Abstract
Hand dominance has long been associated with differences in neural control and motor performance, with the dominant hand typically exhibiting better coordination in reaching tasks. However, the extent to which this dominance influences performance in finger force control remains unclear. This study aimed to examine the behavioural and neural features of the dominant and non-dominant hands during grasping and lifting tasks in healthy young adults, focusing on the synergy index, EEG band power, and EEG–EMG coherence as key measures. Twenty right-handed adults participated in this study. Participants engaged in an experimental task where they grasped a handle for the initial 5 s, followed by lifting and holding it for an additional 5 s. There were two task conditions: fixed (thumb platform secured) and free (thumb platform movable). It was hypothesized that the dominant hand would exhibit greater finger force coordination and enhanced neural features, including higher EEG band power and increased EEG–EMG coherence, compared to the non-dominant hand. Contrary to the hypothesis, we found statistical equivalence in the synergy index, EEG band power, and EEG–EMG coherence between the dominant and non-dominant hands across both fixed and free task conditions. These findings suggest that both hands can achieve similar levels of performance in tasks emphasizing steady-state force maintenance, despite the typical advantages of the dominant hand in other motor tasks. However, a significant difference was observed between task conditions, with the fixed condition showing higher values than the free condition in both behavioural (synergy index—η2 = 0.81, p < 0.0001,) and neural (EEG band power η2 = 0.37, p < 0.05 and EEG–EMG coherence—η2 = 0.49, p < 0.0001) features. These differences were likely due to changes in friction, yet the adjustments remained consistent between the dominant and non-dominant hands.
Similar content being viewed by others
Introduction
Human hands exhibit remarkable dexterity, allowing individuals to manipulate objects using both the dominant and non-dominant hands. Hand dominance plays a crucial role in motor tasks, influencing precision, coordination, and stability. The dynamic dominance hypothesis posits that the dominant (DOM) hand, primarily controlled by the left hemisphere in right-handed individuals, excels in precision and trajectory control, while the non-dominant (NDOM) hand, governed by the right hemisphere, functions as a stabilizer, ensuring object security and controlled movements1,2,3,4. These functional differences emphasize the distinct contributions of each hemisphere in motor control, highlighting the importance of cortical asymmetry in coordinated hand movements5,6,7.
Research indicates that the contralateral hemisphere of the cortex predominantly governs hand movements, reinforcing the role of the brain in motor coordination. These differences reflect the broader neural mechanisms underlying hand function, illustrating how each hand is uniquely adapted for specific motor demands. Cortical asymmetry is a critical factor in motor control. The dominant hemisphere’s primary motor cortex (M1) possesses a larger hand representation area, facilitating enhanced motor learning and dexterity8,9. This connectivity advantage contributes to superior motor practice in the dominant hand. Studies measuring corticomotor excitability during pegboard tasks have shown increased excitability in gripping muscles for both hands, though only the dominant hemisphere exhibited reduced inhibition, linking it to motor learning and refined control10.
The precise regulation of force during object grasping depends on the coordination between the cortex and hand musculature. Corticomuscular coherence measures the degree of statistical dependency between electroencephalography (EEG) and electromyography (EMG) signals, which may suggest a level of coordination between brain and muscle activity, though it does not imply a direct interaction. This can be quantified by calculating coherence between EEG and EMG signals, identical to extending the Pearson correlation coefficient into the frequency domain11,12. Several studies have demonstrated corticomuscular coherence, indicating the relationship between EEG and EMG signals in the 15–30 Hz at the beta frequency range of EEG signal during motor tasks13,14. Research suggests that CMC magnitude increases with stronger EMG signals during maximum voluntary contractions (MVC)15. Additionally, beta-range CMC exhibits significant differences between the left and right hands during gripping tasks, demonstrating laterality effects in neuromuscular control16. However, studies on grip force and isometric force production have reported mixed findings, with some indicating significant inter-hand differences while others do not16,17,18.
Finger force synergies play a crucial role in object manipulation. In this context, we define synergy as a coordinated adjustment of finger forces and moments produced by each finger during multi-finger prehension tasks19. Synergy index is used to quantify the extent of co-variation among the forces and moment of forces produced by individual fingers, called elemental variables. It measures the degree to which the modulation of individual finger forces contributes to the reduction of variability in vital performance variables, such as total grip/normal force, total moment of force, and total tangential/load force20,21. Long-term exposure to object manipulation with the dominant hand leads to distinct force coordination patterns compared to the non-dominant hand22. Zhang et al. demonstrated that the dominant hand is more effective in controlling rapid force changes, indicating its specialization in dynamic tasks, while no strong evidence supports the non-dominant hand’s specialization in stabilizing steady-state force23. Similarly, Rearick and Santello found comparable force control strategies in grasping and lifting tasks across both hands, suggesting that the central nervous system employs similar coordination patterns regardless of hand dominance24. Gorniak et al. further reported no significant differences between hands in kinetic and kinematic adjustments during the vertical transport of fragile objects25.
Laterality studies in proprioception have shown variations in proprioceptive acuity between the dominant and non-dominant limbs. Research indicates that in right-handed individuals, the non-dominant (left) side often exhibits superior proprioceptive performance. Goble et al. found better accuracy in position matching tasks using the non-dominant (left) arm26. Another study assessing multiple joints in the upper and lower limbs confirmed a generalized left-side advantage in proprioceptive utilization27. These findings suggest that proprioception plays a role in influencing hand performance during motor tasks.
Several studies have examined the effects of hand dominance on motor performance, hand selection, and laterality. Bryden et al. explored how task complexity, object location, and object type influence reaching behaviours, finding that right-handed individuals rely more on their non-preferred hand in contralateral space28. Annett’s research highlighted that the non-preferred hand exhibits greater movement variability due to slower corrective movements, rather than differences in movement duration or feedback processing29. Additionally, Gabbard et al. found that right-handers exhibit stronger lateralization, whereas left-handers demonstrate less consistency in hand selection for reaching tasks.
Previous research on finger movements, pegboard tasks, and reaching tasks suggests that the dominant hand excels in precision and dynamic control, whereas the non-dominant hand exhibits greater movement variability. Real-life tasks often require multi-digit prehension, necessitating the coordination of finger forces. This study aimed to compare the behavioural and neural features of lifting and grasping tasks between the dominant and non-dominant hands in healthy young adults under two task conditions: fixed and free. The free condition was more challenging, requiring increased sensorimotor integration and force modulation compared to the fixed condition.
