Abstract
This study investigated the interplay between thrombosis and hemorrhage in critically ill COVID-19 patients, particularly those on extracorporeal membrane oxygenation (ECMO). Forty-three mechanically ventilated patients were divided into ECMO (n = 22) and non-ECMO (n = 21) groups. Thrombotic events occurred similarly in both groups (22.7% in ECMO, 28.6% in non-ECMO), both higher than the approximately 5% observed in patients hospitalized with viral respiratory illnesses other than COVID-19. However, bleeding events were significantly more frequent in the ECMO group (72.7%) compared to the non-ECMO group (14.3%) (P < 0.01). ECMO patients showed decreased platelet counts, fibrinogen, von Willebrand factor (VWF) activity using a ristocetin cofactor (VWF: RCo) assay, and developed acquired von Willebrand syndrome (AvWS) (VWF: RCo/VWF antigen (Ag) ratio < 0.7), along with increased D-dimer and lower high-molecular-weight VWF multimers. In contrast, the non-ECMO group showed no significant changes in platelet counts, fibrinogen, VWF: RCo, or D-dimer. Over time, both VWF: Ag and VWF: RCo increased significantly in both groups, but the VWF: RCo/VWF: Ag ratio remained above 0.7, and high-molecular-weight VWF multimers did not change significantly. These findings emphasize the need for vigilance regarding thrombotic and hemorrhagic complications, particularly in ECMO patients, where ECMO-induced shear stress may lead to AvWS, necessitating monitoring of VWF: Ag and VWF: RCo.
Similar content being viewed by others

Introduction
After three influenza pandemics in the 20th century (Spanish flu, Asian flu, and Hong Kong flu), another influenza pandemic in the 21st century was expected1. However, humankind encountered not a new influenza pandemic but a novel coronavirus pandemic1.
At the end of 2019, an outbreak of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred in Wuhan, China. The outbreak rapidly spread across China and subsequently became endemic worldwide. SARS-CoV-2 led to a global outbreak of coronavirus disease 2019 (COVID-19), which was declared a pandemic by the World Health Organization on 11 March 20202.
By the first half of 2024, approximately 776.9 million people had been infected and over 7.0 million had died globally3,4. This COVID-19 crisis remains not only a medical crisis but also a socioeconomic and political crisis, and it has left deep scars in all affected countries5.
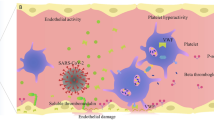
COVID-19 is primarily a lung disease but also affects several other organs such as the kidneys, brain, heart, eye, and gut6. Symptoms are driven by endotheliitis due to direct intracellular invasion by SARS-CoV-2 and/or inflammation and hypoxia7 triggered by the cytokine storm.
Patients with SARS-CoV-2 infection develop a peculiar form of coagulopathy due to severe endothelial damage; this condition is termed COVID-19-associated coagulopathy (CAC). CAC results from complex interactions between regulators of inflammation and coagulation. It is characterized by unique laboratory features different from either disseminated intravascular coagulation (DIC) or sepsis-induced coagulopathy (SIC)8. An increase in the D-dimer concentration is the most common finding. Approximately 45% of patients with COVID-19 have an abnormally elevated D-dimer concentration9,10. An elevated D-dimer concentration is also correlated with a poor patient prognosis and is an independent risk factor for mortality10. By contrast, most patients with COVID-19 have a normal or mildly deviated prothrombin time (PT), activated partial thromboplastin time (aPTT), plasminogen activator inhibitor (PAI)-1 concentration, and antithrombin (AT) concentration on presentation, and these laboratory indices are not reliably associated with disease severity11,12.
Although the main symptoms of COVID-19 are respiratory symptoms, we wish to emphasize that COVID-19 is frequently complicated by thrombosis in arteries, veins, or capillaries and has been described as a blood clotting disorder masquerading as a respiratory disease13.
Our extracorporeal membranous oxygenation (ECMO) center has managed more than 80 patients with COVID-19 in critical condition during the past 3 years. We have noticed an unexpectedly high number of patients who develop bleeding complications in addition to those who develop thrombosis.
We conducted the present study based on the hypothesis that venovenous ECMO (vvECMO) is involved in the development of bleeding complications in patients with severe COVID-19.
The purpose of this study was to determine the characteristics of CAC using biomarkers of coagulation/fibrinolysis, and the von Willebrand factor (VWF) antigen amount (VWF: Ag) and VWF activity (VWF: Ac) using a ristocetin cofactor (VWF: RCo) assay. On the basis of these results, we aimed to evaluate the intricate interplay of thrombosis and hemorrhage in critically ill COVID-19 patients.
Results
Population characteristics
Forty-three patients with severe COVID-19 were enrolled, and all patients were included in the analysis. The patients’ demographic and clinical characteristics are shown in Table 1. The age of the patients (35 men, 8 women) was 61 ± 19 years. Of the 43 patients, 22 were classified into the ECMO group and 21 in the non-ECMO group. Upon ICU admission, the ECMO group had significantly higher Acute Physiology and Chronic Health Evaluation (APACHE II) scores (P = 0.02) and Sequential Organ Failure Assessment (SOFA) scores (P < 0.01), as well as a significantly lower PaO2/FiO2 ratio (P = 0.01) compared to the non-ECMO group. The rate of continuous renal replacement therapy (CRRT) was significantly higher in the ECMO than non-ECMO group (P < 0.01). There was no significant difference between the two groups regarding history, intervention, and contents of medication except CRRT.
Distribution of coagulation/fibrinolysis markers
ICU admission
The differences in baseline coagulation/fibrinolysis biomarkers at intensive care unit (ICU) admission between the ECMO and non-ECMO groups are shown in Table 2. Upon ICU admission, both the total patient group and each cohort displayed platelet counts, PT-international normal ratio (INR), aPTT, and AT activity within or slightly deviating from reference ranges. By contrast, fibrinogen (Fbg), D-dimer, thrombin-antithrombin complex (TAT), plasmin-α2 plasmin inhibitor complex (PIC), and soluble fibrin (SF) diverged from the upper reference limits. The ECMO group had significantly higher value of fibrin/fibrinogen degradation products (FDP) (P = 0.013), D-dimer (P = 0.014), TAT (P = 0.02), and SF (P = 0.12), as well as a significantly lower AT activity (P = 0.014) compared to the non-ECMO group.
