Abstract
Effective malaria vector control is being undermined by the rapid spread of insecticide resistance. VECTRON T500, a new indoor residual spraying (IRS) product containing the active ingredient broflanilide as a 50% wettable powder (WP), was previously shown to be efficacious in experimental hut trials. A two-arm non-inferiority cluster randomized controlled community trial was conducted in Muheza District, Tanga Region, Tanzania. VECTRON T500 was compared to the IRS product Fludora Fusion (clothianidin 50% + deltamethrin 6.25% WP-SB). Sixteen village clusters were pair-matched on baseline vector densities and allocated to reference and intervention arms. Monthly CDC light trapping sampled mosquitoes to estimate vector density, indoor biting, sporozoite and entomological inoculation rate (EIR). The non-inferiority margin of mosquito density was defined as a density ratio of 1.5. Susceptibility to IRS active ingredients was assessed in one of the local vectors, Anopheles gambiae sensu lato (s.l.), using WHO/CDC bottle bioassays. The residual efficacy of both IRS products was monitored for 12 months using susceptible and pyrethroid resistant An. gambiae sensu stricto (s.s.) mosquitoes. This study is registered with ClinicalTrials.gov (NCT05150808). A total of 916 and 844 houses were sprayed with Fludora Fusion and VECTRON T500, respectively, with equitable spray coverage. An. gambiae s.l. was resistant to deltamethrin but susceptible to clothianidin and broflanilide. The density ratio adjusted for baseline Anopheline mosquito density was 0.77 (95% CI: 0.45–1.29). The baseline adjusted sporozoite rate and EIR differences between the two trial arms were 0.84% and 15.61%, respectively. The residual efficacy was > 80% mortality for VECTRON T500 and Fludora Fusion, on both mud and concrete walls, 12 months post spraying. VECTRON T500 was non-inferior to Fludora Fusion in terms of its ability to reduce vector density, sporozoite rate and EIR, providing an additional vector control tool with a new mode of action for malaria prevention and insecticide resistance management.
Similar content being viewed by others
Introduction
Insecticide-treated nets (ITNs) and indoor residual spraying (IRS) remain important components in all malaria vector control programmes1. Between 2000 and 2015, ITNs and IRS contributed to the decline in malaria cases by 68% and 10%, respectively2. In 2021, about 220 million ITNs were delivered to malaria endemic countries, 10 million fewer than in 20203. Whilst the delivery of ITNs by manufacturers to endemic countries in sub-Saharan Africa increased from 145 million in 2010 to about 176 million in 2021, there was a decline in the number of people at risk protected by IRS in sub-Saharan Africa from 153 million to 80 million during the same period3. Several factors, including widespread development of insecticide resistance, lack of alternative insecticides and limited resources, and disruptions caused by the COVID-19 pandemic, have been attributed to this decrease in IRS coverage4.
The Global Plan for Insecticide Resistance Management (GPIRM) proposed the development of new active ingredients (AIs) with different modes of action to support strategies for insecticide resistance management1. These resistance management strategies focus on IRS and propose using mixtures and rotations of insecticides from different chemical classes1. This has stimulated the development of novel IRS products containing new classes of insecticides by private sector manufacturers5,6,7,8.
VECTRON™ T500, a newly developed IRS product containing the active ingredient broflanilide (trade name TENEBENAL™) was recently listed by the World Health Organization Prequalification Vector Control Product Assessment Team (WHO PQT/VCP)9. Broflanilide is a meta-diamide insecticide which is classified by the Insecticide Resistance Action Committee (IRAC) in Group 30, γ-aminobutyric acid gated chloride channel allosteric modulators10,11. It has previously been shown to be efficacious against both insecticide susceptible and pyrethroid resistant Anophelesmosquitoes in small-scale experimental hut trials7,8,12. It has also demonstrated long residual activity and was efficacious against Anopheles (An.) coluzzii and An. gambiae s.s. populations in a community trial in Benin13.
Here, to inform the evaluation of VECTRON™ T500 by WHO PQT/VCP, this IRS product was evaluated for non-inferiority compared to the WHO PQT/VCP listed IRS product Fludora® Fusion (Envu™) in a community-level trial with entomological endpoints. Its residual activity and effect on the malaria entomological inoculation rate (EIR) and other transmission endpoints were also assessed.
Results
The number of houses per study cluster ranged between 100 and 200 houses. In most study clusters, houses with walls made of mud were predominant, i.e. 80.75% (95% CI: 75.0–86.5%) in the Fludora® Fusion and 77.6% (95% CI: 65.2–90.0%) VECTRON™ T500 arms. Coverage of ITNs in study clusters were 85.0% (95% CI: 73.2–96.9%) and 90.8% (95% CI: 81.1–100%) in the Fludora® Fusion and VECTRON™ T500 arms, respectively.
Between February-March 2021, a total of 844 houses were sprayed in the intervention arm and 916 houses in the reference arm. The community accepted the intervention and did not report adverse effects.