These task conditions were based on prior research investigating the mechanical advantage hypothesis, which examines the influence of the thumb in grasping and object manipulation30,31,32. Prior studies have demonstrated task-dependent modulations, particularly in the dominant hand, with evidence of such effects in both neural and behavioural features33. Under demanding conditions, both hands often perform similarly, suggesting that task demands can reduce hand dominance effects34,35. However, previous studies have primarily focused on reaction time and reaching tasks, with limited research on static force maintenance. Based on these findings, it was hypothesized that the dominant hand would exhibit greater finger force coordination (synergy index) and enhanced neural features (EEG band power and EEG–EMG coherence) compared to the non-dominant hand.
Methods
Participants
Twenty young right-handed healthy adults with no history of neuromuscular disorders aged between 20 and 30 years participated in this study. An equal number of male and female participants (10 males, 10 females, mean age: 26.95 ± 2.68 years) participated in this study. This balanced design allows us to examine potential gender-related differences in lifting performance. The experimental procedure was approved by the Institute’s ethics committee at the Indian Institute of Technology, Madras (Approval number: IEC/2021-01/SKM/02/05). Written informed consent was collected from the participants in accordance with the ethical approval. The experiments were conducted in accordance with the relevant guidelines and regulations approved by the Institutional Ethics Committee.
Experimental apparatus and protocol
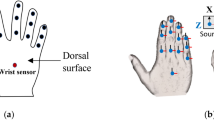
A handle was specifically designed for the experiment to measure the finger force applied by each finger. The handle was equipped with five 6 component force/torque sensors (Nano 17, Force resolution: 0.0125 N, ATI Industrial Automation, NC, USA) as shown in Fig. 1A. Four sensors were fixed on one side of the handle to measure the fingertip forces of index, middle, ring and little fingers. The sensor to measure the thumb digit force was placed on the other side of the handle on a slider platform which can move on a rail. The total weight of the handle including the external weight of 0.25 kg was 0.75 kg. A displacement sensor was used to measure the position of the thumb (OADM 12U6460 sensor with resolution of 5 μm, Baumer, India). An inertial measurement unit (IMU—Model: BNO055 with a resolution of 16 bits and 2000°/s range, BOSCH, Germany), was placed on an acrylic block on top of the handle to measure the orientation of the handle. Additionally, a spirit level was incorporated on the handle, allowing the participants to visually monitor and ensure the orientation of the handle during the experiment. The experimental handle used in this study was previously employed by our research group in other experimental studies30,31,33,36.
(A) Handle. The experimental handle, made up of aluminum frame measuring 21 × 1 × 3 cm was equipped with the five force/torque sensors. An IMU sensor was mounted on an acrylic block on one side of the handle, while a spirit level was positioned on the opposite side. The positions for the five fingers were labelled as I (index), M (middle), R (ring), L (little) and T (thumb). The total weight of the handle is 0.75 kg including the external weight of 0.25 kg. (B) Experimental setup. The designed handle was placed inside an acrylic case. Participants were instructed to grasp and hold the handle for initial 5 s gently. After 5 s they were asked to lift and hold the handle in the air for an additional 5 s.
A bio signal system comprising of two-channels for EMG and twenty-two channels for EEG system was developed using INTAN RHD 2216 bio amplifiers (Intan Technology, USA). The RHD 2216 amplifiers are arrays of 16 channel bipolar bio amplifiers equipped with a serial peripheral interface (SPI) on-chip and a 16-bit analog to digital converter (ADC). A sixty-four channel EEG cap (Waveguard original cap, 64-channel ANT neuro waveguard, The Netherlands; 10/10 electrode montage) was connected to the RHD amplifiers through a custom designed interface board. The EEG signals were recorded from the following 22 channels: Fp1, Fp2, AFz, Fz, F3, F4, F7, F8, Fc1, Fc2, Fc5, Fc6, Cz, C3, C4, Cp5, Cp1, Cp2, Cp6, Pz, P3, and P4. The GND electrode of the EEG cap is connected to the ground of the amplifier. The participants were instructed to clean and dry their hair before the experiment to reduce the effect of impedance. Two surface EMG signals were collected from the flexor digitorum superficialis muscle (FDS) and flexor carpi ulnaris (FCU). We have selected FDS, responsible for finger flexion and FCU, involved in wrist flexion abduction. These muscles play crucial roles in gripping and manipulating objects with multiple digits37. Surface electrodes in bipolar configuration were placed over the belly of the muscle with an inter electrode placement of approximately 1.5 cm38. A ground electrode was placed 4 to 5 cm away from the bi-polar surface electrodes38. The placement of electrodes was determined initially through anatomical descriptions, palpations and assessment of functional contractions39.
Experimental setup
The participants were instructed to sit on a chair and rest their right/left arm in a comfortable position on the table as shown in Fig. 1B. Prior to the experiment a sixty-four channel EEG cap was placed on the participants’ head. Participants were instructed to wash and dry their heads before the start of the experiment and electrode-gel was applied to each channel using a syringe with blunt needle to reduce the electrode impedance, which eliminated the need for additional skin preparation, as the gel was sufficient to maintain impedance below 50 kΩ. The electrode impedance was measured before the experiment and adjusted to be below 50 kΩ. For EMG recordings, standard skin preparation procedures were followed, including cleaning the skin with alcohol wipes to reduce impedance and enhance electrode contact.