Time course of various coagulation/fibrinolysis markers in ECMO and non-ECMO groups
The time course of various coagulation/fibrinolysis markers in the ECMO and non-ECMO groups is shown in Fig. 1.
Comparison of coagulation/fibrinolysis markers at each time point between the ECMO and non-ECMO groups.
Solid lines indicate the ECMO group, and dotted lines indicate the non-ECMO group. P-values indicate comparisons between groups for each period.
*P < 0.05, **P < 0.01 for comparisons between the linked groups at each point. The gray area indicates the reference range.
Point 1 (P-1): day 1 (at intensive care unit admission in both groups, and before starting vvECMO in the ECMO group), Point 2 (P-2): day 3 or 4 (ECMO group: third day after starting vvECMO, non-ECMO group: day 3), and Point 3 (P-3): day 6 to 7 (ECMO group: sixth day after starting vvECMO, non-ECMO group: day 6 or 7).
ECMO, extracorporeal membrane oxygenation; vv, venovenous; PLT, platelet; PT-INR, prothrombin time–international normalized ratio; aPTT, activated partial thromboplastin time; Fbg, fibrinogen; FDP, fibrin/fibrinogen degradation products; TAT, thrombin–antithrombin complex; PIC, plasmin α2–plasmin inhibitor complex; PAI-1, plasminogen activator inhibitor-1; AT, antithrombin; SF, soluble fibrin.
After ICU admission, the ECMO group showed a gradual and significant decrease in the platelet counts and Fbg with each passing day, whereas the AT activity gradually and significantly increased with each passing day. Additionally, the FDP, D-dimer, and SF significantly increased from P-1 to P-2. No significant changes were observed in any other markers in the ECMO group.
The non-ECMO group showed a gradual and significant increase in AT activity with each passing day, and the Fbg significantly decreased from P-1 to P-2. No significant changes were observed in any other markers in the non-ECMO group.
Comparison of various coagulation/fibrinolysis markers at each time point between ECMO and non-ECMO groups
A comparison of coagulation/fibrinolysis markers at each time point between the ECMO and non-ECMO groups is also shown in Fig. 1. There was no significant difference in the platelet counts and Fbg between the two groups at P-1, but these indices were thereafter significantly lower in the ECMO than non-ECMO group at P-2 and P-3, and this difference further increased with each passing day. The FDP, D-dimer, TAT, and SF were significantly higher in the ECMO than non-ECMO group at all points. The AT activity was significantly lower in the ECMO than non-ECMO group at P-1 and P-2 but remained within the normal range in both groups during the observation period.
Cumulative frequency of thrombosis and bleeding events
The cumulative frequency of thrombosis and bleeding events during ICU admission between the ECMO and non-ECMO groups are shown in Table 3.
Thrombosis events occurred in 25.6% (11/43) of all patients in this study [22.7% (5/22) of ECMO group and 28.6% (6/21) of non-ECMO group]. Most of the thrombotic events we encountered were deep venous thrombosis (DVT) and/or pulmonary embolism (PE) [18.2% (4/22) of ECMO group and 28.6% (6/21) of non-ECMO group]. However, there was no significant difference between the two groups. Cerebral infarction, which can be fatal in some cases, occurred in one patient in each group.
Bleeding events occurred in 44.2% (19/43) of all patients and were significantly more frequent in the ECMO group than in the non-ECMO group [72.7% (16/22) of ECMO group and 14.3% (3/21) of non-ECMO group, P < 0.01]. Among them, intramuscular hematoma appeared most frequently, and occurred significantly higher in the ECMO group than in the non-ECMO group. [54.6% (12/22) of ECMO group and 4.8% (1/21) of non-ECMO group, P < 0.01]. Gastrointestinal (GI) bleeding tended to be higher in the ECMO group than in the non-ECMO group [27.3% (6/22) of ECMO group and 4.8% (1/21) of non-ECMO group, P = 0.09]. Cerebral hemorrhage, which can be fatal in some cases, occurred in one patient in each group. No cases of major GI bleeding were observed.
Distribution of VWF: Ag, VWF: RCo, and VWF: RCo/VWF: Ag ratio
Upon ICU admission, VWF: Ag and VWF: RCo were markedly high in both the ECMO and non-ECMO groups, and there was no significant difference in either VWF: Ag or VWF: RCo between the two groups (Fig. 2). After ICU admission in the ECMO group, VWF: Ag slightly increased or decreased within the range of 300–400% and there was a significant increase from P-2 to P-3, but no significant fluctuations were observed during the observation period. VWF: RCo in the ECMO group remained at the upper limit of the reference range, but it significantly decreased as time passed. By contrast, both VWF: Ag and VWF: RCo in the non-ECMO group continued to significantly increase each day (Fig. 2). As a result, the VWF: Ag was significantly lower in the ECMO group compared to the non-ECMO group at P-3, and the VWF: RCo was significantly lower in the ECMO group compared to the non-ECMO group at both P-2 and P-3 (Fig. 2).
Comparison of VWF: Ag, VWF: RCo, and VWF: RCo/VWF: Ag ratio at each time point between the groups.
Solid lines indicate the ECMO group, and dotted lines indicate the non-ECMO group. P-values indicate comparisons between groups for each period.
*P < 0.05, **P < 0.01 for comparisons between the linked groups at each point. The gray area indicates the reference range.
