhibited no pain avoidance behavior; however, the protocol used required that analgesic treatment be provided in the event of pain.
Collagenase models were utilized to create animal models of tendinopathy, as described29,30,31. Briefly, eight-week-old rats were administered 50 μl (700UI) of collagenase type 1 percutaneously into both hindlimbs. Two weeks later, 50 μl of PBS alone or 50 μl of PBS containing 1.0 × 107 ASCL-PLCs was injected percutaneously into the left or right hindlimbs, respectively under anesthesia, and rats were sacrificed at 1, 2, and 4 weeks thereafter. Achilles tendons were collected from both legs, formalin-fixed and paraffin-embedded, and sectioned at 5-μm for histological staining with Hematoxylin & eosin (HE) or Alcian Blue. Stained sections were used to calculate the Bonar score, a semiquantitative assessment of tendon repair, as described32. Samples at 2 and 4 weeks were also used for biomechanical testing as described below. Samples at 1, 2, and 4 weeks were used for gene expression analysis, and samples at 1, 3, and 7 days were used to assay ASCL-PLC clearance, as described below. Rats were euthanized prior to sacrifice by an isoflurane overdose.
Histopathology and fluorescent immunohistochemical analysis
Rat Achilles tendons removed with calcaneus bone were decalcified in 10% EDTA (pH 7.4) for 4 weeks before embedding. Sections were cut horizontally in the direction of the long axis of the tendon, at 4 μm thickness. Sections were prepared with the calcaneal enthesis region of the Achilles tendon to align and define a particular region of the tendon. HE and Alcian Blue staining was performed using standard procedures. The Bonar score was calculated using five parameters: cell morphology, collagen arrangement, cellularity, vascularity, and ground substance, and each parameter was quantified using a 0–3 scale: 0 (normal), 1 (slightly abnormal), 2 (moderately abnormal) and 3 (maximally abnormal), as described32. Three sections were randomly selected from each sample and evaluated blindly by three examiners. The average score was used for comparison.
For immunohistochemical assays, deparaffinized sections were microwaved in 10 mM citrate buffer (pH 6.0) for 10 min for antigen retrieval. After blocking 1 h in 3% BSA in PBS, sections were stained with Rabbit monoclonal antibody against Col-1 [COL-1] (1:100 dilution; Abcam) and mouse monoclonal antibody against Col-3 [COL-3] (1:100 dilution; Abcam) at 4 °C for 18 h. After PBS washing, sections were stained 1 h with Alexa Fluor 488-conjugated goat anti-mouse IgG (1:100 dilution; Invitrogen) and Alexa Fluor 546-conjugated goat anti-rabbit IgG at room temperature. DAPI (1:1000; FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) was used for nuclear staining, and cells were observed under a fluorescence microscope (Keyence, Tokyo, Japan).
Biomechanical testing
Achilles tendons were harvested with the calcaneus bone, and the muscle tissue and tendon sheath were removed (Fig. S7a). Tendons and the calcaneus bone were gripped with gauze and sandpaper to increase frictional force (Fig. S7b–d). The Achilles tendon was then gripped with serrated metal plates and placed in a mechanical testing machine (AGS-10kN; Shimadzu, Tokyo, Japan). A preload was not applied to the tendon. The tendon was then pulled vertically at a constant speed of 50 mm/s until failure. The ultimate failure load (N) was recorded. Parameter data for testing were collected using Trapezium software (Version 2.05; Shimadzu).
Cell culture for RT-PCR
1.0 × 106 NIH3T3 fibroblasts were co-cultured 24 h with 1.0 × 107 ASCL-PLCs in the presence or absence of 10 mM U0126.
Real-time RT-PCR analyses of gene expression
Rat Achilles tendons were washed, cut into small pieces, and homogenized using a syringe and an 18–23 gauge needle. RNA was extracted with TRIzol Reagent and assessed spectrophotometrically for integrity by measuring the OD 260/280 nm. Cultured cells were collected and total RNA was extracted using TRIzol Reagent (Cosmo Bio Company, Tokyo, Japan). First-strand cDNA was prepared using the Prime Script RT Reagent Kit (Takara Bio, Shiga, Japan), according to the manufacturer’s instructions. Quantitative PCR was performed using a Thermal Cycler Dice Real-Time System and SYBR Premix Ex Taq (Takara Bio), and transcript levels were quantified using the ddCt method. Data were normalized to β-actin (Actb) expression levels. Primers used in this study are listed in Table 1.
Assay of ASCL-PLC clearance
Rat Achilles tendons were collected on days 1, 3, and 7 after administration of 1.0 × 107 ASCL-PLCs. Timepoints in the ASCL-PLC clearance assay were evaluated earlier than in animal studies as ASCL-PLC clearance was considered to occur rapidly, prior to a biological response to ASCL-PLC. Human β-actin expression was then analyzed by real-time PCR, using the following primers:
Human β-actin-forward: 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′
Human β-actin-forward: 5′-TGGCACCCAGCACAATGAA-3′.