Experimental protocol
The experiment consists of two task conditions: fixed and free conditions. The thumb platform was positioned on a movable base, with the task conditions defined by the mobility of the platform. In the fixed task condition, the thumb platform was securely fixed with screws between the middle and ring fingers. In the free task condition, the thumb platform was allowed to move freely; however, participants were instructed to maintain the thumb position between the middle and ring fingers. The thumb displacement was measured using a displacement measurement sensor. In the free condition, if the displacement of the thumb platform was more than 1 cm then the trial was rejected. All the participants performed the task with both the dominant hand and non-dominant hand separately. Among the twenty healthy young participants, 50% performed the task with their dominant hand and subsequently switched to the non-dominant hand, while the remaining 50% performed with their non-dominant hand before switching to the dominant hand. The trial order was randomized to control for any potential order effects. During both the fixed and free conditions, initially for a 5 s period, the participants were instructed to hold the handle gently, using all five fingers in a relaxed manner. After this initial 5 s period they were asked to lift the handle and hold it in air for another 5 s. The participants were instructed to maintain the handle in steady state in the lift and hold period by maintaining the bubble of the sprit level at the center. Each participant performed 25 such trials for each condition with each hand. Adequate breaks were provided between the trials and additional breaks were given upon the participants request. The, trials were rejected if the handle tilt exceeded 3 degrees or if the thumb platform displacement exceeded 1 cm in the free condition. Approximately 10% of the data (3 out of 28 trials) were removed, resulting in the inclusion of 25 trials for analysis. Participants were instructed to lift the handle to a minimum height by applying the least amount of normal force necessary.
Data acquisition
The force signals from the five sensors and the displacement signal were digitized using a 16-bit NI USB 6225 and 6210 DAQs (National Instruments, Austin, TX, USA) respectively. The orientation signal from the IMU was recorded through a microcontroller. Force signals, the orientation data and the displacement data were sampled at 100 samples per second. Two-channel EMG and twenty-two channel EEG were recorded using two RHD 2216 (INTAN technologies, USA) through XEM6310 (Opal Kelly, Portland) FPGA interface board. EEG and EMG signals were sampled at 1500 Hz. EEG signal, EMG signal, finger force signal, displacement signal and the orientation data from IMU were recorded simultaneously using a custom LabView code (version 19).
Data analysis
All the recorded signals were analysed offline in MATLAB (Version R2022a, MathWorks, USA).
Synergy analysis
We performed variance analysis as described by Latash et al. (2002); Zhang et al. (2009) to quantify multi finger synergy40,41. An index (ΔV) of synergy was computed as the difference between the sum of the variances of the mechanical variables produced by individual digits (elemental variables) [ΣVar(EV)] and the variance of the total output of these elemental variables [Var(ΣEV)]33. The variance of one of the variables on the left side of Eq. (2), a performance variable (PV), was compared to the sum of the variances of the variables on the right side of those equations, the elemental variables (EVs), and then normalized by the latter value21,23,42 (Eq. 1).
The variance analysis was conducted for performance variable, total moment of force (MTot) due to the normal and tangential forces at the virtual finger (VF) and thumb (TH) level. The Virtual Finger (VF) refers to the combined action of the index, middle, ring, and little fingers, which together function as a single entity in manipulating objects. In the VF-TH level hierarchy, VF forces and moments (sum of index, middle, ring, and little fingers) and the thumb forces and moments were considered as elemental variables. At the VF-TH level, we were interested in examining the co-variation of the following quantities produced by the VF and the opposing effector: the total moment of force, MTot. The total moment of force produced by the normal and tangential force should be equal to zero in equilibrium conditions.
The synergy index was computed for a two second segment of the 5-s hold period, individually for each participant, for each hand and (dominant and non-dominant hand) for each condition (Fixed and Free). Positive values of the synergy index indicate the negative covariation among the elemental variables and, a synergy stabilizing the performance variable. Fisher’s z transformation was applied to the synergy index using the Eq. (3) for the statistical analysis.
EEG band power
The power line interference and its harmonics from the recorded EEG signals were removed using a 50 Hz notch filter. Out of 22 channels, the following 12 EEG channels were taken for further analysis, Fc1, Fc2, Fc5, Fc6, C3, C4, Cp5, Cp1, Cp2, Cp6, P3, and P4. These 12 channels were selected based on their coverage of key brain regions involved in motor control7,43,44. During the experiment, participants were instructed to minimize eye blinks and eye movements as much as possible. This instruction aimed to reduce the occurrence of ocular motion artifacts during data collection. After recording EEG signals, we checked the signal amplitude for each trial manually. If the amplitude exceeded the threshold of 150 μV, indicating the presence of potential artifacts, the trial was rejected. We rejected 10% of the data, specifically 3 out of 28 trials, resulting in the selection of a total of 25 artifact free trials, resulting in the selection of 25 artifact-free trials. The notch filtered 12 channel EEG signals were bandpass filtered to beta band (13–30 Hz) using a fourth order, zero phase lag Butterworth filter. Each band signal was down-sampled to 100 Hz. The total trial duration of the task was 10 s.
The band power value of the EEG signal between 2–4 s of the holding phase was taken to compare the two conditions for the 12 channels mentioned above. For analysis, we focused on the middle 2 s of the 5-s hold period to minimize any minor drift that may occur during the initial and final periods.
When participants performed tasks with their right hand (dominant hand), the EEG band power of the contralateral side (left hemisphere) channels Fc1, Fc5, C3, Cp5, Cp1, and P3 were analysed. When tasks were performed with the left hand (non-dominant hand), the EEG band power of the contralateral side (right hemisphere) channels Fc2, Fc6, C4, Cp6, Cp2, and P4 were analysed. The band power of these channels was analyzed, and the contralateral corresponding channels were compared: Fc1 and Fc2, Fc5 and Fc6, C3 and C4, Cp5 and Cp6, Cp1 and Cp2, P3 and P4.
The following steps were used to calculate the Band Power—BP44 for both the fixed and free condition.
-
1.
The power of the samples was obtained by squaring the amplitude samples of each trial
-
2.
Averaging the power samples across trials
The mean band power during the holding phase was averaged across all participants, and the standard error of the mean was calculated.
EEG–EMG coherence
The artifact removed EEG signal were bandpass filtered between 4–40 Hz using a fourth order, zero phase lag Butterworth filter. The notch filtered EMG signals were band pass filtered between 20–500 Hz using a fourth order, zero phase lag Butterworth filter. EEG and EMG signals during the lift and hold phase between 2–4 s of each trial were taken for the EEG–EMG coherence calculation. EMG signals were not rectified prior to calculating corticomuscular coherence (CMC). Coherence gives a linear relationship between two signals in the frequency domain45. Coherence is obtained through the normalization of the cross-spectrum of the signals x(t) and y(t)46.
where, Pxx (f) is the power spectrum of the time series signal x(t), and Pyy (f) is the power spectrum of the time series signal y(t).
where Xi (f) is the Fourier transform of the i-th signal segment within the total sample of L, where * denotes the complex conjugate. P_xy (f) is the cross spectrum of the two signals x(t) and y(t) is indicated as,
where Yi (f) is the Fourier transform of the signal segment I of the total sample of L and * represents the complex conjugate. Corticomuscular coherence characterizes the coherence of the cortical signals x(t) and the muscle signals y(t).
