Abstract
Regional disparities in the incidence of tuberculosis (TB) pose several challenges to effective TB control. This study aimed to investigate such disparities in Osaka City, Japan, by analyzing the relationship between TB incidence and residents’ socioeconomic indicators. Using 42 indicators from the 2010 population census, the city was clustered into 15 social areas through a factor analysis, and TB incidence during 2012–2016 was compared across the areas in 4,852 cases. Two geographically adjacent areas (Area D and O) exhibited significantly higher TB rates, each with distinct social characteristics. Area D consisted of a high proportion of young, single individuals working in the service sector as well as foreigners. Area O included a high proportion of day laborers, secondary industry workers, and individuals with lower educational levels. TB occurred more frequently in foreign-born patients aged < 60 years, and it was detected during treatment for other diseases in Area D compared with other areas. However, a high proportion of retreated patients aged 40–79 years, with a shorter delay in TB detection, was observed in Area O. The variations in this study provide insights into the relationship between socioeconomic indicators and regional disparities in TB incidence in local settings.
Similar content being viewed by others
Introduction
Tuberculosis (TB), with 9.9 million cases and 1.5 million deaths reported worldwide in 20201, continues to pose a major public health challenge. TB was the leading cause of death from a single infectious disease before the coronavirus disease (COVID-19) pandemic2. The implementation of non-pharmaceutical interventions in response to the COVID-19 crisis has contributed to a decrease in the number of TB cases; however, recent reports have indicated a resurgence of TB1,3. TB control is hindered by several unresolved challenges, including the potential ability of the causative agent (Mycobacterium tuberculosis) to reactivate after remaining latent following infection4. Effective treatment of TB warrants social care in the form of Direct Observation of Drugs and Therapy to ensure proper administration of anti-TB drugs and prevent the emergence and spread of drug-resistant strains5. The COVID-19 pandemic has strained public health resources, resulting in a resurgence in TB incidence and highlighting the continued critical need for effective TB control measures.
Metropolitan cities with large populations are particularly susceptible to the spread of TB owing to the presence of numerous places for gatherings, as well as the concentration of high-risk groups, such as socially vulnerable individuals, in certain areas6. Implementing a public health approach that considers the specific geographical characteristics of patients with TB is crucial for effective control. However, creating a public health system that adequately addresses the disparities within an urban city with a large population is a complex challenge7. Moreover, TB tends to be unevenly distributed in certain areas, in association with urban decline and corresponding inner-city challenges8. Ensuring equitable access to diagnosis and care tailored to local conditions is a key factor in addressing this challenge. Further research is warranted to understand the relationship between the social characteristics of local residents and TB incidence in small regional communities.
The World Health Organization has set a goal to achieve low endemicity of TB globally, with an incidence rate of < 10 per 100,000 people by 20359. Japan successfully met this target in 202110. Despite the steady decrease in TB incidence in Japan, Osaka City, the third largest municipality in Japan11, has exhibited a slower decrease in TB incidence compared to other areas10 (Supplementary Fig. 1), highlighting the need for further improvement in TB control measures. The implementation of measures aimed at reducing TB incidence in large cities, such as Osaka City, is crucial for advancing TB control efforts in the country.
Takatorige et al. (2000)12 analyzed TB incidence rates in 24 administrative wards of Osaka city and demonstrated disparities in the rate of decline among subgroups of wards defined based on the rate of unemployment, number of manufacturing industry workers, and number of construction industry workers. The study also revealed disparities in TB incidence rates within the city, highlighting the importance of adopting area-specific rather than uniform measures to control TB throughout the city. However, since this report, no further studies have been conducted to examine the regional distribution of TB in Osaka City.
Thus, we utilized small-area census data to divide Osaka city into socially segmented areas based on the geodemographic classification and examined the association between socioeconomic indicators of the areas and TB incidence. The study period encompassed the mid-endemic period, which is critical for clarifying the influence of regional characteristics and socioeconomic factors and for assessing the ongoing transition toward low endemicity. Regional segmentation based on geodemographic classification is a common method for analyzing the relationship between area-level socioeconomic factors and health outcomes13. This cross-sectional study will provide basic data for future TB control measures by identifying the characteristics of patients with TB and regional variations in urban areas.