Residual bioefficacy
A total of 12 monthly rounds of in situ wall cone bioassays with pyrethroid-susceptible and pyrethroid-resistant strain mosquitoes were conducted. In most cone bioassays conducted, both VECTRON™ T500 and Fludora® Fusion induced over 90% mortality of susceptible An. gambiae s.s. Kisumu strain and resistant An. gambiae s.s. Muleba-Kis strain mosquitoes on both concrete and mud walls up to 12 months after spraying (Fig. 1). With both VECTRON™ T500 and Fludora® Fusion, relatively higher mortalities were recorded on concrete walls than on mud walls. The residual efficacy for both interventions 12 months post spraying remained above 80% for both concrete and mud walls (Fig. 1).
Vector bionomics and insecticide resistance monitoring
The insecticide susceptibility testing results indicated that An. gambiae s.l. from Muheza was resistant to deltamethrin (mortality rates < 90%) (Supplementary Fig. 1).
A total of 7,127 mosquitoes were collected from the 16 study clusters during the baseline period between January 2021 and the second week of February 2021. Of these 3,262 (46%) were morphologically identified as non-malaria vectors (Culex species) and 3865 were Anopheles mosquitoes. Of the Anopheles mosquitoes, 2357 (32%) and 1488 (21%) were morphologically identified as An. gambiae s.l. and An. funestus s.l., respectively. The other 41 specimens (1%) were identified as other Anophelines.
A sequence of 12 rounds of monthly CDC light trapping was carried out in 5 randomly selected houses per cluster. Proportionally, species of the Culex genus were most abundant, followed by An. gambiae s.l. and then An. funestus s.l. (Fig. 2). The density of An. gambiae s.l. was considerably higher than An. funestus s.l. As expected from its biology, An. funestus s.l. was a late season vector due to its proclivity for breeding in more permanent bodies of water compared to An. gambiae s.l. which, if predominantly An. gambiae s.s. would prefer temporary breeding sites. Both An. gambiae s.l and An. funestus recorded a declining trend in abundance post intervention (Fig. 3). All species declined significantly from the baseline survey to the first month post survey (P = 0.001). With all surveys, the number of Anopheline mosquitoes collected from the VECTRON™ T500 arm were not significantly different to those collected from the Fludora® Fusion arm (Table 1). The prolonged impact of both IRS products over one year is illustrated in Fig. 4.
Monthly violin plots showing An. gambiae s.l. and An. funestus mosquito counts per household across treatment arms. The violin plots illustrate the density distribution of mosquito counts per house, while the boxplots inside each violin show the interquartile range (IQR), with the horizontal line representing the median count per household. The y-axis represents the mean count of An. gambiae (top) and An. funestus (bottom), log-transformed (log(1 + x)) to account for highly skewed mosquito count distribution.
For the pre-intervention period, the results showed that the average number of indoor Anopheline mosquitoes collected per house per night in Fludora® Fusion clusters was 17.4, (95% CI: 0.9–33.9), while the average number of indoor Anopheline mosquitoes collected in VECTRON™ T500 clusters was 15.4 (95% CI: 0.5–30.3). The difference between the two arms pre-intervention was not significant (Table 1). Post-intervention, the results showed that the average number of indoor Anopheline mosquitoes collected per house per night decreased: in Fludora® Fusion clusters it was 1.6 (95% CI: 0.2–3.0), while the average number of indoor Anopheline mosquitoes collected post-intervention in VECTRON TM T500 clusters was 1.5 (95% CI: 0.03–2.9). The difference in vector density between VECTRON™ T500 arm and Fludora® Fusion arm post intervention was not significant (Table 1).
The mean geometric village level density of indoor Anopheline mosquitoes collected per house per night in the Fludora® Fusion treated clusters was 2.87 while the mean geometric village level density of indoor Anopheline mosquitoes collected in the VECTRON™ T500 treated clusters was 2.32. The total Anopheles density ratio was 0.77 (95% CI: 0.45–1.29), after adjustment for baseline density, month of catch, percentage of houses with ITNs and percentage of houses plastered with mud (Table 2). The mosquito density ratios according to taxon are shown in Table 2.
The species identification assays revealed that the majority of Anopheline samples were An. gambiae s.s., being between 60% and 67% in both trial arms before and after spraying (Table 3). The species composition of samples assayed before and after spraying are highlighted in Table 3.
The mean village level P. falciparum sporozoite rates in the VECTRON™ T500 and Fludora® Fusion trial arms were 0.39% and 1.04%, respectively (Table 4). The difference, adjusted for baseline measure, month of catch, percent of houses with ITNs and percent of houses with mud plastered walls, was 0.84% (95% CI: −1.24–2.93%). The mean village level EIR in the VECTRON™ T500 and Fludora® Fusion trial arms were 3.18 and 15.44, respectively (Table 4). The difference adjusted for baseline measure, month of catch, percent of houses with ITNs and percent of mud plastered houses was 15.61 (95% CI: −11.56–42.79).