Coherence was calculated between each EEG channel (14 channels) and each EMG channel (FDS and FCU) using the mscohere function in MATLAB’s Signal Processing Toolbox. For each trial, the data segment of 3000 samples was divided into 8 non-overlapping segments, with a Hamming window applied to each segment. This segmentation provided a window length of 375 samples (equivalent to 250 ms) and a frequency resolution of 4 Hz. The choice of non-overlapping segments was made to ensure independent estimates across segments. Coherence estimates were averaged across these segments to increase the reliability of the measure. The raw coherence values were then transformed into z-scores using the formula (4)47,48,
where, N = 8 denote the number of segments. The 95% confidence intervals (CI) for coherence values were calculated based on non-overlapping segments, using the formula (5)47,48,49
The 95% confidence interval (CI) for coherence values, with N = 8, is approximately 0.348. This means that coherence values above this threshold can be considered significantly different from zero at the 95% confidence level. Mean squared coherence was calculated between 2–4 s of the holding phase of the task for the two conditions. For analysis, we focused on the middle 2 s of the 5-s hold period to minimize any minor drift that may occur during the initial and final periods. The mean squared coherence was averaged across the trials, across the participants and standard error of mean was calculated.
Statistical analysis
All statistical analyses were performed in R. The Shapiro–Wilk normality test was used to assess the normality of all feature datasets. Detailed results of the normality tests are provided in the supplementary section. Given the statistically significant deviation from normality in only one condition per feature, and supported by the robustness of ANOVA to minor violations of normality, parametric two-way repeated measures ANOVAs were conducted. Additionally, Mauchly’s test indicated a violation of sphericity (p < 0.0001), but the Greenhouse–Geisser epsilon was 1.0, indicating no adjustment to degrees of freedom was required. A two-way repeated measures ANOVA was conducted with factors: Conditions (2 conditions: Fixed and Free) × Hand (2 hands: DH and NDH) on EEG band power, synergy index and EEG–EMG coherence. This analysis aimed to examine the changes in EEG band power, synergy index and EEG–EMG coherence. The significant level p < 0.05 was chosen for the statistical analysis. Partial eta-squared (η2) was stated as effect size. Two one sided t test (TOST) analysis was performed on the EEG band power, synergy index and EEG–EMG coherence between the two task conditions with the smallest effect size of interest (SESOI = 1.04) as equivalence bounds. Equivalence bound of ΔV = 1.04 was chosen as lower and upper bound for the statistical power of 95%50. All values are given in mean ± SEM format.
Results
Synergy index of total moment of force
A two-way repeated measures ANOVA by considering factors conditions and hand revealed a significant main effect on the factor conditions (F(1,19) = 81.08, η2 = 0.81, p < 0.0001). However, no main effect was observed for the factor hands (F(1,19) = 2.67, η2 = 0.12, p = 0.11) and the interaction of the factor’s hands x condition (F(1,19) = 1.20, η2 = 0.05, p = 0.28).
Post hoc analysis showed that the synergy index was significantly higher in the fixed condition compared to the free condition in both the left hand (Fixed condition: 1.01 ± 0.06; Free condition: 0.32 ± 0.004; p < 0.001, Cohen’s d = 2.04) and right hand (Fixed condition: 1.23 ± 0.157; Free condition: 0.38 ± 0.108; p < 0.001, Cohen’s d = 2.54). No significant differences were observed between hands (LH vs RH) in either the fixed condition (p = 0.07, Cohen’s d = 0.47) or the free condition (p = 0.63, Cohen’s d = 0.11).
There was no difference observed in the synergy index between the left hand and right (DH and NDH of the right handers) under both task conditions. Since there was no difference observed, TOST analysis was performed by considering the upper and lower bound (ΔU and ΔL) as ± 1.04 between the synergy index of the DH and NDH. TOST analysis showed a statistical equivalence (p < 0.001) on the synergy index between the DH and NDH channel for the fixed (NDH: 1.01 ± 0.066; DH: 1.23 ± 0.157) and free task condition (NDH: 0.32 ± 0.044; DH: 0.38 ± 0.108) as shown in Fig. 2.
Synergy index of moment of force. There was no difference observed in synergy index between the hands in both task conditions. TOST analysis showed statistical equivalence (p < 0.001) of the synergy index of LH and RH (DH and NDH of the right handers). However, synergy index of moment of force in the fixed condition was higher than the free condition for both hands (F(1,19) = 81.08, η2 = 0.81, p < 0.0001).
EEG band power
A two-way repeated measures ANOVA by considering factors conditions and hand revealed a significant main effect on the factor conditions (F(1,19) = 11.07, η2 = 0.37, p < 0.05). However, no main effect was observed for the factor hands (F(1,19) = 1.80, η2 = 0.11, p = 0.19) and the interaction of the factor’s hands x condition (F(1,19) = 1.94, η2 = 0.10, p = 0.17).
Post hoc analysis showed that EEG band power was significantly higher in the fixed condition compared to the free condition in both left hand (Fixed condition: 0.05 ± 0.008; Free condition: 0.03 ± 4e−6; p = 0.008, Cohen’s d = 0.66) and right hand (Fixed condition: 0.06 ± 0.01; Free condition: 0.03 ± 0.005; p < 0.001, Cohen’s d = 0.95). No significant differences were observed between hands (LH vs RH) in either condition (Fixed: p = 0.29, Cohen’s d = 0.12; Free: p = 0.83, Cohen’s d = 0.03).
There was no difference observed between the C4 and C3 EEG band power corresponding to the contralateral channel in both task conditions. Since there was no difference observed, TOST analysis was performed by considering the upper and lower bound (ΔU and ΔL) as ± 1.04 between the C3 and C4 channel EEG band power. TOST analysis showed a statistical equivalence (p < 0.0001) on the EEG band power between the C3 and C4 channel for the fixed (NDH: 0.057 ± 0.008; DH: 0.06 ± 0.011) and free task condition (NDH: 0.032 ± 4e−6; DH: 0.030 ± 0.005) as shown in Fig. 3.