Results
Attributes and geographic distribution of patients with TB
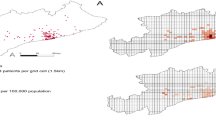
This study included a total of 4,852 individuals, representing over 97% of all patients with TB registered during the study period between 2012 and 2016 in Osaka City. The population was predominantly male, and individuals aged > 60 years accounted for approximately 70% of the sample, which was consistent with previously observed trends among patients with TB in Japan10. To illustrate the spatial distribution of patients with TB, a kernel density distribution map was generated (Fig. 1) based on the patient’s residence. The map revealed that sporadic TB cases occurred in all parts of the city. Additionally, high TB incidence was observed in a region (Airin) in the south-central part of the city. Airin, which has a high proportion of economically underprivileged residents, including residentially unstable day laborers and welfare recipients, has been recognized as a hotspot for TB cases14.
(a) Geographic location of Osaka City in Japan. The city is located about 600 km west of Tokyo and is the largest city economically in the western part of Japan. The map was created by QGIS 3.30 (QGIS Development Team, 2023, http://qgis.org). The administrative boundary data used in this study was obtained from the Humanitarian Data Exchange (HDX, https://data.humdata.org) and is provided under a CC BY-IGO license. (b) Kernel density estimation of tuberculosis patient addresses.
Social area classification
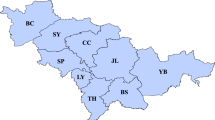
To investigate the socioeconomic residential structure of the city, we conducted a factor analysis of the 42 indicators of town councils (Supplementary Table 1), which yielded six common factors with a cumulative contribution rate of 68.9%. The six factors were interpreted based on large absolute values of the factor loadings (Table 1). Using Ward’s hierarchical clustering with these factor scores, the 332 town councils were classified into 15 social areas (Fig. 2). A dendrogram of the clustering is shown in Supplementary Fig. 2.
The average factor scores of the town councils in each social area are summarized in Table 2. According to this clustering, all 24 administrative wards contained three to seven social areas, which means that they contained different socioeconomic residential neighborhoods as their constituents (Fig. 2).
Regional differences in TB incidence by social area
Standardized TB incidence ratios were significantly higher in two areas (Areas D and O) (Table 3). Area O includes “Airin,” an area with extremely high TB cases. The residents in this area were older, single, short-term residents, and less educated (Table 2). Area D is located in and around “Minami,” a downtown area of Osaka City, adjacent to Area O. Based on the factor scores (Table 2), this area was interpreted as having a large number of young adults, singles, short-term residents, service industry workers, and foreign-born residents (Table 2).
Conversely, two areas (Areas L and M) had a significantly lower ratio (Table 3): Area L had a large population of affluent older adult long-term residents, while Area M had many white-collar commuters with different socioeconomic statuses and industrial composition residents (Table 2).
Characteristics of patients in social areas by TB incidence
With the goal of exploring the social area factors causing higher standardized TB incidence, we examined the regional characteristics thoroughly in the two regions with the highest ratios (Areas D and O). A multinomial logistic regression analysis was performed to examine the characteristics of patients with TB living in these areas. The characteristics of patients with TB were as follows. Regarding age, patients in Area D were more likely to be < 60 years, whereas those in Area O were more likely to be 40–79 years. Significant differences were observed in the number of foreign-born patients, with more patients in Area D than in Area O. Considering the history of TB diagnosis, patients residing in Area D were often diagnosed while undergoing treatment for other diseases. Patients living in Area O were more likely to be registered as recurrent cases; moreover, fewer patients had a delayed diagnosis of > 3 months (Table 4).
