Abstract
The pupillary diameter (PD) clinically observed and measured by conventional devices represents a apparent PD of the anatomical pupil projected onto the corneal plane, yet the relationship between this apparent PD and three-dimensional PD (3D PD) in cataract patients remains unclear. This prospective cross-sectional observational study aimed to investigate the relationship between apparent PD and 3D PD in cataract patients and its clinical impact on multifocal intraocular lens (MIOL) selection. This study included 114 eyes from 72 cataract patients. Apparent PD and 3D PD were measured using Pentacam AXL. Statistical analyses included comparative, correlation, nonlinear relationship, and agreement methods. The clinical impact was assessed through predicted MIOL selections. Apparent PD significantly exceeded 3D PD (3.68 ± 0.76 vs. 2.50 ± 0.44 mm, P < 0.001), with a mean magnification ratio of 47.2 ± 16.5%. The magnification effect increased with pupil size: in eyes with apparent PD < 3.0 mm, 3.0–4.5 mm, and > 4.5 mm, the magnification ratios were 36.18%, 46.93%, and 63.57%, respectively. Strong positive correlation was found between 3D PD and apparent PD (r = 0.84, P < 0.001). For MIOL selection (PD > 2.8 mm threshold), apparent PD identified 90 eyes as suitable while 3D PD identified only 19 eyes, resulting in altered MIOL selection in 71 eyes (62.28%). Apparent PD overestimates 3D PD by 47.2% in cataract patients, increasing the likelihood of MIOL selection due to PD overestimation. Clinicians should consider this magnification effect during preoperative MIOL evaluation to avoid overestimation of functional PD.
Similar content being viewed by others
Introduction
Cataracts, the leading cause of blindness worldwide affecting 15.2 million blind and 78.8 million visually impaired individuals, constitute one of the most commonly performed surgical procedures globally1. In response to growing patient demands for favorable vision at both distance and near ranges following cataract surgery, advanced intraocular lenses (IOLs), including accommodating, extended depth of focus, and multifocal designs, have been extensively adopted in clinical practice3. Notably, when implanting these advanced IOLs, precise pupil size assessment is crucial, given that pupil size significantly influences visual outcomes and patient satisfaction2,3. Specifically, small pupils have been demonstrated to reduce contrast sensitivity at high spatial frequencies and decrease low-contrast visual acuity in patients with diffractive multifocal IOL (MIOL)4. Nevertheless, the pupillary diameter (PD) clinically observed or measured by conventional devices represents a apparent PD projected onto the corneal plane rather than the anatomical pupil size5.
In an effort to evaluate the three-dimensional (3D) PD, previous studies have employed various imaging technologies, including anterior segment optical coherence tomography (OCT), Hartmann-Shack aberrometry, and corneal topography to measure the apparent PD, and then derived the 3D pupil size through mathematical formulas and calculations5,6,7,8. Although these investigations consistently demonstrated that apparent PD exceeds the anatomical pupil size, the magnitude of this disparity has varied among studies employing different mathematical approaches5,6,7,8. Furthermore, this indirect calculation of anatomical pupil size may introduce computational uncertainties and potential system errors8. In addition, previous studies have not comprehensively investigated the relationships between pupil size and various demographic and ocular biometric parameters, nor have they directly demonstrated how different pupil size measurements influence MIOL selection. More importantly, among modern ophthalmic diagnostic devices, the Pentacam AXL Scheimpflug imaging system offers two distinct approaches to pupil measurement: apparent PD, which is derived from iris image analysis by computer recognition, and three-dimensional PD (3D PD), which represents the average of 360° meridional measurements obtained from 3D anterior segment reconstruction using Scheimpflug sectional images9. The Pentacam 3D PD is derived from Scheimpflug camera with 360° reconstruction on the true iris plane, thus providing a closer approximation of the anatomical pupil diameter, whereas the PupilX is an infrared-based video pupillometer that measures the apparent pupil under low illumination10. However, the relationship between these two PD measurements in cataract patients remains unknown.