Point 1 (P-1): day 1 (at intensive care unit admission in both groups, and before starting vvECMO in the ECMO group), Point 2 (P-2): day 3 or 4 (ECMO group: third day after starting vvECMO, non-ECMO group: day 3), and Point 3 (P-3): day 6 to 7 (ECMO group: sixth day after starting vvECMO, non-ECMO group: day 6 or 7).
ECMO, extracorporeal membrane oxygenation; vv, venovenous; VWF, von Willebrand factor; Ag, antigen; RCo, ristocetin cofactor; vv, venovenous.
The VWF: RCo/VWF: Ag ratio in the ECMO group significantly decreased each day (Fig. 2) and remained below 0.7 (defined as AvWS in this study) throughout the observation period. In contrast, the VWF: RCo/VWF: Ag ratio in the non-ECMO group did not decrease but instead gradually and significantly increased above 70% during the observation period (Fig. 2).
Distribution of ADAMTS-13, FVIII, and FIX
A disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS-13) was substantially within the reference range in both the ECMO and non-ECMO groups at ICU admission (reference range: 50–150%) (Fig. 3), and there was no significant difference between the two groups. ADAMTS-13 in the non-ECMO group thereafter remained almost unchanged at 70–75% during the observational period, but it increased in the ECMO group with each passing day. At P-3, ADAMTS-13 was significantly higher in the ECMO than non-ECMO group (Fig. 3).
Comparison of ADAMTS-13, factor VIII, and factor IX in ECMO group and non-ECMO group.
Solid lines indicate the ECMO group, and dotted lines indicate the non-ECMO group.
*P < 0.05, **P < 0.01 for comparisons between the linked groups at each point. The gray area indicates the reference range.
Point 1 (P-1): day 1 (at intensive care unit admission in both groups, and before starting vvECMO in the ECMO group), Point 2 (P-2): day 3 or 4 (ECMO group: third day after starting vvECMO, non-ECMO group: day 3), and Point 3 (P-3): day 6 to 7 (ECMO group: sixth day after starting vvECMO, non-ECMO group: day 6 or 7).
ADAMTS-13, a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13; vv, venovenous; ECMO, extracorporeal membrane oxygenation.
Factor VIII (FVIII) in both the ECMO and non-ECMO groups was about 220%, which was slightly above the upper limit of the reference range, at ICU admission (reference range: 50-200%) (Fig. 3). FVIII in the ECMO group thereafter slightly increased or decreased within the range of 170–220%, but no significant fluctuations were observed. However, FVIII in the non-ECMO group continued to increase each day, exceeding 300% at P-3 (Fig. 3). FVIII was significantly lower in the ECMO than non-ECMO group at P-2 and P-3.
In both groups, factor IX (FIX) remained around the upper end of the reference range throughout the observational period (reference range: 60-130%) (Fig. 3), and no significant difference was observed between the two groups at any time point.
Changes in VWF multimer analysis
The time course of VWF multimer analysis for representative patients from the ECMO and non-ECMO groups is shown in Fig. 4. High-molecular-weight (HMW) VWF multimers were present to the same extent in both the ECMO and non-ECMO groups at the time of ICU admission. The ratio of HMW VWF multimers in the non-ECMO group thereafter remained almost unchanged over time, while the ratio of HMW VWF multimers in the ECMO group gradually decreased and shifted toward low-molecular-weight (LMW) VWF multimers after the start of ECMO.
Time course of VWF multimer analysis for representative patients from ECMO and non-ECMO groups.
HMW VWF multimers were present to the same extent in both the ECMO and non-ECMO groups at P-1. The ratio of HMW VWF multimers in the non-ECMO group thereafter remained almost unchanged over time, while the ratio of HMW VWF multimers in the ECMO group gradually decreased and shifted toward LMW VWF multimers after the start of ECMO (P-2 and P-3).
Point 1 (P-1): day 1 (at intensive care unit admission in both groups, and before starting vvECMO in the ECMO group), Point 2 (P-2): day 3 or 4 (ECMO group: third day after starting vvECMO, non-ECMO group: day 3), and Point 3 (P-3): day 6 to 7 (ECMO group: sixth day after starting vvECMO, non-ECMO group: day 6 or 7).
VWF, von Willebrand factor; ECMO, extracorporeal membrane oxygenation; HMW, high molecular weight; LMW, low molecular weight; IMW, intermediate molecular weight.
Discussion
Thrombosis is a prominent feature of COVID-1911,14. Nearly one in six adults hospitalized with COVID-19 will develop arterial or venous thrombosis during hospital admission14. Ackermann et al.15 reported that alveolar capillary microthrombi are nine times as prevalent at autopsy in COVID-19 than in influenza. In a national analysis of hospitalizations for viral pneumonia in the United States, Smilowitz et al.16 reported that thrombosis occurred in 16% of cases and that this was significantly higher than the incidence of thrombosis observed in patients hospitalized with non-COVID-19 viral respiratory illness from 2002 to 2014 (5%).
In our study, thrombosis events occurred in 25.6% of severe COVID-19 patients. This incidence rate was higher than that in the previous reports mentioned above14,16. We suspect that this result may be because our study enrolled only severe COVID-19 patients. Alternatively, the divergent result may be owing to frequent ultrasonographic examinations to assess the presence or absence of DVT in the lower extremities. Regardless of the reason, thrombosis is a prominent feature of COVID-19.
On the other hand, few studies have determined the association between COVID-19 and bleeding. However, in a Danish nationwide population-based cohort study, the diagnosis rate of major bleeding was 0.5% (47/9,460) of all SARS-CoV-2-positive individuals and 2.3% in hospitalized SARS-CoV-2 patients17. In a self-controlled case series and matched cohort study using national registries in Sweden, the absolute risk for bleeding among patients with COVID-19 was 0.101% (1002 events). The rate ratios were highest in critically ill COVID-19 patients18. A multicenter retrospective study from the USA reported a rate of major bleeding of 5.6% in critically ill patients with COVID-19. One bleeding event, an intracranial hemorrhage, was fatal. A baseline platelet count of < 150 × 109/L and D-dimer levels > 2500 ng/mL were identified as independent predictors of a threefold increase in the risk of major bleeding with anticoagulant thrombo-prophylaxis19.