A total of 1,341 An. gambiae s.s. and An. arabiensis were analysed for the kdr mutation L1014S. Of these, 812 and 529 were collected at baseline and after IRS, respectively. Molecular analysis for kdr in An. gambiae s.s. and An. arabiensis samples showed a significant difference in the frequency of homozygous resistant (RR), heterozygous resistant (RS), and homozygous susceptible (SS) after IRS compared to the baseline in the Fludora® Fusion arm (X2 = 27.77; P < 0.001; Table 5). Similarly, in the VECTRON™ T500 arm, there was a significant difference in the frequency of RR, RS and SS after IRS compared to baseline (X2 = 14.39; P = 0.001; Table 5). The frequency of RR, RS and SS pre- and post-intervention for each species are shown in Supplementary Tables 1 and 2.
Adverse effects and acceptability
Participants in all groups reported that the spray exercise was organized in such a way that it did not interfere with any of their routine household activities. It was reported that before the spray team arrived at a consented house, one person visited the targeted houses a few hours prior to the spray team to remind house owners about house preparations for spraying. According to participants, the reminder also gave them the opportunity to shift some of the in-house activities such as cooking to outside the house. This served to minimise the interference of the spraying on household activities.
“There is someone who passed earlier to check if the properties in the house have been arranged properly, then later on the sprayer came for the spraying, therefore, they found us we have already taken out all properties…….” (Male, p6, Muungano).
Another female participant added that;
“The activities indeed were neither stopped nor affected, for us who resides in the house since there were early pass of the information, hence making all issues such as preparing of children food was conducted earlier before the arrival of the team at the house…….” (Female, p4, Misozwe).
All group discussants in intervention and reference clusters expressed a preference for village residents to serve as spray operators. They noted that, having known the sprayers for years, it would not be likely for them to disclose household secrets, as the community shares living conditions. Consequently, revealing another household’s secrets would be regarded the same as revealing their own. In addition, discussants highlighted that the spraying activity provided income for their children and youths, which contributed to the village development. Finally, some participants mentioned that using sprayers from within the village enhanced security, as their behaviour was known and trusted, unlike that of strangers.
“I think the inside village sprayers should continue since they already knew our life in the house, it is easy for them to keep our secrets….”(Female, p5, Misozwe).
All group discussants reported initially having great interest and high expectations for the spray exercise, believing that the intervention could completely eliminate mosquitoes and malaria in the village. In all the intervention clusters, group discussants reported a tremendous decline in mosquito density, similar to group discussants from the Fludora® Fusion clusters, where discussants noted that mosquitoes disappeared in the first three weeks after spraying. They continued by adding that after the first three weeks of the spray, mosquitoes returned into the houses. Opinions varied on why mosquitoes returned; some discussants believed it was due to standing water and bushes which surround their houses. In one male group from the intervention cluster, it was suggested that the product which was sprayed in their village was fake, since they did not observe any changes in the mosquito abundance.
“Since we were told that there would be no mosquitoes we expected to see no mosquitoes indeed, and frankly speaking at first there were no mosquitoes at all, but in the coming days say after three weeks the mosquitoes returned back….” (Male, p6, Mtindiro).
All discussants showed interest in having their entire house sprayed. In some clusters, house owners decided which room should be sprayed or given priority, but in other clusters the sprayers were the one who made these decisions. For the clusters where homeowners chose, the whole house was sprayed. However, in other clusters in which the sprayers decided, some of the preferred rooms were not sprayed. A few discussants, especially in the female groups, commented that toilets/bathrooms and kitchen should not be left unsprayed to ensure comprehensive protection from mosquito bites, especially as the bathrooms were marked as areas with a lot of resting mosquitoes.
“I also prefer the spray to be sprayed even in the toilets and bathrooms as well as other surrounding places, because if you go in the toilets mosquitoes can bite you there, because there are so many mosquitoes there, they can miss you in the seating room but they will get you in the toilets….”(Female, p8, Misozwe).
Most did not experience or hear of any adverse events after the spray campaign. A few women in the intervention arm reported having experienced an increased heartbeat, unpleasant smells and skin itching. Minor reactions were noted by others which included physical fatigue, burning face sensation, dry throat and dizziness. Some discussants admitted entering their houses before the recommended waiting period after spraying, accidentally coming into contact with the chemicals, or sleeping on wet sprayed beds. These mild reactions typically lasted only a few hours and claimed were alleviated by washing with water and drinking milk. No medical attention was required for these adverse events.
“I passed near the window during the spraying and I was accidentally sprayed on the face, at that time I felt normal but later on I started feeling face itching, hence I decided to wash by water but after few minutes of washing the face my throat became dried and started feeling some fire burning in the stomach….” (Female, p6, Mtindiro).
“…….there after we were told to wait outside the house for two hours, but after they left I decided to go inside and take some of my two oranges, I picked them washed and peel, after peeling I ate them, after eating them I started feeling dizzy and loss of bodily strength….” (Female, p9, Misozwe).