EEG band power. There was no difference observed in EEG band power between the channel C3 and C4 in both task conditions. TOST analysis showed statistical equivalence (p < 0.0001) of the EEG band power of C4 and C3 activities corresponding to the contralateral hand in both fixed and free task conditions. However, EEG band power of fixed condition was higher than the free condition for both hands (F(1,19) = 11.07, η2 = 0.37, p < 0.05).
EEG–EMG coherence
A two-way repeated measures ANOVA by considering factors conditions and hand revealed a significant main effect on the factor conditions (F(1,19) = 26.17, η2 = 0.49, p < 0.0001). However, no main effect was observed for the factor hands (F(1,19) = 1.54, η2 = 0.07, p = 0.22) and the interaction of the factor’s hands x condition (F(1,19) = 0.24, η2 = 0.01, p = 0.62).
Post hoc analysis showed that EEG–EMG coherence was significantly higher in the fixed condition compared to the free condition in both the left hand (Fixed: 2.17 ± 0.12; Free: 0.72 ± 0.13; p < 0.0001, Cohen’s d = 1.86) and the right hand (Fixed: 2.32 ± 0.15; Free: 0.76 ± 0.09; p < 0.0001, Cohen’s d = 2.07). No significant differences were found between left and right hands in either fixed (p = 0.40, Cohen’s d = 0.23) or free condition (p = 0.85, Cohen’s d = 0.05).
The magnitude of EEG–EMG coherence between the hands exhibited comparable values under both task conditions. Therefore, TOST analysis was performed by considering the upper and lower bound (ΔU and ΔL) as ± 1.04 between the left and right hand for both task conditions. TOST analysis showed a statistical equivalence (p < 0.0001) on the EEG–EMG coherence between the hands in the fixed (NDH: 2.17 ± 0.120; DH: 2.32 ± 0.153) and free task condition (NDH: 0.72 ± 0.13; DH: 0.76 ± 0.09) as shown in Fig. 4.
EEG–EMG coherence. The magnitude of EEG–EMG coherence was comparable between the hands in both task conditions. TOST analysis revealed a statistical equivalence (p < 0.0001) between the left and right hand in both task conditions. However, EEG–EMG coherence was higher in the fixed condition compared to the free condition (F(1,19) = 26.17, η2 = 0.49, p < 0.0001).
Discussion
The primary aim of this study was to compare the behavioural and neural features during lifting and grasping tasks between the dominant and non-dominant hands in healthy young adults. It was hypothesized that the dominant hand would exhibit greater finger force coordination (synergy index) and enhanced neural features (EEG band power and EEG–EMG coherence) compared to the non-dominant hand. However, our results contradicted the hypothesis, as no differences were observed in synergy index, EEG band power, and EEG–EMG coherence, between the dominant and non-dominant hands. These findings suggest that the non-dominant hand can function similarly to the dominant hand for the given task. The implications of these findings are discussed in the following paragraphs.
In our study, participants had to maintain the handle in a steady state during both task conditions. The fixed task condition is similar to isometric tasks, where constant force is required to keep the handle steady. However, in the free task condition, the thumb platform is freely movable due to the frictionless setup, which introduces a level of dynamic interaction. The results indicate a clear task-level difference in the synergy index for both dominant and non-dominant hand, suggesting that the modulation of force coordination varies between the two task conditions. Despite the hypothesized differences, the similar synergy indices observed between the dominant and non-dominant hands suggest that both hands are capable of achieving comparable levels of coordination and stability during the task. This lack of difference in the synergy index aligns with studies on handling fragile objects with both hands25 and isometric conditions23. In isometric conditions, muscle contraction occurs without changing muscle (muscle + tendon) length. Performance differences typically associated with hand used in dynamic reaching tasks may not be evident in this task, as it does not involve complex movements or dynamic adjustments.
These results suggest that, despite the differing nature of the tasks, the underlying motor control mechanisms may be similarly effective across both hands. Notably, the non-dominant hand demonstrated a level of performance comparable to the dominant hand, even under the dynamic thumb condition which was supported by TOST analysis. This finding is particularly intriguing, as the dynamic condition introduces greater challenges in thumb control due to the need for constant adjustments. The ability of the non-dominant hand to match the performance of the dominant hand in this condition suggests that the cortical activity and finger force coordination are not exclusively reliant on hand used. Instead, both hands appear to utilize similar adaptive strategies to achieve coordinated control of finger forces across varying task demands. These results highlight the robustness of the motor control system in facilitating task-specific adjustments in finger force coordination, regardless of hand being used. Furthermore, this suggests that the non-dominant hand can compensate effectively, potentially reducing reliance on the dominant hand, even in tasks requiring fine motor adjustments.
A possible explanation for this lack of observed differences lies in the different roles of the visual and motor systems during the task. Reaching tasks require significant coordination between these systems, but after grasping and lifting the handle in both task conditions, their involvement reduces. This reduced engagement of the visual system might elucidate the lack of differences observed between the hands in both task conditions in our study. These results are partially consistent with another study from our group, where finger force synergies were similar between hands in non-challenging tasks, while the dominant hand showed better coordination during more challenging tasks. However, the task in the above-mentioned study also involved continuous monitoring of the thumb platform displacement, which might have influenced the observed outcomes36.
However, our findings contradict the dynamic dominance hypothesis, which suggests that the dominant arm uses more sophisticated neural control mechanisms and coordination strategies. According to this hypothesis, differences in muscle torques and hand path trajectories should be evident between the dominant and non-dominant hands1,3,4. The absence of such differences in the present study suggests that the task demands may not have been sufficiently complex or dynamic to elicit dominance-related disparities. Alternatively, these findings might indicate that, for tasks involving steady-state force maintenance and limited visual-motor engagement, both hands are capable of achieving comparable performance and stability.