Based on these considerations, this study therefore aims to: (1) investigate the relationship between apparent PD and 3D PD measured simultaneously using Pentacam AXL in cataract patients, (2) examine their associations with demographic and ocular biometric parameters, and (3) evaluate the impact of different PD measurements on MIOL selection. In contrast to previous research that relied on mathematical derivations, our study uniquely utilizes Pentacam AXL’s Scheimpflug imaging technology to directly obtain 3D PD through image reconstruction. Ultimately, these findings could enhance the accuracy of preoperative PD assessment and MIOL selection, thereby contributing to improved surgical outcomes in cataract patients.
Methods
Study design and participants
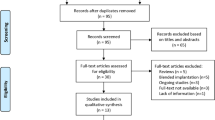
This prospective cross-sectional observational study was conducted at Chongqing Emergency Medical Center from October 2024 to December 2024. The study protocol adhered to the tenets of the Declaration of Helsinki11 and was approved by the Institutional Review Board of Chongqing Emergency Medical Center (IRB No. 2025-16). All participants provided written informed consent prior to study participation. A total of 156 consecutive patients scheduled for age-related cataract surgery were screened. After applying the inclusion and exclusion criteria listed below and retaining only Pentacam AXL measurements with “OK” quality specification, 72 patients (114 eyes; 42 bilateral, 30 unilateral) were included in the final analysis (Supplementary Fig. S1).
The inclusion criteria were: (1) age ≥ 50 years; (2) bilateral age-related cataract; (3) normal consciousness with clear communication ability and unimpaired mobility. Notably, Patients with diabetes mellitus were not excluded from this study12. The exclusion criteria comprised: (1) history of ocular surgery or trauma; (2) active ocular inflammation; (3) conditions affecting corneal transparency, including corneal disease; (4) moderate to severe dry eye disease; (5) presence of retinal or macular pathology; and (6) contact lens wear within 2 weeks prior to examination; and (7) measurements failing quality control criteria. Quality control was rigorously implemented to ensure data reliability. Measurements were deemed acceptable only when meeting specific standards: proper eye alignment, complete corneal visibility, absence of artifacts or distortions, and a “quality specification” parameter displaying “OK” on the Pentacam AXL. To minimize potential bias from repeated measurements, only one valid acquisition was performed and retained per eye. Pupil diameter has been identified as the least repeatable of all Pentacam automated parameters in within-session testing, with poor repeatability attributed primarily to physiologic pupil dynamics and pupillary unrest rather than measurement error or examiner variability13. Only measurements satisfying both clinical and quality control criteria were included in the final analysis.
Examination protocol
Initial comprehensive ophthalmic examination was performed for all potential participants. Subsequently, those who met the inclusion criteria were scheduled for Pentacam AXL® (Oculus, Germany) measurement after a 2-day interval. All Pentacam AXL measurements were conducted in a darkened examination room between 09:00 and 12:00 h to minimize diurnal variations14, with built-in illumination of 14.5 ± 0.1 lx under mesopic conditions. Prior to measurement, participants underwent a 15-minute dark adaptation period15. For the examination, participants were instructed to maintain a stable seated position with their chin on the chin rest and forehead against the forehead strap, while fixating on the target. Furthermore, participants were instructed to blink completely just before each measurement.
Data collection
The following parameters were obtained from the Pentacam AXL: Apparent PD, 3D PD, corneal white-to-white distance (WTW), central corneal thickness (CCT), flattest and steepest corneal axis refractive power (CARP), anterior chamber depth (ACD), axial length (AL), signal-to-noise ratio (SNR), kappa angle, and alpha angles. The absolute difference between apparent PD and 3D PD (PD difference = apparent PD − 3D PD) and the magnification ratio (calculated as (apparent PD − 3D PD) / 3D PD × 100%) were calculated. In addition to the Pentacam AXL measurements, best-corrected visual acuity (BCVA) was assessed using standard Early Treatment of Diabetic Retinopathy Study charts and expressed in logarithm of the minimum angle of resolution (LogMAR) units. Demographic information, including age, gender, ethnicity, and medical history, was collected through participant interviews.