Discussion
For the first time since the Millenium there has been an upsurge of malaria in parts of sub-Saharan Africa. Over the last 20 years there has been a steady increase in ITN coverage of various classes, but the last decade has also seen a gradual decrease in IRS coverage13. If Africa is to eliminate malaria, the international community should revise its malaria vector control policy and consider combining dual interventions of different classes of ITNs and IRS in areas where malaria transmission remains persistently high and resources are sufficient. There has been only one formal community randomised trial of Dual-AI ITNs and long-lasting IRS – that of a PBO ITNs combined with pirimiphos-methyl IRS (Actellic®300 CS) in a malaria hot spot in North West Tanzania14. These were the only long-lasting IRS and ITN products approved by WHO and available at the time. Regrettably, there was a negative interaction between the PBO on the net and the mosquito cytochrome P450 enzymes responsible for the activation of the pro-insecticide pirimiphos-methyl to the active metabolite in the pyrethroid resistant vector population14. Hence the mixture of PBO net and pirimiphos-methyl IRS failed to be additive in terms of reducing vector density and this has set back any further evaluations of the possible benefit of dual ITN plus IRS interventions.
VECTRON™ T500 effectively controls vectors which have target site resistance mechanisms such as kdr found in An. gambiae or metabolic upregulated P450-based resistance mechanisms found in An. funestus, and therefore would be appropriate for combining with new classes of ITN. According to WHO for a new candidate IRS product to be listed in the IRS intervention class, it must demonstrate non-inferiority to an IRS product which has already demonstrated public health value (Fludora® Fusion) in a community (Phase III) cluster randomized indoor residual spraying trial15,16.
In this trial, VECTRON™ T500 was non-inferior to Fludora® Fusion in terms of its ability to reduce the vector density, sporozoite rate, entomological inoculation rate, and malaria transmission for one year.
In terms of residual efficacy, at twelve months post spraying, both VECTRON™ T500 and Fludora®Fusion remained effective based on cone bioassay mortality criteria which remained above the 80% threshold against insecticide susceptible and pyrethroid resistant mosquitoes. Although efficacy was relatively higher on concrete walls compared to mud walls, the differences were not significant. Implementing IRS with long-lasting insecticide formulations that have residual activity and can reduce vector density throughout the year is essential in reducing the overall cost of IRS, which is a key challenge to universal coverage of any IRS intervention. The long-lasting residual efficacy of at least 12 months of the candidate VECTRON™ T500 and reference Fludora Fusion present an important development to the IRS intervention class. In Benin the residual efficacy of VECTRON™ T500 as shown in an experimental hut trial and in a two‑arm non‑inferiority community randomised evaluation of VECTRON™ T500, the residual efficacy was up to 18 months and up to 24 months, respectively12,13. However, it should be noted that in Benin, the local practice when using mud to plaster houses is to mix the mud with cement, which is different to the practice in Tanzania where only mud plaster is used.
Molecular assays for Anopheline species identification revealed that the majority of vectors were from the An. gambiae complex (An. gambiae s.s. and An. arabiensis) with the minority from the An. funestus complex (An. funestus s.s., An. parensis, An. leesoni and An. rivulorum). Where An. gambiae s.s. and An. arabiensis are sympatric in Tanzania, such as around Lake Victoria, the sporozoite rate of An. arabiensis is lower than that of An. gambiaes.s. and its zoophilic/exophilic tendencies are greater17,18,19. Less is known about the vector status of sibling species of the An. funestus complex; An funestus s.l.has increased in frequency in North Tanzania20,21. Considering that all CDC light traps were conducted inside houses, this could be an indication of the importance of some of these sibling species as malaria vectors in the study area.
The increased frequency of kdr-VGSC RR and RS genotype in the Fludora® Fusion arm could be an indication of further pyrethroid selection in this arm. Conversely, the small increase kdr-VGSC SS genotype frequency in VECTRON™ T500 arm may indicate a restoration of pyrethroid susceptibility in the An. gambiae s.l. populations. In a community trial conducted in Benin, there was no significant change in kdr frequency in either VECTRON™ T500 or Fludora®Fusion arms13.
In conclusion, the impact of the VECTRON™ T500 IRS intervention on vector density and EIR was non-inferior to that recorded for the WHO reference product, Fludora®Fusion, thereby fulfilling the WHO criterion for an IRS product. Secondly, its long residual efficacy of up to 12 months post spraying makes it more cost effective when compared to many other WHO approved IRS products. Study findings provide important operational information for National Malaria Control and regional malaria elimination programmes to utilise when designing prospective, pragmatic insecticide resistance management schemes and regional elimination programmes. This supports the strategy of rotation IRS products containing insecticides with different modes of action for insecticide resistance management in combination with standard ITN or dual-active ingredient ITNs17. To further enhance malaria control gains, there is a need for new IRS products to be safe to use, and additive in effect to combine with PBO ITNs. VECTRON™ T500 and Fludora® Fusion with their novel modes of action and, unlike pirimiphos-methyl IRS, not requiring metabolic activation, could be used as IRS partners in a rotation strategy to regain control of malaria in Africa.