This interpretation is further supported by the EEG results, which revealed comparable cortical activity in the contralateral channels for both hands in both task conditions. Additionally, no differences were observed in EEG band power between the hands in either task condition. These findings imply that the cortical control mechanisms involved in the task are similar for both hands, regardless of the hand used. The comparable EEG band power suggests that both hands employ equivalent neural resources to meet the demands of the task, further underscoring the uniformity in their performance. The lack of observed differences between the hands in EEG band power may also reflect the task’s emphasis on steady-state force maintenance rather than dynamic adjustments or fine motor precision. Tasks involving greater variability or rapid transitions might engage the cortex differently and reveal dominance-related differences. The findings of this study contradict the expected differences based on the dominant hemisphere’s known anatomical and functional advantages9. The hemispheric differences suggested by previous findings could be related to the specialized neural mechanisms for skilled movements in the dominant hand. However, EEG band power in the contralateral channels corresponding to hand movement (C3 for the right hand and C4 for the left hand) was considerably higher in the fixed condition compared to the free condition. This observation aligns with the findings on finger force coordination and suggests task-level modulation in cortical activity specific to the contralateral hand.
Moreover, the similar corticomuscular coherence (CMC) magnitude observed between the dominant and non-dominant hands further supports the notion of comparable performance of the hands. Despite differences in hemispheric representation for the dominant and non-dominant hands, the overall performance and functionality of the hands did not differ considerably. These findings align with previous studies, which observed no differences between the two hands during the steady-state phase17. The similarity in EEG band power across both hands suggests that equivalent neural resources are engaged, regardless of hand dominance. This uniform cortical activity likely supports the observed comparable EEG–EMG coherence, as the synchronization of cortical and muscle activity depends on the consistency and efficacy of cortical signals reaching the muscles.
Additionally, task-level modulations in corticomuscular coherence (CMC) were observed for both hands. In the free condition, the altered friction between the platform and the thumb is likely perceived by proprioceptors and cutaneous receptors in the fingertip. This sensory input prompts adjustments in grip and tangential forces, allowing the fingers to maintain the handle’s equilibrium. These adjustments demonstrate how finger coordination adapts dynamically to meet task-specific requirements33. Previous studies demonstrated that synergistically activated muscles are driven by a shared beta-band cortical drive, which can be quantified using intramuscular coherence51. The observed reduction in CMC during the free condition can thus be interpreted as a decrease in finger coordination due to changes in friction. This reduction likely reflects a shift in neural control strategies, where the need for finer adjustments to changing friction demands results in less synchronous cortical input to the muscles. These results align with previous findings, where beta-band CMC was reduced during tasks involving object compliance. Specifically, studies demonstrated that CMC was lower when using a compliant object like a spring compared to a rigid object such as a wooden dowel during a two-finger pinch grasp47. Additionally, differences in the neural and behavioural features between the task conditions in the dominant hand of young adults have been extensively discussed in our previous findings33.
Previous studies have suggested that interhemispheric monitoring and performance evaluation play a key role in motor control and hand dominance, with hemispheric interactions influencing how each hand’s performance is monitored and adjusted52,53. However, the coordination and force control required in our tasks may necessitate similar motor strategies from both hands, enabling them to perform the task effectively regardless of dominance. Taken together, task-level modulation was observed between the task conditions in both hands, specifically in cortical activity, finger force coordination, and EEG–EMG coherence. The lack of differences in synergy indices, combined with the comparable neural features observed across task conditions, underscores the adaptability of motor control strategies and the uniformity in how both hands respond to task demands. The lack of interaction effect between the hand and conditions signify that the hands adjust to task demands in a non-different manner. These findings highlight the capacity of both hands to achieve comparable performance in tasks requiring steady-state force control, despite their typical dominance-related roles in other contexts.
Limitations
One limitation of this study is the exclusive focus on contralateral hemisphere activity. As this work extends our prior research, our primary objective was to investigate the role of the contralateral hemisphere in neural control during force maintenance tasks. While analyzing ipsilateral activity was beyond the primary scope of this study, we are currently conducting a separate analysis of ipsilateral channels. Future studies should further explore the role of ipsilateral activation in force control to provide a more comprehensive understanding of laterality effects. Additionally, EEG band power and finger force synergies were analyzed separately, without directly testing the explicit relationship between these two aspects. Our synergy analysis was based on variances across trials, a methodology that limits direct comparisons between EEG data and synergy indices. While this approach provides valuable insights into motor variability and coordination, it imposes constraints on investigating the precise neural mechanisms underlying force synergies. Future research should integrate these measures more explicitly to enhance our understanding of the neural control of hand function.
Furthermore, our study primarily examined steady-state force maintenance, which may not fully capture functional differences between the dominant and non-dominant hands. While our findings suggest that both hands are equally capable of maintaining steady-state force, a task requiring dynamic force adjustments such as rapid grasping, unpredictable perturbations, or object manipulation under varying loads might better highlight the specialization of the dominant hand in dynamic control. Future research should explore such task conditions to better illustrate the potential limitations of the non-dominant hand and further test the applicability of the dynamic dominance hypothesis.
Conclusion
In this study we compared the behavioural and neural features between the dominant hand and non-dominant hand during grasping and lifting tasks under two task conditions. Specifically, we compared the synergy index and EEG band power, EEG–EMG coherence as behavioural and neural features. Our study revealed that there was no difference observed between the dominant and non-dominant hands in both behavioural and neural features. In conclusion, these findings indicate that both the dominant and non-dominant hands can achieve comparable performance levels in the given tasks, despite the dominant hand’s typical advantages in dynamic motor tasks. Task-dependent modulations were observed, particularly in the free condition, where variations in friction led to adjustments in both behavioural and neural features. Further studies should explore how different task complexities and dynamic conditions might reveal more pronounced differences between the hands.
Data availability
The authors do not report data and therefore the pre-registration and data availability requirements are not applicable. The datasets generated and analyzed during the current study are not publicly available as further analyses are ongoing. However, they are available from the corresponding author upon reasonable request.
References
Sainburg, R. L. & Kalakanis, D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J. Neurophysiol. 83(5), 2661–2675. https://doi.org/10.1152/jn.2000.83.5.2661 (2000).
Bagesteiro, L. B. & Sainburg, R. L. Handedness: Dominant arm advantages in control of limb dynamics. J. Neurophysiol. 88(5), 2408–2421. https://doi.org/10.1152/jn.00901.2001 (2002).
Sainburg, R. Evidence for a dynamic-dominance hypothesis of handedness. Exp. Brain Res. 142(2), 241–258. https://doi.org/10.1007/s00221-001-0913-8 (2002).