In our study, bleeding events occurred in 44.2% of the severe COVID-19 patients. This incidence rate was much higher than that in the previous reports mentioned above17,19. We suspect that the differing results can be attributed to the fact that our study exclusively enrolled severe COVID-19 patients. All 42 patients were managed with mechanical ventilation, and additionally, 22 patients (52.4%) were receiving ECMO.
We measured various coagulation/fibrinolysis biomarkers in severe COVID-19 patients in this study. We confirmed that severe COVID-19 patients had normal or mildly deviated platelet counts, PT-INR, aPTT, and AT activity. In contrast, the coagulation/fibrinolysis markers that deviated significantly from the normal reference range were FDP, D-dimer, TAT, PIC, and SF. In addition, Fbg and PAI-1 levels were slightly above the upper normal limit. This result suggests that the pattern of CAC is definitely different from the pattern of conventional sepsis-induced DIC or SIC, which usually presents with suppressed fibrinolytic-type coagulopathy20. In particular, in the ECMO group, platelet counts and Fbg levels decreased significantly over time, while FDP and D-dimer levels remained high, which may be associated with the development of bleeding events.
In this study, we also focused on the time course of both VWF: Ag and VWF: RCo. VWF is a multimeric glycoprotein that facilitates primary hemostasis by promoting platelet adhesion and aggregation at sites of vascular injury21. Upon contact with collagen at sites of endothelial injury, HMW VWF multimers unfold, with each multimeric subunit of VWF exposing its binding sites to platelet glycoprotein receptors22 Larger VWF multimers engage in more effective binding to collagen and platelets. This is the main mechanism of primary hemostasis23.
Most previous reports of COVID-19 have provided values for only VWF: Ag, but to date fewer reports have provided values for VWF: RCo. We measured not only VWF: Ag but also VWF: Ac using a VWF: RCo assay, and confirmed both values were invariably higher than the normal reference ranges in both the ECMO and the non-ECMO group at all measurement points including ICU admission. Based on previous reports, we hypothesized that the several factors may be involved in the elevated levels of both VWF: Ag and VWF: RCo in severe COVID-19 patients. In general, in COVID-19 patients, higher levels of VWF: Ag or VWF: RCo were associated with more severe cases and non-survival24. Although no studies have been conducted specifically in COVID-19 patients, several previous studies on the blood clotting effects of steroids have reported significant increases in VWF: Ag and/or FVIII levels following steroid administration in patients with multiple sclerosis, systemic lupus erythematosus, and even in healthy individuals25,26,27. These findings suggest that steroid administration may result in an increase in coagulation factor levels or activity. All patients in this study had severe COVID-19 and over 90% patients were treated with dexamethasone according to COVID-19 treatment guidelines. For these reasons, VWF: Ag and VWF: RCo levels might be elevated above the upper limit of the normal range. Finally, we concluded that elevated levels of both VWF: Ag and VWF: RCo value were deeply involved in the reason for the higher incidence of thrombosis events compared to patients hospitalized with non-COVID-19 viral respiratory illness.
In addition, we also calculated the VWF: RCo/VWF: Ag ratio. We believe that this is probably the first valuable assessment of the VWF: RCo/VWF: Ag ratio in patients with severe COVID-19 treated with and without vvECMO.
After ICU admission, in the ECMO group, VWF: Ag hardly changed and VWF: RCo decreased; in the non-ECMO group, however, both VWF: Ag and VWF: RCo steadily increased. The VWF: RCo/VWF: Ag ratio significantly decreased in the ECMO group but significantly increased in the non-ECMO group. In addition, the VWF: RCo/VWF: Ag ratio was significantly lower in the ECMO than non-ECMO group at P-2 and P-3.
In the absence of a family history of bleeding, the diagnosis of AvWS is usually based on the laboratory tests used to diagnose inherited von Willebrand disease25,28,29. Patients with acquired von Willebrand syndrome (AvWS ) typically exhibit normal or mildly decreased VWF: Ag levels, in contrast to a more pronounced reduction in VWF: RCo28. This trend was observed not only in our results, but also in a previous report concerning severe COVID-19-induced acute respiratory distress syndrome (ARDS) patients who underwent ECMO30. And as a result, patients with AvWS often exhibit a VWF: RCo/VWF: Ag ratio < 0.7, resembling that observed in patients with von Willebrand disease type 2 A31,32,33. Our ECMO group showed similar results. In our study, the VWF: RCo/VWF: Ag ratio was almost 0.7 in both groups at the time of ICU admission, but this ratio in the ECMO group thereafter declined and remained below 0.7. By contrast, the VWF: RCo/VWF: Ag ratio in the non-ECMO group remained above 0.7. Therefore, when AvWS is defined as a VWF: RCo/VWF: Ag ratio of ≤ 0.731, it should be noted that severe COVID-19 often coexists with AvWS during ECMO management and may be complicated with bleeding complications. And we suspected that even if the values of VWF: RCo and VWF: Ag do not decrease but rather increase slightly, if the VWF: RCo/VWF: Ag ratio decreases to less than 0.7, we should be on alarm against the onset of AvWS and be on precaution against not only thrombosis but hemorrhage. We believe that calculation of the VWF: RCo/VWF: Ag ratio is important for detecting complications in critically ill COVID-19 patients, especially those receiving ECMO management.
ECMO is well known to induce AvWS34, which severely affects primary hemostasis. Previous reports have shown that approximately 90–100% of patients undergoing ECMO management develop AvWS35. Larger VWF multimers more effectively bind to collagen and platelets, which is the main mechanism underlying primary hemostasis23. On the other hand, when the level of HMW VWF multimers decreases, the primary hemostasis mechanism is disrupted, resulting in an increased the risk of spontaneous bleeding. With respect to the time course of the VWF multimers in our patients, HMW VWF multimers were present to the same extent in both the ECMO and non-ECMO groups at the time of ICU admission. The HMW VWF multimers in the non-ECMO group remained almost unchanged over time, while those in the ECMO group gradually decreased after the start of ECMO. This decrease occurred because initiation of ECMO caused high shear stress within the cannulas and extracorporeal circuit, enhancing cleavage of the HMW VWF multimers by the protease ADAMTS-13.