Methodology
Study area
This study was carried out in Muheza District, Tanga Region, Northeast Tanzania22. The population of Muheza District consists primarily of subsistence farmers23. The region has a tropical climate with a long rainy season from March to June and a short rainy season from October to December. Most houses within Muheza District are constructed from mud or concrete with a thatched or iron roof. In 2014, surveys conducted in Muheza District found that 83% of households owned at least one ITN, and there have been two universal ITN distribution campaigns since this date24. Muheza District had received nets from the targeted mass replacement campaign, which took place about a month before spraying for this study was carried out. Therefore, the majority (80%) of the ITNs were Olyset Plus and the average household coverage was 87% (Supplementary Table 4). Malaria transmission occurs all year round with two peaks in transmission following the two rainy seasons. The main malaria vectors in Muheza District are An. gambiae s.s., An. arabiensis and An. funestuss.s24. Wild An. gambiaes.l. in Muheza District are resistant to pyrethroids25.
Study design
This study was a mixed method design that employed both quantitative and qualitative methods. The study protocol was published earlier22. The quantitative component utilized a matched cluster randomized controlled non-inferiority study design, appropriate for evaluating IRS as a community-level intervention. The study monitoring period was for 12 months post-intervention, between April 2021 and March 2022. Baseline data collection on vector densities was conducted in January and February 2021. At the onset of the long rainy season in March 2021, one round of IRS was completed, which was followed by monthly vector density monitoring and in situ wall cone bioassays. The primary trial outcome was Anopheline indoor vector density post-intervention, adjusted for baseline density, month of collection, proportion of houses with ITNs and proportion of mud houses. The secondary outcomes were: (1) EIR, an indicator of malaria transmission, defined as the average number of sporozoite-positive mosquitoes per night per household, and weighted to account for the proportion of collected Anopheles mosquitoes processed for analysis; (2) sporozoite rate, defined as the proportion of Anopheles mosquitoes found to be infected with Plasmodium falciparum; and (3) intervention residual bio-efficacy. The qualitative component of the trial included a social-cultural and acceptability study. Baseline household enumeration, along with social-cultural and acceptability assessments, were conducted prior to the IRS intervention. After the IRS campaign, these factors were monitored and compared to baseline data22.
Power calculation, estimation of effect size and cluster selection
The sample size calculation was based on the study primary endpoint i.e., indoor density of Anopheles mosquitoes. An estimated mean of 3.0 female Anophelesmosquitoes per light trap per night was used, referencing data from recent studies14. A paired design was used in which clusters were matched on the baseline indoor density of Anophelesmosquitoes. Previous data suggested a within-pair coefficient of variation of 0.2326, but a more conservative value of 0.3 was used in power calculations for this study.
The trial was designed to demonstrate that spraying the intervention product, VECTRON™ T500, would not result in higher vector densities, by a prespecified non-inferiority margin of 50%, than those per trap per night in the reference arm sprayed with Fludora®Fusion22. Using the methods described in Hayes and Moulton26 and the assumptions listed above, 8 clusters per treatment arm (16 in total) were needed for the 50% margin to have 80% power to demonstrate non-inferiority. The calculations were performed based on 49 trap nights per cluster. The number of clusters considered the loss of degrees of freedom caused by matching.
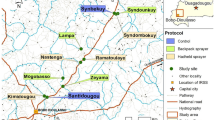
Sixteen clusters, containing 75–200 households per cluster, were selected for spraying. Core areas of clusters were at least 1 km apart to prevent infiltration of mosquitoes from other villages22. During baseline vector density monitoring, 20 clusters were included to enable adequate pair-matching of the 16 clusters needed to power the study (Fig. 5). Four clusters were dropped or ineligible based on different reasons including organic farming that was been practised in or near the two clusters, extremely low vector density that was observed in one cluster and reluctancy to participate in the study in one cluster. The 16 selected clusters were pair-matched based on vector densities and randomly allocated to the two trial arms: one arm (8 clusters) was sprayed with the novel intervention VECTRON™ T500 and the other arm (8 clusters) with the reference product Fludora® Fusion. All possible randomisations were stratified on ITN coverage and wall surface type to ensure a minimum difference of 15% or less in mean ITN coverage and mean ratio of concrete houses to mud houses per arm.
Procedures
Fifty-six 11 L Hudson®X-pert compression sprayers (H.D Hudson Manufacturing Company, Foreman St. Lowel, U.S.), each fitted with a 1.5 bar control flow valve and an 8002E nozzle were used. Calibration tests were done three times by filling each sprayer with 7.5 L of water and pressurising it to 40 psi. The target spray discharge was 550 mL per minute according to the WHO recommendations27.
Consenting households in the intervention arm were sprayed with VECTRON™ T500 (100 mg/m2 broflanilide) and consenting households in the reference arm received IRS with Fludora® Fusion (200 mg/m2 clothianidin and 25 mg/m2deltamethrin). A total of 100 spray operators were recruited from the clusters and trained according to the WHO IRS operational manual by a company from South Africa which had many years of experience with IRS in the national MCP27. Spray operators were provided with personal protective equipment and clothing, and health checks were performed on them before and after spraying. All sleeping and living rooms in houses were sprayed, whilst kitchens and storerooms were excluded.