Wang, J. & Sainburg, R. L. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp. Brain Res. 178(4), 565–570. https://doi.org/10.1007/s00221-007-0936-x (2007).
Gut, M. et al. Brain correlates of right-handedness. Acta Neurobiol. Exp. 67(1), 1. https://doi.org/10.55782/ane-2007-1631 (2007).
Nam, C. S., Jeon, Y., Kim, Y.-J., Lee, I. & Park, K. Movement imagery-related lateralization of event-related (de)synchronization (ERD/ERS): Motor-imagery duration effects. Clin. Neurophysiol. 122(3), 567–577. https://doi.org/10.1016/j.clinph.2010.08.002 (2011).
Pfurtscheller, G. & Lopes Da Silva, F. H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 110(11), 1842–1857. https://doi.org/10.1016/S1388-2457(99)00141-8 (1999).
Amunts, K. et al. Asymmetry in the human motor cortex and handedness. Neuroimage 4(3), 216–222. https://doi.org/10.1006/nimg.1996.0073 (1996).
Volkmann, J., Schnitzler, A., Witte, O. W. & Freund, H.-J. Handedness and asymmetry of hand representation in human motor cortex. J. Neurophysiol. 79(4), 2149–2154. https://doi.org/10.1152/jn.1998.79.4.2149 (1998).
Garry, M. I., Kamen, G. & Nordstrom, M. A. Hemispheric differences in the relationship between corticomotor excitability changes following a fine-motor task and motor learning. J. Neurophysiol. 91(4), 1570–1578. https://doi.org/10.1152/jn.00595.2003 (2004).
Liu, J., Sheng, Y. & Liu, H. Corticomuscular coherence and its applications: A review. Front. Hum. Neurosci. 13, 100. https://doi.org/10.3389/fnhum.2019.00100 (2019).
Mima, T. & Hallett, M. Corticomuscular coherence: A review. J. Clin. Neurophysiol. 16(6), 501. https://doi.org/10.1097/00004691-199911000-00002 (1999).
Boonstra, T. W., Van Wijk, B. C. M., Praamstra, P. & Daffertshofer, A. Corticomuscular and bilateral EMG coherence reflect distinct aspects of neural synchronization. Neurosci. Lett. 463(1), 17–21. https://doi.org/10.1016/j.neulet.2009.07.043 (2009).
Chakarov, V. et al. Beta-range EEG–EMG coherence with isometric compensation for increasing modulated low-level forces. J. Neurophysiol. 102(2), 1115–1120. https://doi.org/10.1152/jn.91095.2008 (2009).
Fu, A. et al. Corticomuscular coherence analysis on the static and dynamic tasks of hand movement. In 2014 19th International Conference on Digital Signal Processing, 715–718. https://doi.org/10.1109/ICDSP.2014.6900757 (2014).
Chen, X., Zhang, Y., Yang, Y., Li, X. & Xie, P. Beta-range corticomuscular coupling reflects asymmetries in hand movement. IEEE Trans. Neural Syst. Rehabil. Eng. 28(11), 2575–2585. https://doi.org/10.1109/TNSRE.2020.3022364 (2020).
Tecchio, F. et al. Sensory-motor interaction in primary hand cortical areas: A magnetoencephalography assessment. Neuroscience 141(1), 533–542. https://doi.org/10.1016/j.neuroscience.2006.03.059 (2006).
L’Abbate, T. et al. Corticomuscular coherence dependence on body side and visual feedback. Neuroscience 490, 144–154. https://doi.org/10.1016/j.neuroscience.2022.02.019 (2022).
Latash, M. L., Scholz, J. F., Danion, F. & Schöner, G. Structure of motor variability in marginally redundant multifinger force production tasks. Exp. Brain Res. 141(2), 153–165. https://doi.org/10.1007/s002210100861 (2001).
Latash, M. Stages in learning motor synergies: A view based on the equilibrium-point hypothesis. Hum. Mov. Sci. 29, 642–654. https://doi.org/10.1016/j.humov.2009.11.002 (2010).
Latash, M. L. Synergy (Oxford University Press, 2008).
Adam, A., Luca, C. J. D. & Erim, Z. Hand dominance and motor unit firing behavior. J. Neurophysiol. 80(3), 1373–1382. https://doi.org/10.1152/jn.1998.80.3.1373 (1998).
Zhang, W., Sainburg, R. L., Zatsiorsky, V. M. & Latash, M. L. Hand dominance and multi-finger synergies. Neurosci. Lett. 409(3), 200–204. https://doi.org/10.1016/j.neulet.2006.09.048 (2006).
Rearick, M. P. & Santello, M. Force synergies for multifingered grasping: Effect of predictability in object center of mass and handedness. Exp. Brain Res. 144(1), 38–49. https://doi.org/10.1007/s00221-002-1024-x (2002).
Gorniak, S. L., Zatsiorsky, V. M. & Latash, M. L. Manipulation of a fragile object. Exp. Brain Res. 202(2), 413–430. https://doi.org/10.1007/s00221-009-2148-z (2010).
Goble, D. J., Lewis, C. A. & Brown, S. H. Upper limb asymmetries in the utilization of proprioceptive feedback. Exp. Brain Res. 168(1), 307–311. https://doi.org/10.1007/s00221-005-0280-y (2006).
Han, J., Anson, J., Waddington, G. & Adams, R. Proprioceptive performance of bilateral upper and lower limb joints: Side-general and site-specific effects. Exp. Brain Res. 226(3), 313–323. https://doi.org/10.1007/s00221-013-3437-0 (2013).
Bryden, P. J. & Roy, E. A. Unimanual performance across the age span. Brain Cogn. 57(1), 26–29. https://doi.org/10.1016/j.bandc.2004.08.016 (2005).
Annett, J., Annett, M., Hudson, P. T. W. & Turner, A. The control of movement in the preferred and non-preferred hands. Q. J. Exp. Psychol. 31(4), 641–652. https://doi.org/10.1080/14640747908400755 (1979).
Rajakumar, B. & Skm, V. A test of the mechanical advantage hypothesis during artificial reduction in thumb contribution to hold objects. bioRxiv https://doi.org/10.1101/2020.03.28.013896 (2020).