Discussion
This comparative study of TB incidence among social areas classified using geographic segmentation revealed the potential for geodemographic analysis to represent TB incidence patterns across cities more accurately. In this study, Osaka City was found to have significantly higher TB incidence rates limited to two social areas that vary in nature. Area O had the highest TB incidence rate (146.4 per 65,506 population), higher than that of the highest-level administrative ward, Nishinari (180.6 per 100,000 population, 2012–2016 average). These findings indicate that geographic classifications can identify TB hotspots more accurately compared to traditional administrative boundaries.
Area O, an identified TB hotspot, encompasses Airin—a historic urban center known for its concentration of day laborers15. Unlike regions where immigrants and people living with human immunodeficiency virus (HIV) are typically considered the primary TB risk groups16, Airin has a unique profile. Here, foreign-born patients and people living with HIV accounted for only 0.55% and 0.41% (data not shown) of the TB cases, respectively, which starkly contrasts with international patterns. Paradoxically, less delay in TB detection was observed in Airin, which could be attributed to the enhanced screening program in this area. Despite the introduction of an effective detection system, TB incidence rates remain at an alarmingly high level. This persistent transmission is likely due to district-specific environmental factors. Specifically, the presence of communal spaces and homeless shelters, the spread of localized infections confirmed by recent genetic studies of patient isolates17, and the higher prevalence of latent TB infection compared to the general Japanese population18 all contribute to the sustained transmission. Thus, the distinctive TB situation in area O, particularly within the Airin area, underscores the importance of targeted interventions to disrupt local transmission chains. This unique study offers valuable insights into urban public health strategies and highlights the need for context-specific approaches for TB control.
Delays in TB diagnosis may be influenced by socioeconomic factors, including patient recall bias and disparities in healthcare access19,20. This potential bias necessitates a cautious interpretation of the disparities observed in area O (Table 4). While Japan’s urban areas have historically exhibited less pronounced socioeconomic gaps compared to those in Western countries, recent trends indicate a significant widening of these disparities. For instance, Tokyo is experiencing increasing residential segregation based on occupation21. This shift is particularly relevant to TB, given its strong correlation with socioeconomic status22,23. To address these evolving dynamics, future TB research should incorporate more nuanced, region-specific analyses. Researchers should remain vigilant of confounding factors arising from demographic variations linked to widening regional disparities. These considerations are crucial for developing effective targeted TB control strategies that account for the complex interplay between socioeconomic factors and disease prevalence.
Considering the demographic changes, a detailed geographic analysis of TB incidence could provide valuable insights. Area D stands out for its large number of young foreign-born and short-term residents. Notably, a significant proportion of TB cases in this region included young foreigners (Table 4). Although surveillance data from Tokyo currently show limited cases of TB infection originating from foreign-born individuals, highlighting its unlikely role as a major risk factor in Japan24, the recent surge in foreign-born arrivals cannot be overlooked. The increased proportion of foreign-born patients with TB has highlighted the importance of foreign-born patients with TB in Japan’s TB control strategies. By examining the spatial distribution of patients in different population groups, we can better understand the unique challenges faced by regions such as Area D and adjust relevant strategies accordingly.
Combining a geodemographic analysis of TB incidence with a genotypic analysis of clinical strains of M. tuberculosis may provide valuable insights into the transmission dynamics of TB and help adjust strategies accordingly. As a limitation of the study, it is important to note that areas D and O, which showed significantly higher TB incidences, were geographically adjacent to each other. This suggests the possibility of large-scale transmission in this area, warranting further investigation. In addition, endogenous reactivation and recent transmission cases could not be differentiated based on patient data. Such a distinction could help identify geographic areas prone to TB outbreaks and enable the development of targeted and effective interventions. Because this is a cross-sectional study, only basic demographic data can be presented, and a causal relationship cannot be definitively established. Although an association was found between local residents’ demographic characteristics and TB cases, regional differences are likely to fluctuate over time. Therefore, continuous reanalysis and comparative studies using the most recent data are essential to examine the variation in TB incidence more comprehensively.