Whatman® No. 1 filter papers were attached to the walls of households before spraying and then removed post-spraying for chemical analysis at the Liverpool School of Tropical Medicine, UK. The results of the chemical analysis will be reported elsewhere.
WHO cone bioassays on IRS treated walls of houses were used to assess residual activity in both trial arms at monthly intervals for 12 months post spraying22,28. Four households were randomly selected from each cluster for monthly monitoring of residual efficacy using the wall-cone bioassay. Following exposure, mosquitoes were placed in paper cups and monitored for up to 72 h to assess mortality at 24, 48 and 72 h. WHO cones were affixed to four walls per house in a diagonal arrangement across the walls, and one additional cone was affixed to an outside untreated wall to act as a control. Of the four selected households per cluster, the walls of two were constructed out of concrete and the other two out of mud, to compare residual activity on these substrate materials. To determine IRS product bioefficacy, both insecticide susceptible (An. gambiae s.s. Kisumu) and pyrethroid resistant (An. gambiaes.s. Muleba-kis) strains were used7,8. The Muleba-Kis strain was resistant to pyrethroids based on the kdr (L1014S) target site mutation and a metabolic mechanism associated with overexpression of the cytochrome P450-dependent monooxygenase CYP6P329. The strain was susceptible to carbamates and organophosphates and did not express the Ace-1mutation7,29.
In each cone bioassay test, 10 An. gambiaes.s. adult, unfed, 2–5-day old female mosquitoes28 were aspirated into each cone and exposed for 30 min. After exposure, mosquitoes were held in paper cups with access to cotton wool soaked in 10% glucose solution. Immediate knockdown and delayed mortality at 24, 48 and 72-hours post-exposure was recorded.
The WHO/CDC bottle bioassays were used to determine any phenotypic resistance of wild Anopheles mosquitoes to broflanilide (6 µg/bottle), deltamethrin (12.5 µg/bottle) and clothianidin (90 µg/bottle without Mero®), according to WHO/CDC methods. The above concentrations of these insecticides have been shown to kill 100% of insecticide susceptible mosquitoes13,30,31. The broflanilide bottle bioassay dosage was determined by conducting dose response bioassays with the insecticide susceptible An. gambiae s.s. Kisumu strain. Discriminating concentration determination was undertaken using BioRssay in RStudio v4.0.2. The preliminary discriminating concentration for use in the broflanilide susceptibility monitoring associated with this community trial was defined as twice the LC99at 72 h post exposure as stipulated by WHO Control of Neglected Tropical Diseases (NTD) and WHO Global Malaria Programme (GMP)32.
Broflanilide bottles were coated mixed with the adjuvant Mero®(Bayer Crop Science) at 800 ppm. Insecticide solutions were prepared from technical grade material dissolved in acetone33.
An. gambiae s.l. were collected as larvae from breeding sites within the study area and reared in the insectary. Progeny of these wild An. gambiaes.l. were then exposed to insecticide according to standard operating procedures (using insecticide-coated Wheaton bottles)31. Two-to-five-day old, unfed female mosquitoes were exposed in bottles for 1 h. Knockdown was recorded for all insecticides after 1 h. Mortality was recorded after 24 h for deltamethrin, and at 48 and 72 h for broflanilide and clothianidin. Assays were conducted with wild An. gambiae s.l. at baseline and 3 months after IRS. CDC/WHO bottle bioassays were also performed with An. gambiaes.s. Kisumu (insecticide susceptible strain) from lab colonies for comparison and quality control16. An. gambiae Kisumu is a standard susceptible laboratory strain originally colonised in 1953 from Kenya that is widely used in Africa. Muleba-kis is a cross between Kisumu and a wild pyrethroid resistant strain colonised in Muleba, Kagera, and pressurised using permethrin every generation to maintain pyrethroid resistance. The strain is widely used in the PAMVERC laboratories in Tanzania.
CDC light traps, hung inside houses next to occupied ITNs, were used to collect mosquitoes for species identification18 and P. falciparumcircumsporozoite protein enzyme linked immunosorbent assay (CSP-ELISA)19,34. Four operatives were allocated with 5 light traps each and given responsibility for trapping in 4 of the 16 clusters. In each cluster each month, there were 3 rounds of CDC light trap collections spaced over 3 weeks in 15 randomly selected households. Therefore, in each treatment arm of 8 clusters there were 15 light trap nights in each month. All households in each cluster were re-randomised at each monthly surveillance point. Therefore, in each cluster there were 180 light-trap nights spread across the 12-month post intervention period, from which non-inferiority was determined. All mosquitoes collected were identified morphologically to genus and species complex level (An. gambiae s.l., An. funestus s.l. and Culexspp.). Sub-samples taken from each cluster each month were molecular identified to species level using Taqman real-time PCR assays18; numbers and proportions identified depended on the total numbers caught; P. falciparumCSP-ELISA was performed on all molecular identified Anopheline species22. A subsample of molecularly identified An. gambiae s.s. and An. arabiensis were subjected to Taqman real-time PCR assays to detect the kdrtarget site mutation (L1014S)35.