In addition, ADAMTS-13 activity was significantly higher in the ECMO than non-ECMO group at P-3. This enhancement of ADAMTS-13 activity is also considered to be one of the reasons for the decrease in the ratio of HMW VWF multimers in the ECMO group. We thought these changes in the ECMO group led to the loss of HMW VWF multimers, and a part to significant increase in the cumulative bleeding complication rate compared to the non-ECMO group.
Furthermore, the SOFA and APACHE II scores at admission in the ECMO group were significantly higher than those in the non-ECMO group, and the patients in the ECMO group were severely ill and frequently underwent CRRT. Therefore, we believe that the ECMO group received higher doses of anticoagulant therapy compared to the non-ECMO group to prevent intracircuit coagulation associated with CRRT. We speculate that these circumstances are also the cause of the frequent bleeding complications in the ECMO group.
In particular, the reason for the high incidence of intramuscular hematoma in the ECMO group is that repositioning, including frequent prone therapy, was mandatory for patients with COVID-19 who had developed severe ARDS.
This study has several limitations. First, this was a retrospective study, and each biomarker for each patient was measured at different times from the beginning of COVID-19 infection. Second, the patients with severe COVID-19 were usually administered an anticoagulant, mainly heparin, upon ICU admission. Because anticoagulants affect hemostatic alterations, their potential effects on the outcomes of this study cannot be ignored. Third, the ECMO circuits were managed using several types of cannulas, oxygenators, and centrifugal pumps with or without a heparin coating and regulated with different pump flow and sweep gas rates. As the ECMO circuit may influence changes in hemostasis, the potential impact of the equipment on the results of this study cannot be ignored. Fourth, because this study focused on measurable markers in our hospital, few other markers were examined. Fifth, this was a relatively short-term single-center study. Therefore, further research is required to confirm our findings.
Conclusions
Several reports have emphasized that CAC is characterized not only by a hypercoagulable state with thrombosis but also by a pre-hemorrhagic state with endothelial leakage and intracranial hemorrhage, particularly in critically ill patients36,37,38,39,40. Based on the results of this study, a comprehensive understanding of CAC and the prevention of thrombotic or hemorrhagic complications require not only the measurement of coagulation/fibrinolysis markers but also the assessment of VWF: Ag and VWF: RCo levels, along with the calculation of the VWF: RCo/VWF: Ag ratio. This is especially important in critically ill COVID-19 patients undergoing ECMO, as the shear stress induced by ECMO can contribute to the development of AvWS, thereby increasing the risk of bleeding and coagulopathy. Regular monitoring of VWF: Ag and VWF: RCo levels, ideally on a daily basis, is strongly recommended to enable the early detection of AvWS and to mitigate the risk of bleeding and thrombotic complications.
Methods
This retrospective, single-center, observational study was conducted at the ECMO Center of Fukuoka University Hospital in Fukuoka, Japan, which is a 915-bed referral and tertiary hospital, from June 2020 to August 2021. The ethics review boards of Fukuoka University Hospital (U22-988; registered on 22 October 2020) and Sysmex Corporation (2020 − 242; registered on 13 November 2020) approved the study protocol. Informed consent was obtained from all subjects and/or their legal guardian(s). The present study adhered to the guidelines of the Declaration of Helsinki. The study involved ≥ 18-year-old patients with severe COVID-19 who were diagnosed with SARS-CoV-2 infection as detected by reverse transcription–polymerase chain reaction from a nasopharyngeal swab sample. Severe COVID-19 was defined as admission to the ICU for treatment of ARDS and a requirement for mechanical ventilation management or additional vvECMO management.
Patients were evaluated for the presence of ARDS according to the Berlin definition41. The illness severity was evaluated using the APACHE II (APACHE II) score42. Organ failure was assessed using the SOFA score43. These scores are useful for evaluating the morbidity and mortality of patients with critical illnesses. Patient demographics and clinical characteristics were extracted from the electronic medical records. Patients with severe COVID-19 were divided into the ECMO group (vvECMO management within 24 h after admission) and the non-ECMO group (absence of vvECMO management during hospitalization). We also investigated the complications of hemorrhagic and thrombotic events and their details during the ICU stay in each group.
Bleeding and thrombotic events
The incidence of thrombotic and bleeding events in COVID-19 patients was assessed. Cerebral infarction, DVT, and PE were confirmed using radiographic imaging, including ultrasonography, computed tomography, and pulmonary angiography. Synchronously diagnosed DVT and PE in the same patient were considered a single venous thromboembolism event. Patients with thrombotic events clearly associated with central venous or other indwelling catheters were excluded.
Cerebral hemorrhage and intramuscular hematoma were also confirmed radiographically. GI bleeding during hospitalization was identified using the electronic medical record system. Only those patients with overt manifestations of bleeding in the form of bleeding from an indwelling GI tube, hematochezia, or melena, were classified as having GI bleeding. Patients with signs of GI bleeding on admission were excluded. Major GI bleeding was defined as requiring transfusion of more than two units of packed red blood cells or a decrease in hemoglobin by 2 g/dL attributed to hemorrhage.
Study procedures
Blood samples were routinely collected from patients with severe COVID-19 to measure various markers. We collected peripheral blood and measured coagulation/fibrinolysis biomarkers including the platelet counts, PT-INR, Fbg, FDP, D-dimer, TAT, PIC, PAI-1, AT activity, and SF.
We also measured VWF: Ag, VWF: RCo, ADAMTS-13, FVIII, and FIX.