Conclusions
Our geodemographic analysis of patients with TB in Osaka City revealed distinct socioeconomic patterns in neighboring areas with high TB incidences. This approach, which considers the social characteristics of each region, offers valuable insights into the development of targeted and effective TB control strategies. These analyses are meaningful in urban environments with localized social disparities, enabling tailored interventions to reduce TB incidence while accounting for each city’s unique social characteristics.
Methods
Geodemographic clustering of social areas
In this study, data was obtained from the public resources of the Osaka City Planning and Coordination Bureau25. Based on these tabulation data of Osaka City from the 2010 National Census, social area clustering was established. Osaka City has 332 town councils. The Z-score normalization for 42 indicators reflected the familial, socioeconomic, and ethnic statuses of the residents of each town council (Supplementary Table 1). These indicators of the abovementioned three statuses have traditionally been employed in various studies on social area analysis and factorial ecology in urban sociology and geography26,27. We applied factor analysis to the 42 indicators using the maximum likelihood method and varimax rotation to extract common factors. Geodemographic clustering of the 332 town councils into social areas was performed using Ward’s method and the scores of common factors.
Geocoding of the residence of patients with TB
Data on TB patients was obtained from the Osaka City Public Health Office. The number of patients with TB who lived in each social area and were registered as new cases in Osaka City between 2012 and 2016 was counted based on their residential address. The town councils of 4,990 patients with TB were identified from their residential addresses, but those of 138 patients could not be detected. Consequently, data from 4,852 individuals were used in this study.
Standardization of TB incidence ratio in social areas
The standardized annual TB incidence ratio for each social area (Si, where i denotes the respective social area) during the study period was calculated by dividing the number of registered TB cases in each area (Pi) by the expected number of TB cases per year (Ei).
Si = Pi/Ei.
Here, Ei was calculated based on the ratio of the population of each area (Ni) to the total population of Osaka City (Nt) from the total number of registered patients with TB (4,852 persons) in the entire city. It was calculated based on the age distribution of registered patients with TB (Supplementary Table 2) to consider the age structure of each area. Hence, for a given age group j (in increments of 10 years), if the population of Osaka City as a whole is Ntj, the population of each area is Nij, and the number of patients with TB in Osaka City as a whole is Ptj, then Ei is the sum of all age groups as follows:
The 95% confidence interval for Si was calculated based on a Poisson distribution28.
Characteristics of patients with TB in social areas with significantly higher standardized TB incidence ratios
Multinomial logistic regression was used to examine the characteristics of patients with TB in social areas where the standardized TB incidence ratio was significantly higher. Two social areas with high TB incidence ratios and other social areas were used as objective variables. The explanatory variables selected for the study included sex, age group, national origin, respiratory symptoms, history of medical treatment, history of TB diagnosis, and delay in the diagnosis from symptom onset (Table 4). The adjusted odds ratio, along with the 95% confidence interval and p-value, was calculated after adjusting for all explanatory variables.
Data analysis
R ver. 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) was used for the statistical analysis. ArcGIS ver. 10.5 (ESRI, Redlands, CA) was used for mapping, unless otherwise noted in the figure legend.
Ethical considerations
This study was a retrospective observational study. Two types of data were used: one, small area aggregated results from the national census obtained from the Osaka City Planning and Coordination Bureau; two, tuberculosis patient data collected by the Osaka City Health Center through active epidemiological investigation based on the Infectious Diseases Control Law.
This study was conducted in accordance with the “Ethical Guidelines for Life Science and Medical Research Involving Human Subjects” established by the Ministry of Health, Labour, and Welfare of Japan (effective March 23, 2021). Ethical approval was obtained from the Ethical Review Committee of the first author’s institution (approval number: 1812-01-4, approval date: April 1, 2023).
As this study was a retrospective observational study using existing data, the requirement for obtaining individual informed consent was waived. However, in accordance with the aforementioned ethical guidelines, an overview of the research and opt-out procedures was posted on the institution’s website and made publicly available for a specified period. During this period, no requests to opt out of the study were received.