Adverse effects and acceptability assessment
The social-cultural and acceptability study was conducted in four of the sixteen sprayed clusters, selected at random, that is, two clusters each in the intervention and reference study arms. The selected clusters in the VECTRON™ T500 intervention arm were the villages Muungano and Pangamlima whilst in the Fludora® Fusion arm Misozwe and Mtindiro were selected. Eight community focus group discussions (FGDs), two in each cluster were conducted, with 63 study participants.
Statistical analysis
Data analysis was carried out using cluster level summaries22, using Stata (Version 16) since random effects models perform poorly in a matched design with fewer than 20 clusters per arm26. For the primary endpoint of mosquito density, the ratio of densities in each matched pair was calculated and inference was based on a paired t-test. Adjustments were made for time post-spraying, ITN use and wall type using the two-stage method described by Hayes and Moulton26. The secondary outcomes of P. falciparum sporozoite rate and estimated number of infective bites per person per year (EIR) were analysed in the same way.
Data availability
All data associated with this study are present in the paper or the appendix. All other relevant data are available from the corresponding author upon reasonable request.
References
World Health Organization. Global Plan for Insecticide Resistance Management in Malaria Vectors. (2012). https://apps.who.int/iris/handle/10665/44846
Bhatt, S. et al. The effect of malaria control on plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015).
WHO. World Malaria Report 2022 (WHO, 2022).
Oxborough, R. M. Trends in US president’s malaria Initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008–2015): urgent need for affordable, long-lasting insecticides. Malar. J. 15, 1–9 (2016).
Kleinschmidt, I. & Rowland, M. Insecticides and malaria. In Innovative Strategies for Vector Control- Ecology and Control of Vector-borne Diseases Vol. 6 (eds Koenraadt, C. J. M. et al.) 17–32 (Wageningen Academic, 2021).
Ngufor, C. et al. Efficacy of broflanilide (VECTRON T500), a new meta-diamide insecticide, for indoor residual spraying against pyrethroid-resistant malaria vectors. Sci. Rep. 11, 7976 (2021).
Snetselaar, J. et al. Efficacy of indoor residual spraying with broflanilide (TENEBENAL), a novel meta- diamide insecticide, against pyrethroid- resistant anopheline vectors in Northern Tanzania : an experimental hut trial. PLoS One. 16, e0248026 (2021).
Mbewe, N. J. et al. A non-inferiority and GLP-compliant study of broflanilide IRS (VECTRON™ T500), a novel meta-diamide insecticide against Anopheles arabiensis. Front. Trop. Dis. 4 (2023).
WHO. Prequalified Vector Control Products. Preprint at (2023). https://extranet.who.int/pqweb/vector-control-products/prequalified-product-list?field_product_type_tid=83&field_pqt_vc_ref_number_value=&title=&field_applicant_tid=&field_active_ingredient_synergis_tid=&page=1
Nakao, T. & Banba, S. Broflanilide: A meta-diamide insecticide with a novel mode of action. Bioorg. Med. Chem. 24, 372–377 (2016).
IRAC. IRAC Mode of Action Classification Scheme (IRAC, 2021).
Govoetchan, R. et al. VECTRON™ T500, a new broflanilide insecticide for indoor residual spraying, provides prolonged control of pyrethroid-resistant malaria vectors. Malar. J. 21, 324 (2022).
Ngufor, C. et al. Community evaluation of VECTRON™ T500, a broflanilide insecticide, for indoor residual spraying for malaria vector control in central Benin; a two arm non-inferiority cluster randomised trial. Sci. Rep. 13, 17852 (2023).
Protopopoff, N. et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two fact. Lancet 391, 1577–1588 (2018).
WHO. Determining Non-Inferiority of Insecticide-Treated Nets and Indoor Residual Spray Products within an Established Product Class (2018).
Portwood, N. M. et al. Multi-centre discriminating concentration determination of broflanilide and potential for cross-resistance to other public health insecticides in Anopheles vector populations. Sci. Rep. 12, 22359 (2022).
Oxborough, R. M., Chilito, K. C. F., Tokponnon, F. & Messenger, L. A. Malaria vector control in sub-Saharan Africa: complex trade-offs to combat the growing threat of insecticide resistance. Lancet Planet. Health. 8, e804–e812 (2024).
Bass, C., Williamson, M. S., Wilding, C. S., Donnelly, M. J. & Field, L. M. Identification of the main malaria vectors in the Anopheles gambiae species complex using a TaqMan real-time PCR assay. Malar. J. 6, 155 (2007).
Wirtz, R., Avery, M., Benedict, M. & Sutcliffe, A. Plasmodium Sporozoite ELISA. in Field Techniques (ed. Dotson, E. M.) (2015).
Matowo, N. S. et al. An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the lake zone, Tanzania. Sci. Rep. 11, 13457 (2021).