Predicted MIOL suitability based on PD threshold
Predicted MIOL suitability was assessed by comparing categorizations derived from apparent PD versus 3D PD in a simulation framework. The currently recommended threshold of photopic pupil diameter > 2.8 mm for MIOL candidacy, established by a modified Delphi consensus among refractive surgery experts through anonymous multi-round questionnaires, was applied (i.e., photopic PD > 2.8 mm = suitable; photopic PD ≤ 2.8 mm = unsuitable)16. Consistency of the predicted MIOL suitability between apparent PD and 3D PD was calculated using the following formulas:
-
(1)
Consistency for Suitable MIOL = (Number of eyes categorized as suitable by both apparent PD and 3D PD) / (Number of eyes categorized as suitable by apparent PD) × 100%.
-
(2)
Consistency for Unsuitable MIOL = (Number of eyes categorized as unsuitable by both apparent PD and 3D PD) / (Number of eyes categorized as unsuitable by 3D PD) × 100%.
-
(3)
Overall Consistency = (Total number of eyes with consistent categorizations) / (Total number of eyes) × 100%.
Statistical analysis
For descriptive statistics, continuous variables are presented as mean ± standard deviation (SD), and categorical variables as frequency (percentage). Skewness and kurtosis were evaluated for the PD measurements to assess their distribution. The normality of the data was assessed using the Shapiro-Wilk test. Between-group comparisons were performed using the following tests: (a) chi-square test for categorical variables; (b) Mann-Whitney U test for non-normally distributed continuous variables in two independent samples; (c) independent samples t-test for normally distributed continuous variables in two independent samples; (d) Wilcoxon signed-rank test for non-normally distributed paired data; and (e) paired t-test for normally distributed paired data.
Correlation analyses were performed using the following methods: (a) Pearson correlation coefficient for normally distributed continuous variables; (b) Spearman’s rank correlation coefficient for non-normally distributed continuous variables; (c) independent samples t-test for comparisons between continuous variables and binary variables; and (d) Mann-Whitney U test for comparisons between non-normally distributed continuous variables and binary variables. Non-linear relationships were explored using generalized additive models (GAMs). Multivariable linear regression analysis, with prior assessment of multicollinearity, was performed to investigate factors influencing the 3D PD. Agreement between apparent PD and 3D PD was evaluated using intraclass correlation coefficient (ICC)16 and Bland-Altman analysis17.
All statistical analyses were conducted using R software (version 4.3.1). Statistical significance was defined as P < 0.05 for two-sided tests.
Results
Demographics and clinical characteristics of participants
This study enrolled 72 patients (114 eyes, 60 right and 54 left), with a mean age of 70.05 ± 9.18 years (range: 50–91 years), including 44 males and 70 females (Table 1). The participants’ ocular parameters showed a mean CCT of 538.15 ± 32.67 μm, WTW of 11.33 ± 0.44 mm, ACD of 3.16 ± 0.42 mm, and AL of 24.43 ± 1.92 mm. The flattest, steepest, and mean CARP measurements were 43.45 ± 1.60 D, 44.16 ± 1.59 D, and 43.81 ± 1.59, respectively. The analysis of PD measurements showed a mean apparent PD of 3.68 ± 0.76 mm (range: 2.04–5.65 mm) and a mean 3D PD of 2.50 ± 0.44 mm (range: 1.48–3.65 mm). The mean difference between apparent PD and 3D PD was 1.18 ± 0.47 mm (range: 0.36–2.56 mm), corresponding to a substantial mean magnification ratio of 47.2 ± 16.5% (range: 12.0–89.5%).
Statistical analysis demonstrated that both apparent PD and 3D PD exhibited non-normal distributions (Fig. 1). Apparent PD showed a positive skewness of 0.55 and kurtosis of 2.95 (Shapiro-Wilk test, P = 0.0168), while 3D PD demonstrated a skewness of 0.37 and kurtosis of 2.57 (Shapiro-Wilk test, P = 0.0462).
Distribution characteristics of apparent and three-dimensional pupillary diameter measurements. (A) Distribution histogram of apparent pupillary diameter. (B) Distribution histogram of three-dimensional pupillary diameter. (C) Quantile-Quantile plot of apparent pupillary diameter. (D) Quantile-Quantile plot of three-dimensional pupillary diameter.