Measurement of VWF: ag and VWF: RCo
The level of VWF: Ag in the plasma was determined by a VWF: Ag immunoassay (VWF Ag reagent, Siemens Healthcare Diagnostics, Germany). The level of VWF: RCo in the plasma was determined as the VWF ristocetin cofactor activity by an aggregation assay using BC Von Willebrand Reagent (Siemens Healthcare Diagnostics, Germany). Both VWF: Ag and VWF: RCo were measured using an automated coagulation analyzer, CN-6000 system (Sysmex Corporation, Kobe, Japan), according to the manufacturer’s instructions.
Measurement of ADAMTS-13 activity
The level of ADAMTS-13 activity was determined by a chromogenic ADAMTS-13 activity enzyme-linked immunosorbent assay (Kainos Laboratories, Tokyo, Japan).
Measurement of FVIII and FIX
Revohem FVIII Chromogenic and Revohem FIX Chromogenic (Sysmex Corporation, Kobe, Japan) were used to measure the activity of FVIII and FIX, respectively. All reactions were performed using the CN-6000 system (Sysmex Corporation, Kobe, Japan).
VWF multimer analysis
VWF multimers were determined by sodium dodecyl-sulfate gel electrophoresis with subsequent transfer of the resolved multimers to a polyvinylidene fluoride membrane. The content of the VWF multimers was classified as HMW, intermediate-molecular-weight (reduced but not entirely absent), or LMW if they corresponded to bands > 10, bands 5 to 7, or bands 1 to 4 in the VWF multimer analysis, respectively.
Usually, we routinely determined the coagulation/fibrinolysis markers, especially global markers, in ICU patients. In particular, we measured markers and carefully observe patients who have coagulation/fibrinolysis abnormalities upon ICU admission every day. In addition, special markers such as VWF: Ag, VWF: RCo, ADAMTS13 etc. are also retrospectively measured from time to time.
In this study, we compared various coagulation/fibrinolysis markers as well as VWF: Ag, VWF: RCo, and the VWF multimer between the ECMO and non-ECMO groups at three time points: Point 1 (P-1): day 1 (at ICU admission in both groups, and before starting vvECMO in the ECMO group), Point 2 (P-2): day 3 or 4 (ECMO group: 3rd day after starting vvECMO, non-ECMO group: day 3 after admission), and Point 3 (P-3): day 6 to 7 (ECMO group: 6th day after starting vvECMO, non-ECMO group: day 6 or 7 after admission).
Definition of acquired von willebrand syndrome
In this study, AvWS was defined to have developed when the VWF: RCo/VWF: Ag ratio was < 0.729,32.
Management of VvECMO
vvECMO was initiated within 24 h of patient admission to the ICU of the ECMO center and then maintained at the ECMO center until ECMO and mechanical ventilation management were completed. The indications for vvECMO in patients with COVID-19 were decided based on the criteria of the ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial22 despite lung-protective ventilation and prone positioning, taking into account age, comorbidities, and clinical course. The patients were connected to the vvECMO circuit via two single-lumen HLS catheters (Maquet Holding GmbH KG, Rastatt, Germany).
Treatment followed a standardized protocol. The most commonly used drainage cannulas were 23 to 25 Fr, and the return cannulas were 19 to 21 Fr. The vvECMO circuit consisted of several oxygenator and centrifugal pump combinations as follows. The oxygenator used in the vvECMO circuit was either the CAPIOX LX [poly(2-methoxyethyl acrylate (PMEA)-coated polymethylpentene membrane] (Terumo Corporation, Tokyo, Japan) or the MERA NHP Exelung NSH-R (heparin- and silicone-coated polypropylene membrane) (Senko Medical Instrument Manufacturing Co., Ltd., Tokyo, Japan). The centrifugal pump used in the vvECMO circuit was the PMEA-coated CAPIOX SL (Terumo Corporation), heparin-coated MERA centrifugal blood pump (Senko Medical Instrument Manufacturing Co., Ltd.), or the uncoated Gyro pump (Kyocera Corporation, Kyoto, Japan). In addition, a heparin-coated HLS Module (Maquet Cardiopulmonary GmbH, Hechingen, Germany), which integrates an oxygenator, a centrifugal pump, and a heat exchanger, was also used.
The system was actively warmed by heated water. During vvECMO management, the pump flow was routinely set at 3 to 5 L/min.
For coagulation and blood management during vvECMO, patients received unfractionated heparin targeting an aPTT of 40 to 60 s.
Thresholds for transfusion of packed red blood cells, fresh frozen plasma, and platelet concentrates were a hemoglobin concentration of ≤ 8 g/dL, fibrinogen level of ≤ 150 mg/dL, and platelet count of ≤ 50,000/µL, respectively.
Statistical analysis
Unless otherwise indicated, all data are expressed as mean ± standard deviation. SPSS 15.0 J (SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Nonparametric statistical tests were used because these tests are more appropriate than traditional parametric tests for analysis of data sets with high variability. Differences in biomarker levels between the ECMO and non-ECMO groups were analyzed using the Mann–Whitney U test. Comparisons between three or more groups were carried out using the Bonferroni method. Unless otherwise indicated, the level of statistical significance was set at P < 0.05.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to protect the privacy of severe COVID-19 patients as much as possible but are available from the corresponding author (HI) on reasonable request.