Matowo, N. S. et al. Differential impact of dual-active ingredient long-lasting insecticidal Nets on primary malaria vectors: a secondary analysis of a 3-year, single-blind, cluster-randomised controlled trial in rural Tanzania. Lancet Planet. Health. 7, e370–e380 (2023).
Tungu, P. K. et al. Large-scale (Phase III) evaluation of broflanilide 50WP (VECTRON™ T500) for indoor residual spraying for malaria vector control in Northeast Tanzania: study protocol for a two-arm, non-inferiority, cluster-randomised community trial. BMC Infect. Dis. 22, 171 (2022).
Statistics, N. B. O. 2012 Population and Housing Census. (2012).
Mtove, G. et al. The effectiveness of non-pyrethroid insecticide-treated durable wall lining to control malaria in rural Tanzania: study protocol for a two-armed cluster randomized trial. BMC Public. Health. 16, 1–15 (2016).
Kabula, B. et al. Distribution and spread of pyrethroid and DDT resistance among the Anopheles gambiae complex in Tanzania. Med. Vet. Entomol. 28, 244–252 (2014).
Hayes, R. J. & Moulton, L. H. Cluster Randomised Trials. Cluster Randomised Trials, Second Edition (Chapman and Hall/CRC, 2017). https://doi.org/10.4324/9781315370286
WHO. Indoor Residual Spraying, an Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination (WHO, 2015).
WHO. Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets (WHO, 2006).
Azizi, S. et al. Colonization and authentication of the Pyrethroid-Resistant Anopheles gambiae S.s. Muleba-Kis strain; an important test system for laboratory screening of new insecticides. Insects 12, 710 (2021).
Govoetchan, R. et al. Investigating discriminating concentrations for monitoring susceptibility to broflanilide and cross resistance to other insecticide classes in Anopheles gambiae sensu Lato, using the new WHO bottle bioassay method. PLoS One 18, (2023).
CDC. Guideline for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassay (CDC, 2010).
WHO. Determining Discriminating Concentrations of Insecticides for Monitoring Resistance in Mosquitoes: Report of a Multi-Centre Laboratory Study and WHO Expert Consultation. (2022).
WHO. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes (WHO, 2016).
Durnez, L. et al. False positive circumsporozoite protein ELISA: a challenge for the Estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar. J. 10, 195 (2011).
Bass, C. et al. Detection of knockdown resistance (kdr) mutations in Anopheles Gambiae: a comparison of two new high-throughput assays with existing methods. Malar. J. 6, 111 (2007).
Acknowledgements
This study was funded by IVCC through support from the Bill & Melinda Gates Foundation (grant: INV-007509), the Swiss Agency for Development and Cooperation (SDC) (grant: 81067480) and UK Aid (grant: 30041-105). The findings and conclusions contained within are those of the authors and do not necessarily reflect the positions or policies of the Bill & Melinda Gates Foundation, SDC, UK Aid or IVCC.
Funding
This study was funded by the Bill and Melinda Gates Foundation (grant no. INV-007509) through the Innovative Vector Control Consortium (IVCC). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
N.J.M., P.K.T., L.A.M., M.W.R. designed the study, and led the quantitative analysis and data collection. John Bradley led the statistical analysis. N.J.M., M.W.R. led the trial. P.E.M. and M.S. conducted the qualitative analysis and social science. N.J.M., P.K.T., L.A.M., M.W.R., were responsible for management and delivery of the trial. M.J.K., J.M., G.S., J.S., F.W.M., P.M., F.M., W.S., W.K. provided infrastructure, resources and ensured GLP systems at the two PAMVERC project sites. N.M.P., M.F.S., G.M., S.A., F.M., P.M., W.S., maintained mosquito colonies and participated in the data collection. B.S., O.M., and N.M.P. performed the molecular analysis, under the supervision of N.J.M. and L.A.M. N.J.M., P.K.T., L.A.M. and M.W.R. wrote the manuscript which was reviewed by co-authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the ethical review committee of the Ministry of Health in Tanzania (NIMR/HQ/R.8a/VOL.IX/3520), and the institutional review board of LSHTM (N° 22,459). The study was registered on clinicaltrials.gov (NCT05150808, registered on 26 November 2021). The study was conducted according to the Declaration of Helsinki and the International Guidelines for Ethical Review of Epidemiological Studies. The protocol of the study was reviewed by the independent Expert Scientific Advisory Committee of the IVCC and by the WHO Pre-qualification Vector Control Team (WHO PQT/VCT). Community consent was obtained from village leaders. Individual verbal and written informed consent were obtained from family heads, household members and qualitative study participants. All households within each community were offered IRS.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mbewe, N.J., Tungu, P.K., Messenger, L.A. et al. A noninferiority cluster randomised evaluation of a broflanilide indoor residual spraying insecticide, VECTRON T500, for malaria vector control in Tanzania. Sci Rep 15, 15013 (2025). https://doi.org/10.1038/s41598-025-99809-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99809-9