Baseline characteristics stratified by gender are presented in Supplementary Table S1. Males were slightly older, had longer axial length and deeper anterior chamber depth, whereas females exhibited steeper corneas and larger apparent PD (all P < 0.05); no significant gender difference was observed for 3D PD (P = 0.108).
Comparison of apparent PD and 3D PD
Significant differences were observed between apparent PD and 3D PD in both the overall analysis and subgroup comparisons (Table 2). Apparent PD was consistently and significantly larger than 3D PD in all eyes and in every subgroup (all P < 0.001; Table 2). The mean difference increased with larger apparent PD (from 0.72 ± 0.18 mm in eyes with apparent PD < 3.0 mm to 1.93 ± 0.38 mm in eyes with apparent PD > 4.5 mm), corresponding to magnification ratios ranging from 36% to 64% (Table 2).
Correlations between PD measurements and clinical parameters
Apparent PD showed significant correlations with gender (3.46 ± 0.76 mm in males versus 3.82 ± 0.74 mm in females, P = 0.015; Table S2). Both PD difference (1.04 ± 0.46 mm in males versus 1.26 ± 0.46 mm in females, P = 0.005) and magnification ratio (43.2 ± 16.6% in males versus 49.6 ± 16.1% in females, P = 0.043) were significantly smaller in males. The 3D PD showed no gender-related difference (P = 0.108).
Age showed a weak negative correlation with PD difference (r = -0.19, P = 0.042) but no significant correlations with apparent PD, 3D PD, or magnification ratio (P = 0.061, P = 0.248, and P = 0.114, respectively). Other clinical parameters, including eye laterality, diabetes status, corneal WTW distance, CCT, three CARP values, ACD, SNR, AL, kappa and alpha angles, and BCVA, showed no significant correlations with any PD measurements (P > 0.05).
Apparent PD exhibited strong positive correlations with both 3D PD (r = 0.84, P < 0.001) and PD difference (r = 0.82, P < 0.001), and moderate correlation with magnification ratio (r = 0.51, P < 0.001). The relationship between apparent PD and 3D PD is illustrated in Fig. S2. Additionally, PD difference showed positive correlation with 3D PD (r = 0.40, P < 0.001).
GAMs were applied to explore potential nonlinear relationships between 3D PD and demographic or clinical variables (Fig. S3). However, no significant nonlinear associations were observed (P for nonlinearity > 0.05). Further analysis of the correlation between age and various ocular biological parameters revealed that age was negatively correlated with ACD (P = 0.011), SNR (P = 0.047), and kappa angle (P = 0.039), while showing a positive correlation with BCVA (P < 0.001; Table S3).
Multivariable linear regression analysis
To identify factors affecting 3D PD, a multivariable linear regression model was constructed based on correlation analysis results (Table S4). With 3D PD as the dependent variable, apparent PD, age, gender, and diabetes mellitus status were included as independent variables. Apparent PD emerged as the strongest predictor of 3D PD (P < 0.001), while age, gender, and diabetes status showed no significant effects (P = 0.501, P = 0.608, and P = 0.636, respectively).
Predicted MIOL suitability based on apparent vs. 3D PD assessment
In the assessment for MIOL suitability (Table 3), apparent PD-based evaluation categorized 90 eyes as suitable and 24 as unsuitable for MIOL implantation. However, 3D PD-based evaluation produced markedly different categorizations, with only 19 eyes predicted suitable and 95 as unsuitable. This represents a discrepancy in predicted suitability for 71 eyes (62.28%). Furthermore, predicted consistency between apparent PD and 3D PD was observed in 19 eyes (21.11%) for suitable MIOLs and 24 eyes (25.26%) for unsuitable MIOLs. Overall, the consistency between the two methods was found in 43 eyes (37.72%).
Agreement analysis between apparent PD and 3D PD
Agreement analysis revealed ICC values of 0.257 for single random raters and 0.714 for single fixed raters (P < 0.001; Table S5). With averaged measurements, ICC values improved to 0.408 for random raters and 0.833 for fixed raters (P < 0.001). Bland-Altman analysis demonstrated that 94.7% of measurements (108/114) fell within the limits of agreement, with six measurements (5.3%) outside these limits (Fig. 2).