Rajakumar, B. & Skm, V. Comparable behaviour of ring and little fingers due to an artificial reduction in thumb contribution to hold objects. PeerJ 8, e9962. https://doi.org/10.7717/peerj.9962 (2020).
Rajakumar, B., Dutta, S. & Varadhan, S. K. M. Support for mechanical advantage hypothesis of grasping cannot be explained only by task mechanics. Sci. Rep. 12(1), 10242. https://doi.org/10.1038/s41598-022-14014-2 (2022).
Eswari, B., Balasubramanian, S. & Skm, V. Older individuals do not show task specific variations in EEG band power and finger force coordination. IEEE Trans. Biomed. Eng. https://doi.org/10.1109/TBME.2024.3435480 (2024).
Négyesi, J. et al. Handedness did not affect motor skill acquisition by the dominant hand or interlimb transfer to the non-dominant hand regardless of task complexity level. Sci. Rep. 12(1), 18181. https://doi.org/10.1038/s41598-022-21962-2 (2022).
Mathew, J., Sarlegna, F. R., Bernier, P.-M. & Danion, F. R. Handedness matters for motor control but not for prediction. eNeuro https://doi.org/10.1523/ENEURO.0136-19.2019 (2019).
Shenoy, P. & Varadhan, S. K. M. Task demands modulate distal limb handedness: A comparative analysis of prehensile synergies of the dominant and non-dominant hand. Sci. Rep. 14(1), 25565. https://doi.org/10.1038/s41598-024-75001-3 (2024).
Chaudhry, F., Ahmad, H., Sinkler, M. A. & Arain, A. Anatomy, shoulder and upper limb, forearm compartments. In StatPearls (StatPearls Publishing, 2024) [Online]. http://www.ncbi.nlm.nih.gov/books/NBK539784/. Accessed: Jul. 05, 2024.
Meyer, J. Standards for Reporting EMG Data (1999).
Hermens, H. J., Freriks, B., Disselhorst-Klug, C. & Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 10(5), 361–374. https://doi.org/10.1016/S1050-6411(00)00027-4 (2000).
Zhang, W., Olafsdottir, H. B., Zatsiorsky, V. M. & Latash, M. L. Mechanical analysis and hierarchies of multidigit synergies during accurate object rotation. Mot. Control 13(3), 251–279. https://doi.org/10.1123/mcj.13.3.251 (2009).
Latash, M. L., Scholz, J. P. & Schöner, G. Motor control strategies revealed in the structure of motor variability. Exerc. Sport Sci. Rev. 30(1), 26 (2002).
Skm, V., Zhang, W., Zatsiorsky, V. & Latash, M. Age effects on rotational hand action. Hum. Mov. Sci. 31, 502–518. https://doi.org/10.1016/j.humov.2011.07.005 (2012).
Pfurtscheller, G. & Neuper, C. Simultaneous EEG 10 Hz desynchronization and 40 Hz synchronization during finger movements. NeuroReport 3(12), 1057 (1992).
Pfurtscheller, G., Neuper, C. & Kalcher, J. 40-Hz oscillations during motor behavior in man. Neurosci. Lett. 164(1–2), 179–182. https://doi.org/10.1016/0304-3940(93)90886-P (1993).
Grosse, P. et al. Abnormal corticomuscular and intermuscular coupling in high-frequency rhythmic myoclonus. Brain 126(2), 326–342. https://doi.org/10.1093/brain/awg043 (2003).
Baker, S. N., Olivier, E. & Lemon, R. N. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J. Physiol. 501(Pt 1), 225–241 (1997).
Reyes, A., Laine, C. M., Kutch, J. J. & Valero-Cuevas, F. J. Beta band corticomuscular drive reflects muscle coordination strategies. Front. Comput. Neurosci. https://doi.org/10.3389/fncom.2017.00017 (2017).
Laine, C. M. & Valero-Cuevas, F. J. Intermuscular coherence reflects functional coordination. J. Neurophysiol. 118(3), 1775–1783. https://doi.org/10.1152/jn.00204.2017 (2017).
Bayram, M. B., Siemionow, V. & Yue, G. H. Weakening of corticomuscular signal coupling during voluntary motor action in aging. GERONA 70(8), 1037–1043. https://doi.org/10.1093/gerona/glv014 (2015).
Lakens, D. Equivalence tests: A practical primer for t tests, correlations, and meta-analyses. Soc. Psychol. Pers. Sci. 8(4), 355–362. https://doi.org/10.1177/1948550617697177 (2017).
Kilner, J. M. et al. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J. Physiol. 516(2), 559–570. https://doi.org/10.1111/j.1469-7793.1999.0559v.x (1999).
Hochman, E. Y. & Eviatar, Z. Does each hemisphere monitor the ongoing process in the contralateral one?. Brain Cogn. 55(2), 314–321. https://doi.org/10.1016/j.bandc.2004.02.030 (2004).
Hochman, E. Y. & Eviatar, Z. Do the hemispheres watch each other? Evidence for a between-hemispheres performance monitoring. Neuropsychology 20(6), 666–674. https://doi.org/10.1037/0894-4105.20.6.666 (2006).
Acknowledgements
This work was supported by the American Express Lab for Data Analytics, Risk and Technology, IIT Madras, vide reference no SB20210346CPAMEXAMEHOC (awarded to Varadhan SKM). The funders played no role in the experimental design, protocol, data collection, analysis and in the manuscript preparation. We thank Thomas Jacob for his valuable contribution to the data collection process.
Author information
Authors and Affiliations
Contributions
Data collection—B.E; Methodology—B.E., V.S.K.M., S.B.; Formal analysis—B.E.; Writing original draft—B.E.; Writing review, Review and Editing—B.E., V.S.K.M., S.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Eswari, B., Balasubramanian, S. & Varadhan, S.K.M. Comparable neural and behavioural performance in dominant and non-dominant hands during grasping tasks. Sci Rep 15, 14690 (2025). https://doi.org/10.1038/s41598-025-99941-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99941-6
Keywords
This article is cited by
-
The influence of hand dominance, object distance, and size on reach-to-grasp coordination in a virtual environment
Experimental Brain Research (2026)
-
One third of people exert greater handgrip strength with their non-dominant hand
Scientific Reports (2025)