All information handled in this study was anonymized and did not contain personally identifiable information. Data handling strictly adhered to the policies of the Ethics Committee, and the analysis was conducted in a secure environment. Address data were converted to coordinate values in a stand-alone environment to avoid the risk of online data breach, using the basic geocoding function of ArcGIS Pro 2.4 (ESRI, Redlands, CA) with the Japan Data Contents Starter Pack (2018) (ESRI Japan, Tokyo, Japan).
Data availability
The tabulation data of Osaka City from the 2010 National Census and shapefiles of the 332 town councils in Osaka City are not publicly available but can be obtained from the Urban Planning Bureau of Osaka City (https://www.city.osaka.lg.jp/toshikeikaku/) upon reasonable request. The patient information obtained through the Infectious Disease Surveillance under the Infectious Diseases Control Law is not publicly available due to the sensitive nature of the data and restrictions on access. The data may not be available for public use due to legal and privacy restrictions, and is generally not accessible.
References
World Health Organization. Global Tuberculosis Report 2022. (2022). https://www.who.int/publications/i/item/9789240061729
Bagcchi, S. WHO’s global tuberculosis report 2022. Lancet Microbe. 4, e20 (2023).
Pai, M., Kasaeva, T. & Swaminathan, S. Covid-19’s devastating effect on tuberculosis care – a path to recovery. N Engl. J. Med. 386, 1490–1493 (2022).
Barry, C. E. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 7, 845–855 (2009).
Jeffries, C., Lobue, P., Chorba, T., Metchock, B. & Kashef, I. Role of the health department in tuberculosis prevention and control-legal and public health considerations. Microbiol. Spectr. 5, 261–282 (2017).
de Vries, G. et al. Epidemiology of tuberculosis in big cities of the European union and European economic area countries. Euro. Surveill. 19, 20726 (2014).
Cole, B. et al. Essential components of a public health tuberculosis prevention, control, and elimination program: recommendations of the advisory Council for the elimination of tuberculosis and the National tuberculosis controllers association. MMWR Recomm Rep. 69, 1–27 (2020).
Sotir, M. J. et al. Tuberculosis in the inner City: impact of a continuing epidemic in the 1990s. Clin. Infect. Dis. 29, 1138–1144 (1999).
World Health Organization. Global strategy and targets for tuberculosis prevention, care and control after 2015. (2013). https://apps.who.int/gb/ebwha/pdf_files/EB134/B134_12-en.pdf
Tuberculosis Surveillance Center. Tuberculosis in Japan: Annual Report 2022. (2023). https://jata-ekigaku.jp/wp-content/uploads/2022/11/TB-in-Japan_2022.pdf
Statistics Bureau. Ministry of internal affairs and communications Japan. Statistical handbook of Japan 2022. (2023). https://www.stat.go.jp/english/data/handbook/pdf/2022all.pdf
Takatorige, T., Aoki, Y., Tanigake, C., Ruful, A. & Tatara, K. Factors related to the slowdown in the reduction of the tuberculosis incidence rate in Osaka City–structure of the high incidence rate of tuberculosis in Osaka City analyzed by administrative-ward group, five-year period and age group. Kekkaku 75, 533–544 (2000).
Singleton, A. D. & Spielman, S. E. The past, present and future of geodemographic research in the united States and united Kingdom. Prof. Geogr. 66, 558–567 (2014).
Shimouchi, A. et al. Factors associated with mortality among patients with culture-positive pulmonary tuberculosis in the urban poor population of Osaka City, Japan. Western Pac. Surveill Response J. 12, 25–33 (2021).
Mizuuchi, T., Hirakawa, T. & Tominaga, T. Fifty years’ history of the Osaka Prefecture urban hostels’ association. Osaka City University (2011). https://www.hannan-u.ac.jp/doctor/tourism/matsumura/mrrf4300000091ub-att/mrrf43000000bfpm.pdf
Hardie, R. Inner City tuberculosis: an increasing problem? Crit. Public. Health. 3, 21–28 (1992).