Cell culture for Western blotting
Prior to cell stimulation, the culture medium of NIH3T3 cells was changed to serum-free medium for 24 h. Cells were then stimulated with 1.0 × 107 of ASCL-PLCs and samples collected thereafter at 5, 10, 15, 30, 60, and 90 min.
Western blotting
Total cell lysates were obtained from cultured cells using a tissue protein extraction reagent (Thermo Fisher Scientific, Waltham, MA). All buffers contained a protease inhibitor cocktail (Roche), NaF (1 M), and Na3VO4 (50 μM). Cell lysates were mixed with loading buffer (Tris–Glycine SDS Sample Buffer, Invitrogen) containing 5% 2-mercaptoethanol and electrophoresed through 15% SDS-PAGE gels. Proteins were electroblotted onto polyvinylidene fluoride membranes (ATTO Corporation, Tokyo, Japan). Membranes were blocked in 5% nonfat dry milk in Tris-buffered saline containing Tween 20 (TBST) (50 mM Tris, pH 7.6, 150 mM NaCl, 0.1% Tween 20) and incubated overnight at 4 °C in 5% nonfat dry milk in TBST with the designated primary antibody. Primary antibodies (all from Cell Signaling, Beverly, MA) were as follows: mouse-anti-ERK antibody (9102, 1:1000 dilution), mouse-anti-pERK antibody (9101S, 1:1000 dilution), mouse-anti-p-38 antibody (9212S, 1:1000 dilution), mouse-anti-pp-38 antibody (9211S, 1:1000 dilution), mouse-anti-JNK antibody (9252S, 1:1000 dilution) and rabbit-anti-pJNK antibody (9255S, 1:2000 dilution) or mouse-anti-actin antibody (A2066, 1:100 dilution, Sigma-Aldrich Co., St Louis, MO, USA). Secondary antibodies were HRP-conjugated goat-anti-mouse IgG (G21040, 1:10,000 dilution, Thermo Fisher Scientific, Waltham, Massachusetts, USA) or goat-anti-rabbit IgG (G21234, 1:10,000 dilution, Thermo Fisher Scientific). Membranes were incubated using appropriate secondary antibodies, and immune complexes were visualized using the ECL Western Blotting Analysis System (GE Healthcare, Tokyo, Japan). Membranes were exposed to X-ray film for various times (from 30 s to 5 min) to optimize signal intensity and minimize background noise, and then developed. Band intensities obtained from Western blot results were quantified using ImageJ software (version [1.54 h], National Institutes of Health, Bethesda, MD, USA).
ASCL-PLC clearance time assay
Collagenase type 1 (700UI) was injected percutaneously into hindlimbs of 8-week-old rats. Two weeks later, rats were injected with 50 μl PBS containing 1.0 × 107/50 μl ASCL-PLCs in the same region. Rats were then sacrificed 1, 3 or 7 days later, because ASCL-PLC clearance was considered to occur very rapidly, prior to the biological response evoked by ASCL-PLCs, and Achilles tendons were analyzed for human β-actin expression by real-time PCR.
Statistical analysis
All numerical data are shown as means ± SD. The Shapiro–Wilk test was performed to analyze data normality. The normality of the distribution of the data by the Shapiro–Wilk test showed that only Col1a1 expression at 4w was nonparametric, while all other time and gene comparisons were parametrically distributed. If the data was parametric, we performed a paired or unpaired Student’s t-test using Excel; if the data was nonparametric, we performed Wilcoxon signed-rank sum test in R. (*p < 0.05; **p < 0.01; ***p < 0.001; NS, not significant, throughout the paper).
Data availability
The datasets collected during the current study are available from the corresponding author upon reasonable request.
References
Józsa, L. & Kannus, P. Histopathological findings in spontaneous tendon ruptures. Scand. J. Med. Sci. Sports 7, 113–118 (1997).
Kannus, P. Tendons—a source of major concern in competitive and recreational athletes. Scand. J. Med. Sci. Sports 7, 53–54 (1997).
Nakama, L. H., King, K. B., Abrahamsson, S. & Rempel, D. M. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J. Orthop. Res. 23, 1199–1205 (2005).
Screen, H. R., Berk, D. E., Kadler, K. E., Ramirez, F. & Young, M. F. Tendon functional extracellular matrix. J. Orthop. Res. 33, 793–799 (2015).
Singh, D., Rai, V. & Agrawal, D. K. Regulation of collagen I and collagen III in tissue injury and regeneration. Cardiol. Cardiovasc. Med. 7(1), 5–16 (2023).
Walden, G., Liao, X. & Donell, S. A clinical biological, and biomaterials perspective into tendon injuries and regeneration. Tissue Eng. Part B Rev. 23(1), 44–58 (2017).