Discussion
The primary objective of this study was to investigate the relationship between apparent PD and 3D PD in cataract patients. Our findings revealed that apparent PD was consistently larger than 3D PD, with the difference tending to increase with apparent PD—a mean magnification of 47.2%. These findings indicate the importance of considering magnification effects of apparent PD when interpreting conventional PD measurements, as it may overestimate the anatomical pupil size and influence IOL selection in cataract surgery.
To quantify these observations, quantitative analysis revealed a mean magnification of 47.2% between apparent PD and 3D PD. Previous studies have also demonstrated that apparent PD exceeds the anatomical PD, though with lower magnification rates. Specifically, using MS-39 AS-OCT (CSO, Italy), researchers reported apparent PD and 3D PD of 3.51 ± 0.71 mm and 3.13 ± 0.63 mm respectively, showing a 12% magnification5. A study using KR-9000 KR-9000 (Topcon, Japan) has found entrance pupil of 6.24 mm versus anatomical pupil of 5.44 mm, indicating a 14.7% magnification6. In keratoconus patients, TMS-1 topographer (Tomey, Japan) measurements showed apparent pupil exceeded anatomical pupil by 11–35% depending on disease severity7. The higher magnification rate observed in this study may be attributed to several factors. A key methodological difference is that our study measured anatomical PD directly through Pentacam AXL’s Scheimpflug imaging with 3D reconstruction, whereas previous studies relied on optical formulas for indirect calculations5,6,7. Additionally, variations in measurement illumination and image processing algorithms may have contributed to this difference18.
More importantly, the observed discrepancy between apparent PD and 3D PD had important implications for clinical decision-making regarding MIOL implantation. While 90 eyes were initially categorized as suitable for MIOL based on apparent PD, this number dropped to only 19 eyes when assessed by 3D PD, resulting in changed clinical selections for 62.28% of cases. The 2.8 mm photopic pupil diameter threshold applied in this study originates from an international expert Delphi consensus19. The consensus does not specify whether this 2.8 mm cutoff refers to conventional apparent pupil diameter or 3D/anatomical pupil diameter19. Therefore, direct application of the same numeric threshold to 3D PD may be overly conservative and requires prospective validation. This conservative outcome aligns with the Delphi consensus’s cautious recommendations, aimed at minimizing near-vision deficits in small-pupil patients19. Given the consistent ≈ 47% magnification of apparent PD observed in this study, we suggest prioritizing 3D PD as the clinical reference for pupil evaluation; When only apparent PD is available, a simple approximation of the anatomical pupil diameter may be obtained by dividing by ≈ 1.47. In clinical practice, however, the lower limit could be flexibly adjusted based on individual factors, as small pupils primarily impair near acuity with presbyopia-correcting IOLs without severe complications3. Furthermore, for small-pupil cases, pupil-independent IOLs such as Vivity DFT015 (Alcon), ZFR00 (Johnson & Johnson), and AT LISA tri 839MP (Zeiss) offer viable alternatives providing excellent presbyopia correction, whereas pupil-dependent models like AT LARA 829MP/929MP (Zeiss) and Tecnis Symfony ZXR00 (Johnson & Johnson) may require larger pupils for optimal performance3. Additionally, for patients scheduled for presbyopia-correcting IOLs, a scotopic PD < 6.0 mm is also recommended in addition to a photopic PD preferably > 2.8 mm, as excessively large pupils increase the risk of postoperative visual disturbances19.
The entrance pupil, as observed clinically, represents a apparent image of the anatomical pupil that undergoes anterior displacement and magnification due to the refractive power of the cornea6. This optical phenomenon resulted in the disparity between apparent PD and 3D PD observed in our study5. The magnitude of this magnification primarily depends on both corneal refractive power and ACD8. A higher refractive power (steeper corneal curvature) results in greater magnification, while a deeper anterior chamber also contributes to increased magnification5. Moreover, the disparity between apparent PD and 3D PD tends to increase with pupil size, possibly due to the decreased angle between the pupillary margin, corneal surface, and external observation point, which may enhance the optical magnification effect through the cornea8. The understanding of optical magnification effects is crucial for clinical applications, as it explains why simple pupillometry without considering these optical factors may overestimate the anatomical pupil size.