Abbreviations
- Ac:
-
activity
- ADAMTS-13:
-
a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13
- Ag:
-
antigen
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- aPTT:
-
activated partial thromboplastin time
- ARDS:
-
acute respiratory distress syndrome
- AT:
-
antithrombin
- AvWS:
-
acquired von Willebrand syndrome
- CAC:
-
COVID-19-associated coagulopathy
- COVID-19:
-
coronavirus disease 2019
- CRRT:
-
continuous renal replacement therapy
- DIC:
-
disseminated intravascular coagulation
- DVT:
-
deep venous thrombosis
- ECMO:
-
extracorporeal membrane oxygenation
- Fbg:
-
fibrinogen
- FDP:
-
fibrin/fibrinogen degradation products
- FVIII:
-
factor VIII
- FIX:
-
factor IX
- GI:
-
gastrointestinal
- HMW:
-
high molecular weight
- ICU:
-
intensive care unit
- IMW:
-
intermediate molecular weight
- INR:
-
international normal ratio
- LMW:
-
low molecular weight
- PAI:
-
plasminogen activator inhibitor
- PE:
-
pulmonary embolism
- PIC:
-
plasmin-α2 plasmin inhibitor complex
- PT:
-
prothrombin time
- RCo:
-
ristocetin cofactor activity
- SF:
-
soluble fibrin
- SOFA:
-
Sequential Organ Failure Assessment
- TAT:
-
thrombin-antithrombin complex
- VTE:
-
venous thromboembolism
- vvECMO:
-
venovenous ECMO
- VWF:
-
von Willebrand factor
References
Akin, L. & Gözel, M. G. Understanding dynamics of pandemics. Turk. J. Med. Sci. 50, 515–519. https://doi.org/10.3906/sag-2004-133 (2020).
WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020. World Health Organization. Geneva. (2020). http://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed 24 Dec. 2024.
WHO COVID-19 dashboard. Number of COVID-19 cases reported to WHO. Dec. (2024). https://data.who.int/dashboards/covid19/cases; Accessed 24.
WHO COVID-19 dashboard. Number of COVID-19 deaths reported to WHO. Dec. (2024). https://data.who.int/dashboards/covid19/deaths; Accessed 24.
Perico, L. et al. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 17, 46–64. https://doi.org/10.1038/s41581-020-00357-4 (2021).
Kalbhenn, J., Glonnegger, H., Wilke, M., Bansbach, J. & Zieger, B. Hypercoagulopathy, acquired coagulation disorders and anticoagulation before, during and after extracorporeal membrane oxygenation in COVID-19: a case series. Perfusion 36, 592–602. https://doi.org/10.1177/02676591211001791 (2021).
Pavoni, V., Gianesello, L., Pazzi, M., Dattolo, P. & Prisco, D. Questions about COVID-19 associated coagulopathy: possible answers from the viscoelastic tests. J. Clin. Monit. Comput. 36, 55–69. https://doi.org/10.1007/s10877-021-00744-7 (2022).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl. J. Med. 382, 1708–1720. https://doi.org/10.1056/NEJMoa2002032 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3 (2020).
Colling, M. E. & Kanthi, Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med. 25, 471–478. https://doi.org/10.1177/1358863X20932640 (2020).
Iba, T., Levy, J. H., Levi, M., Connors, J. M. & Thachil, J. Coagulopathy of coronavirus disease 2019. Crit. Care Med. 48, 1358–1364. https://doi.org/10.1097/CCM.0000000000004458 (2020).
Ishikura, H. et al. Daily combined measurement of platelet count and presepsin concentration can predict in-hospital death of patients with severe coronavirus disease 2019 (COVID-19). Int. J. Hematol. 117, 845–855. https://doi.org/10.1007/s12185-023-03555-5 (2023).
Janardhan, V., Janardhan, V. & Kalousek, V. COVID-19 as a blood clotting disorder masquerading as a respiratory illness: a cerebrovascular perspective and therapeutic implications for stroke thrombectomy. J. Neuroimaging. 30, 555–561. https://doi.org/10.1111/jon.12770 (2020).
Bilaloglu, S. Thrombosis in hospitalized patients with COVID-19 in a new York City health system. JAMA 324, 799–801. https://doi.org/10.1001/jama.2020.13372 (2020).
Ackermann, M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl. J. Med. 383, 120–128. https://doi.org/10.1056/NEJMoa2015432 (2020).
Smilowitz, N. R. et al. Thrombosis in hospitalized patients with viral respiratory infections versus COVID-19. Am. Heart J. 231, 93–95. https://doi.org/10.1016/j.ahj.2020.10.075 (2021).
Dalager-Pedersen, M. et al. Venous thromboembolism and major bleeding in patients with coronavirus disease 2019 (COVID-19): a nationwide, population- based cohort study. Clin. Infect. Dis. 73, 2283–2293. https://doi.org/10.1093/cid/ciab003 (2021).
Katsoularis, I. et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: nationwide self-controlled cases series and matched cohort study. BMJ 377, e069590. https://doi.org/10.1136/bmj-2021-069590 (2022).
Al-Samkari, H. et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 136, 489–500. https://doi.org/10.1182/blood.2020006520 (2020).
Asakura, H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J. Intensive Care. 2, 20. https://doi.org/10.1186/2052-0492-2-20 (2014).
Stockschlaeder, M., Schneppenheim, R. & Budde, U. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis. 25, 206–216. https://doi.org/10.1097/MBC.0000000000000065 (2014).
Combes, A. et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl. J. Med. 378, 1965–1975. https://doi.org/10.1056/NEJMoa1800385 (2018).
Lenting, P. J., Christophe, O. D. & Denis, C. V. Von Willebrand factor biosynthesis, secretion, and clearance: connecting the Far ends. Blood 125, 2019–2028. https://doi.org/10.1182/blood-2014-06-528406 (2015).
Favaloro, E. J., Henry, B. M., Lippi, G., Increased, V. W. F. & Decreased ADAMTS-13 in COVID-19: creating a milieu for (Micro)Thrombosis. Semin Thromb. Hemost. 47, 400–418. https://doi.org/10.1055/s-0041-1727282 (2021).
Federici, A. B. Acquired von Willebrand syndrome: is it an extremely rare disorder or do we see only the tip of the iceberg? J. Thromb. Haemost. 6, 565–568. https://doi.org/10.1111/j.1538-7836.2008.02917.x (2008).
Frank, R. D., Altenwerth, B., Brandenburg, V. M., Nolden-Koch, M. & Block, F. Effect of intravenous high-dose Methylprednisolone on coagulation and fibrinolysis markers. Thromb. Haemost. 94, 467–468 (2005).