Yamamoto, K. et al. Longitudinal genotyping surveillance of Mycobacterium tuberculosis in an area with high tuberculosis incidence shows high transmission rate of the modern Beijing subfamily in Japan. Infect. Genet. Evol. 72, 25–30 (2019).
Tabuchi, T. et al. Tuberculosis infection among homeless persons and caregivers in a high-tuberculosis-prevalence area in Japan: a cross-sectional study. BMC Infect. Dis. 11, 22 (2011).
Jensen, P. A., Lambert, L. A., Iademarco, M. F. & Ridzon, R. D. C. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 54, 1–141 (2005).
Kiwuwa, M. S., Charles, K. & Harriet, M. K. Patient and health service delay in pulmonary tuberculosis patients attending a referral hospital: a cross-sectional study. BMC Public. Health. 5, 122 (2005).
Masaya, U. Socio-spatial segregation in the era of growing economic disparities: the case of Tokyo. Geog Rev. Japan B. 94, 18–30 (2021).
Holtgrave, D. R. & Crosby, R. A. Social determinants of tuberculosis case rates in the united States. Am. J. Prev. Med. 26, 159–162 (2004).
Ploubidis, G. B. et al. Social determinants of tuberculosis in Europe: a prospective ecological study. Eur. Respir J. 40, 925–930 (2012).
Murase, Y., Ohkado, A., Watanabe, Y. & Kaguraoka, S. Transmission dynamics of Mycobacterium tuberculosis between foreign-nationals and Japanese tuberculosis patients living in Shinjuku-City, Tokyo. Kekkaku 92, 431–439 (2017).
Planning, Bureau, C. & Planning and Promotion Department. Population census. (2010). https://www.city.osaka.lg.jp/toshikeikaku/page/0000214424.html
Ueno, K. A review on the study of urban residential structures: on the relationships between factorial ecology and urban geography. Geogr. Rev. Jpn. 55, 715–734 (1982).
Kurasawa, S. Social Atlas of Tokyo Japanese-Tokyo No Shakai Chizu (University of Tokyo, 1986).
Nakamura, Y. A textbook of epidemiology without tears in Kiso Kara Manabu Tanoshii Ekigaku (in Japanese). Igaku-Shoin 2nd ed, 114–126 (2006).
Acknowledgements
We would like to acknowledge the Division of Infectious Disease Control, Osaka City Public Health Center, and the Department of Microbiology, Osaka Institute of Public Health, for their cooperation in this research. We are grateful to the late Dr. Kenji Matsumoto (Department of Infectious Disease Control, Osaka City Public Health Office, Osaka, Japan) for his valuable discussions and contributions. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This research was partly supported by the Japan Society for the Promotion of Science (JSPS), Grant-in-Aid for Young Scientists (18K17410 and 22K17318), Grant-in-Aid for Scientific Research C (21K10502), Grant-in-Aid for Scientific Research B (17H04136), and Grant-in-Aid for Scientific Research A (20H00040). This study was partially supported by the Japan Agency for Medical Research and Development (AMED; grant ID 24fk0108703h). N.F. was partially supported by JST PRESTO Grant Number JPMJPR21RA, Japan.
Author information
Authors and Affiliations
Contributions
KY, TW designed the study. JK, YT, HY collected epidemiological data. KY collected geographic and census data. KY, ST, TN, NF, JS and TW performed data analysis. KY, TW prepared the figures and tables. KY did the literature search. All authors read and provided feedback on the manuscript, and they approved the final version after incorporating the suggested revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamamoto, K., Takeuchi, S., Nakaya, T. et al. Geodemographic analysis of socioeconomic area disparities in tuberculosis incidence in Osaka City, Japan. Sci Rep 15, 15968 (2025). https://doi.org/10.1038/s41598-025-99711-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99711-4