The positive correlation between 3D PD and apparent PD in our study aligns with previous research on topographic apparent pupil versus iris plane5 and entrance versus physical pupil measurements8, while the PD difference increased with pupil size, consistent with geometric optical analyses8. Demographic factors revealed gender differences in apparent PD and magnification (higher in females than males), with no gender effect on 3D PD; baseline analysis showed males had greater ACD but females higher corneal power in flattest and steepest meridians, where corneal power’s stronger influence on magnification8 explains females’ greater effect despite shallower ACD, validating Pentacam AXL’s 3D reconstruction accuracy. Similarly, age negatively correlated with PD difference but not 3D PD, aligning with age-related shallower ACD reducing magnification8. Reliability analysis indicated substantial agreement between apparent PD and 3D PD (ICC > 0.90 for averaged measures; 94.7% within Bland-Altman limits), suggesting consistency under standardized conditions.
This study has several strengths, including the direct and simultaneous acquisition of apparent and true 3D pupil diameters using Pentacam AXL Scheimpflug tomography in a single measurement—eliminating the indirect mathematical approximations required by prior methods—as well as comprehensive correlation analyses with multiple biometric parameters and demonstration of substantial clinical impact on MIOL selection.
Nevertheless, certain limitations should be acknowledged. Pupil measurements were performed only under standard Pentacam illumination, the cohort consisted predominantly of elderly Han Chinese patients in a single center, and scans of insufficient quality were excluded. Furthermore, the ≥ 2.8 mm mesopic/photopic suitability threshold used in the present study was derived from consensus statements referring to conventional apparent pupil diameter; its optimal cutoff when using true 3D PD, as well as potential modifications required for younger patients or different measurement devices and illumination levels, remains to be prospectively determined. Most importantly, this study did not assess postoperative visual or functional outcomes; therefore, the clinical implications of using 3D PD for MIOL selection remain inferential and require prospective validation in future longitudinal studies incorporating actual postoperative results. Accordingly, future investigations should also validate these findings in more diverse populations, evaluate pupil size across varying luminance conditions, and compare Pentacam 3D PD with other high-precision devices.
Conclusion
Apparent PD overestimates 3D PD by approximately 47% in cataract patients, potentially leading to inappropriate MIOL selection. Clinicians should account for this magnification effect in preoperative assessments to optimize IOL choices and surgical outcomes.
Data availability
The original datasets used in this study have been deposited in the Zenodo repository with the persistent identifier DOI 10.5281/zenodo.14736998.
References
Hashemi, H., Fayaz, F., Hashemi, A. & Khabazkhoob, M. Global prevalence of cataract surgery. Curr. Opin. Ophthalmol. 36 (1), 10–17. https://doi.org/10.1097/ICU.0000000000001092 (2025).
Wu, P. et al. Influence of ocular biometric parameters such as effective lens position, keratometry, and axial length on near add power of multifocal intraocular lens. J. Cataract Refract. Surg. 48 (11), 1331–1334. https://doi.org/10.1097/j.jcrs.0000000000000947 (2022).
Rampat, R. & Gatinel, D. Multifocal and extended depth-of-focus intraocular lenses in 2020. Ophthalmology 128 (11), E164–E185. https://doi.org/10.1016/j.ophtha.2020.09.026 (2021).
Ouchi, M. & Shiba, T. Diffractive multifocal intraocular lens implantation in eyes with a small-diameter pupil. Sci. Rep. 8, 11686. https://doi.org/10.1038/s41598-018-30141-1 (2018).
Camellin, U. et al. Estimation of pupil size at Iris plane and its magnification after cataract surgery. Indian J. Ophthalmol. 72 (SUPPL 5), S831–S837. https://doi.org/10.4103/IJO.IJO_544_24 (2024).
Kamiya, K., Shimizu, K., Igarashi, A. & Ishikawa, H. Evaluation of pupil diameter after posterior chamber Phakic intraocular lens implantation. Eye 24 (4), 588–594. https://doi.org/10.1038/eye.2009.170 (2010).