Garg, A., Gupta, G., Gupta, R. & Mishra, R. K. Converging pathways: acquired von Willebrand disease in systemic lupus erythematosus with antiphospholipid antibodies presenting with persistent menstrual bleeding. BMJ Case Rep. 17, e260824. https://doi.org/10.1136/bcr-2024-260824 (2024).
Favaloro, E. J., Facey, D. & Grispo, L. Laboratory assessment of von Willebrand factor. Use of different assays can influence the diagnosis of von Willebrand’s disease, dependent on differing sensitivity of sample Preparation and differential recognition of high molecular weight VWF forms. Am. J. Clin. Pathol. 104, 264–271. https://doi.org/10.1093/ajcp/104.3.264 (1995).
Franchini, M. & Mannucci, P. M. Acquired von Willebrand syndrome: focused for hematologists. Haematologica 105, 2032–2037. https://doi.org/10.3324/haematol.2020.255117 (2020).
Kalbhenn, J., Glonnegger, H., Wilke, M., Bansbach, J. & Zieger, B. Hypercoagulopathy, acquired coagulation disorders and anticoagulation before, during and after extracorporeal membrane oxygenation in COVID-19: A case series. Perfusion 36, 592–602 (2021).
Roth, N. et al. The impact of bicuspid aortic valve morphology on von Willebrand factor function in patients with severe aortic stenosis and its change after TAVI. Clin. Res. Cardiol. 111, 1348–1357. https://doi.org/10.1007/s00392-022-02047-6 (2022).
Kalbhenn, J., Glonnegger, H., Büchsel, M., Priebe, H. J. & Zieger, B. Acquired von Willebrand syndrome and Desmopressin resistance during venovenous extracorporeal membrane oxygenation in patients with COVID-19: a prospective observational study. Crit. Care Med. 50, 1246–1255. https://doi.org/10.1097/CCM.0000000000005467 (2022).
James, P. D. et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 5, 280–300. https://doi.org/10.1182/bloodadvances.2020003265 (2021).
Kalbhenn, J., Schlagenhauf, A., Rosenfelder, S., Schmutz, A. & Zieger, B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: rapid onset and fast recovery. J. Heart Lung Transpl. 37, 985–991. https://doi.org/10.1016/j.healun.2018.03.013 (2018).
Panholzer, B. et al. Acquired von Willebrand syndrome in ECMO patients: a 3-year cohort study. Blood Cells Mol. Dis. 87, 102526. https://doi.org/10.1016/j.bcmd.2020.102526 (2021).
Dixon, L. et al. Cerebral microhaemorrhage in COVID-19: a critical illness related phenomenon? Stroke Vasc Neurol. 5, 315–322. https://doi.org/10.1136/svn-2020-000652 (2020).
Mousa-Ibrahim, F., Berg, S., Od`TPDetola, O., Teitcher, M. & Ruland, S. Intracranial hemorrhage in hospitalized SARS-CoV-2 patients: a case series. J. Stroke Cerebrovasc. Dis. 30, 105428. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105428 (2021).
Fayed, I., Pivazyan, G., Conte, A. G., Chang, J. & Mai, J. C. Intracranial hemorrhage in critically ill patients hospitalized for COVID-19. J. Clin. Neurosci. 81, 192–195. https://doi.org/10.1016/j.jocn.2020.08.026 (2020).
Fraiman, P., Godeiro-Junior, C., Moro, E., Cavallieri, F. & Zedde, M. COVID-19 and cerebrovascular diseases: a systematic review and perspectives for stroke management. Front. Neurol. 11, 574694. https://doi.org/10.3389/fneur.2020.574694 (2020).
Cezar-Junior, A. B. et al. Subarachnoid hemorrhage and COVID-19: association or coincidence? Med. (Baltim). 99, e23862. https://doi.org/10.1097/MD.0000000000023862 (2020).
ARDS Definition Task Force et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 307, 2526–2533. https://doi.org/10.1001/jama.2012.5669 (2012).
Knaus, W. A., Draper, E. A. & Wagner, D. P. Zimmerman. J, E, APACHE II: a severity of disease classification system. Crit. Care Med. 13, 818–829 (1985).
Vincent, J. L. et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on sepsis-related problems of the European society of intensive care medicine. Crit. Care Med. 26, 1793–1800. https://doi.org/10.1097/00003246-199811000-00016 (1998).
Acknowledgements
We thank Angela Morben, DVM, ELS, and Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Funding
There were no external sources of funding for this study.
Author information
Authors and Affiliations
Contributions
HI contributed to the study design, statistical analysis, interpretation of the results, drafting of the manuscript, and critical revision of the manuscript for intellectual content. YI participated in designing the study and interpreting the results. MN and JM participated in interpreting the results and drafting of the manuscript. YN was involved in data acquisition. TY and MY participated in the measurement of samples and statistical analysis. HH performed the blood sampling and collected the data for the ECMO patients. SY participated in the data collection for the ECMO patients and the revision of the manuscript drafts. KH participated in designing the study and interpreting the results and drafting of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The following ethics review boards approved the protocol for this study: Fukuoka University Hospital (U20-10-008; registered on 22 October 2020); and Sysmex Corporation (2020 − 242; registered on 13 November 2020). We obtained signed informed consent from the patients or their proxies for publication of this study and the study was conducted in accordance to the principles of the Declaration of Helsinki. A copy of the informed consent document can be provided upon request.
Competing interests
The authors declare no competing interests.
Authors’ information
Hiroyasu Ishikura (HI) is a member of the Scientific Standardization Committee for disseminated intravascular coagulation of the Japanese Society for Thrombosis and Hemostasis.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ishikura, H., Irie, Y., Nakashio, M. et al. Management of COVID-19 associated coagulopathy in critically ill patients and the risk of acquired von willebrand syndrome. Sci Rep 15, 19321 (2025). https://doi.org/10.1038/s41598-025-99786-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99786-z