Yang, G., Rothrauff, B. B. & Tuan, R. S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. Part C Embryo Today Rev. 99, 203–222 (2013).
Dohan Ehrenfest, D. M., Rasmusson, L. & Albrektsson, T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 27, 158–167 (2009).
de Mos, M. et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am. J. Sports Med. 36(6), 1171–1178 (2008).
Zhang, J. & Wang, J. H. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am. J. Sports Med. 38(12), 2477–2486 (2010).
Burnham, T. et al. The effectiveness of platelet-rich plasma injection for the treatment of suspected sacroiliac joint complex pain; a systematic review. Pain Med. 21(10), 2518–2528 (2020).
Tozawa, K. et al. Megakaryocytes and platelets from a novel human adipose tissue-derived mesenchymal stem cell line. Blood 14, 633–643 (2019).
Harrison, P. Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 16, 1895–1900 (2018).
Pan, L. et al. Growth factor release from lyophilized porcine platelet-rich plasma: Quantitative analysis and implications for clinical applications. Aesthetic Plast. Surg. 40(1), 157–163 (2016).
Kahlenberg, C. A., Knesek, M. & Terry, M. A. New developments in the use of biologics and other modalities in the management of lateral epicondylitis. Biomed Res. Int. 439309 (2015).
Degen, R. M. et al. Three or more preoperative injections is the most significant risk factor for revision surgery after operative treatment of lateral epicondylitis: An analysis of 3863 patients. J. Shoulder Elbow Surg. 26, 704–709 (2017).
Li, A. et al. Platelet-rich plasma vs corticosteroids for elbow epicondylitis: A systematic review and meta-analysis. Medicine 98, e18358 (2019).
Marx, R. E. Platelet-rich plasma (PRP): What is PRP and what is not PRP?. Implant Dent 10(4), 225–228 (2001).
Zaragosi, L. E., Ailhaud, G. & Dani, C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adipose-derived stem cells. Stem Cells 24, 2412–2419 (2006).
Baer, P. C., Schubert, R., Bereiter-Hahn, J., Plosser, M. & Geiger, H. Expression of a functional epidermal growth factor receptor on human adipose-derived mesenchymal stem cells and its signaling mechanism. Eur. J. Cell Biol. 88, 273–283 (2009).
Song, S. Y., Chung, H. M. & Sung, J. H. The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin. Biol. Ther 10, 1529–1537 (2010).
Lai, F. et al. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res. Ther. 16, 107 (2018).
Etulain, J. et al. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci. Rep. 8(1), 1513 (2018).
Cheng, Y. S. et al. Simultaneous binding of bFGF to both FGFR and integrin maintains properties of primed human induced pluripotent stem cells. Regen. Ther. 25, 113–127 (2023).
Tang, J. B. et al. Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. An in vivo study. J. Bone Joint Surg. Am. 90, 1078–1089 (2008).
Havis, E. et al. TGFβ and FGF promote tendon progenitor fate and act downstream of muscle contraction to regulate tendon differentiation during chick limb development. Development 143(20), 3839–3851 (2016).
Shiga, Y. et al. Freeze-dried human platelet-rich plasma retains activation and growth factor expression after an eight-week preservation period. Asian Spine J. 11(3), 329–336 (2017).
Rossi, L. et al. Substantial variability in platelet-rich plasma composition is based on patient age and baseline platelet count. Arthrosc. Sports Med. Rehabil. 5(3), e853–e858 (2023).
Lui, P. P., Fu, S. C., Chan, L. S., Hung, L. K. & Chan, K. M. Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration. J. Histochem. Cytochem. 57(2), 91–100 (2009).
Chen, L. et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat Achilles tendinopathy. Cell Physiol. Biochem. 34(6), 2153–2168 (2014).
Chen, J. et al. Autologous tenocyte therapy for experimental Achilles tendinopathy in a rabbit model. Tissue Eng. Part A 17, 2037–2048 (2011).
Fearon, A., Dahlstrom, J. E., Twin, J., Cook, J. & Scott, A. The Bonar score revisited: region of evaluation significantly influences the standardized assessment of tendon degeneration. J. Sci. Med. Sport 17, 346–350 (2014).
Acknowledgements
K. Sato was supported by a grant-in-aid for Scientific Research in Japan.
Author information
Authors and Affiliations
Contributions
Investigation: YY, YU and YS; conceptualization: YM, MN, KS and TM; resources: YU and YM; data curation: AT; funding acquisition: YM, KS and TM; supervision: YM, MM, MN, KS and TM; writing: TM.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yamada, Y., Torii, A., Uruga, Y. et al. Platelet like cells differentiated from human adipose derived mesenchymal stem cells promote healing of tendinopathy in rats. Sci Rep 15, 15015 (2025). https://doi.org/10.1038/s41598-025-99657-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99657-7