Langenbucher, A., Neumann, J., Kus, M. M. & Seitz, B. Calculation of pupil localization and dimension in keratoconus using exact Raytracing of corneal topography height data. Klin. Monatsbl. Augenheilkd. 215 (3), 163–168. https://doi.org/10.1055/s-2008-1034693 (1999).
Freedman, K. A., Brown, S. M., Mathews, S. M. & Young, R. S. L. Pupil size and the ablation zone in laser refractive surgery: considerations based on geometric optics. J. Cataract Refract. Surg. 29 (10), 1924–1931. https://doi.org/10.1016/S0886-3350(03)00214-1 (2003).
OCULUS & Pentacam AXL Instruction Manual. Article no. G/70020/EN, Rev. 08. Wetzlar: OCULUS Optikgeräte GmbH. https://documents.oculus.de/G-70100-EN-04.pdf (2022).
Davidova, P., Biller, M., Ademmer, V. & Kohnen, T. Pupil size measurements with a multifunctional aberrometer/coherence interferometer/tomographer and two infrared-based pupillometers. J. Cataract Refract. Surg. 50 (6), 631–636. https://doi.org/10.1097/j.jcrs.0000000000001432 (2024).
Wen, B. et al. The 2024 revision of the declaration of helsinki: A modern ethical framework for medical research. Postgrad. Med. J. https://doi.org/10.1093/postmj/qgae181 (2024). (Early Access)).
Simsek, A. & Toptan, M. The evaluation of pupil diameter by using Sirius before and after phacoemulsification in healthy, diabetic and hypertension patients. Medicine 102 (16), e33223. https://doi.org/10.1097/MD.0000000000033223 (2023).
Shankar, H., Taranath, D., Santhirathelagan, C. T. & Pesudovs, K. Anterior segment biometry with the pentacam: comprehensive assessment of repeatability of automated measurements. J. Cataract Refract. Surg. 34 (1), 103–113. https://doi.org/10.1016/j.jcrs.2007.09.013 (2008).
Redondo, B. et al. Diurnal variation in accommodation, binocular vergence, and pupil size. Optom. Vis. Sci. 100 (12), 847–854. https://doi.org/10.1097/OPX.0000000000002091 (2023).
Lu, C. D. et al. Photoreceptor layer thickness changes during dark adaptation observed with ultrahigh-resolution optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 58 (11), 4632–4643. https://doi.org/10.1167/iovs.17-22171 (2017).
Bunce, C. et al. Ophthalmic statistics note 14: method agreement studies in ophthalmology: the intraclass correlation coefficient? Br. J. Ophthalmol. 104 (8), 1033–1035. https://doi.org/10.1136/bjophthalmol-2019-314947 (2020).
Chatfield, M. D. et al. Blandaltman: A command to create variants of bland-altman plots. Stata J. 23 (3), 851–874. https://doi.org/10.1177/1536867X231196488 (2023).
Altan, C. et al. Comparison of 3 pupillometers for determining scotopic pupil diameter. Eur. J. Ophthalmol. 22 (6), 904–910. https://doi.org/10.5301/ejo.5000150 (2012).
Romano, V. et al. Recommendation for presbyopia-correcting intraocular lenses: A Delphi consensus statement by the Esaso study group. Am. J. Ophthalmol. 253, 169–180. https://doi.org/10.1016/j.ajo.2023.05.002 (2023).
Acknowledgements
We would like to express our sincere gratitude to all the participants who contributed to this research. We also thank the staff at Chongqing Emergency Medical Center for their support throughout the study.
Author information
Authors and Affiliations
Contributions
Bin Wang: Methodology, Software, Visualization, Investigation, Validation, Writing—Original draft preparation; Zaihong Chen: Supervision, Writing—Reviewing and Editing; Huafeng Ma: Conceptualization; Lin Wang: Data curation. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
This study was approved by the Ethics Committee of Chongqing Emergency Medical Center(IRB No. 2025-16). All participants provided informed consent and signed consent forms prior to their participation in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, B., Ma, H., Wang, L. et al. Comparison of apparent and three-dimensional pupillary diameter measurements in cataract patients and their impact on multifocal intraocular lens selection. Sci Rep 16, 5064 (2026). https://doi.org/10.1038/s41598-026-35975-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-026-35975-8