Abstract
Auditory events occurring in the peripersonal space (PPS) or near specific body parts such as the peritrunk (PTS) and perihand (PHS) space, have been shown to facilitate tactile processing in a somatotopic manner. Furthermore, previous research has demonstrated that audio-tactile intersensory effects are influenced by the execution of full body transport movements (i.e., walking or cycling), so that far-off auditory stimuli that approach the body in a direction coherent with the movement facilitate tactile processing on the body. However, whether these motor-related intersensory effects are modulated by non-transport movements is not known. Here, in two experiments, we sought to determine whether audio-tactile intersensory effects for looming sounds in the PHS and PTS are somatotopically modulated by arm movement compared to when being still. By controlling the role of temporal expectation, we confirm that looming sounds enhance tactile reactivity as they approach the participants’ hand and trunk. We further show that this facilitation is eliminated by limb movements, regardless of somatotopy, and that executing movements blur the distinction between intersensory effects in extrapersonal and peripersonal space. These results contribute to understanding the complex relation between motor execution and intersensory processing.
Similar content being viewed by others
Introduction
Interacting with the environment requires the brain to continuously integrate large amounts of multisensory information from both the external world and the body. Events occurring within the space immediately surrounding the body, known as the peripersonal space (PPS), are particularly relevant for selecting effective actions to achieve goals1 or to execute defensive behaviors2,3. Perception and action are tightly intertwined: coherent perception of the environment guides motor behavior, while motor planning and execution, in turn, influence sensory processing4,5 and perception6. Consequently, perception and action are regarded as interdependent functions7,8,9.
The impact of movement execution on perception is especially evident in the tactile domain. Movement planning and execution can both facilitate10,11,12 and reduce13,14,15,16 tactile processing through gating/suppression/attenuation mechanisms17,18,19,20, which are characterized by a reduced detection rate21, decreased subjective intensity of externally generated stimuli17 and lower detection precision15, when the stimulated body part is moving compared to when it is static. Although sometimes gating/suppression and attenuation are used interchangeably, they are other times described as different mechanisms where gating/suppression refers to a reduction of sensory processing for irrelevant or redundant stimuli, while attenuation refers to reduced sensory processing for self-generated sensations or sensory consequences of own movements and actions19,20,22. These effects may depend on central and/or peripheral mechanisms, as suggested by studies using active movements (highlighting central processes)13,23,24 and passive movements (highlighting peripheral contributions)25. The interplay between movement execution and tactile gating has been shown during upper-limb motion: tactile suppression at the finger emerges once the elbow movement reaches a critical speed, a threshold typically not reached during exploratory actions that rely on sensory feedback17,21,26,27,28. Interestingly, blind individuals exhibit stronger tactile suppression during active finger movements than during passive conditions29, suggesting a modulatory role of visual calibration in motor–sensory coupling. Additionally, the context and goal of a movement, and the relevance of tactile feedback for guiding it, critically shape the timing and somatic specificity of tactile facilitation or inhibition15,16,30,31,32,33.
Although movement-related effects on unisensory (tactile) processing are well established, it remains unclear whether and how motor execution modulates intersensory (multisensory) processes34, and whether such effects depend on peripheral gating/suppression or on central mechanisms of sensory attenuation and/or multisensory integration. Multisensory integration underlies coherent perception and effective action control35,36. The spatial dependency of visuo- and audio-tactile effects has been used to map the extent of PPS, reflected in behavioral1 and neural37 modulations of tactile responses elicited by nearby visual or auditory cues. PPS representations are thought to be body-part specific-perihand (PHS), peritrunk (PTS), and periface (PFS), and anchored to their respective effectors1,38.
A well-documented multisensory phenomenon is the facilitation of tactile perception by concurrently presented looming visual39,40,41 or acoustic stimuli42,43,44. Looming sounds also increase corticospinal excitability45, consistent with the idea that such stimuli activate defensive motor responses2. Approaching stimuli thus enhance tactile processing at the location where the external stimulus is expected to impact the body39,40. Moreover, movement preparation and execution can extend these facilitatory effects to far locations when those locations correspond to movement targets. For instance, visual cues on an object that a person is reaching for facilitate tactile perception on the approaching fingertip46,47,48. Similarly, tactile perception on the chest improves during cycling when visual cues appear at distant locations49,50, and comparable effects have been shown for audio-tactile interactions during walking51. In this case, looming sounds enhance chest tactile processing even for far sound sources, an effect also reproduced when auditory cues merely simulate walking52. A similar multisensory-motor coupling has been observed in studies showing that sounds modulate the preparation of ecological movements such as stepping53.
Previous work has examined audio-tactile interactions mainly in relation to whole-body movements and sound sources. However, the influence of limb movement on audio-tactile integration, particularly when tactile stimuli are applied to either the moving limb or a static body part (chest), has not been addressed. This approach can reveal whether motor-related multisensory effects are (1) somatotopic, depending on spatial correspondence between the moving effector and the stimulation site, or (2) central, reflecting more general motor-planning mechanisms. It may also clarify how perihand and peritrunk spaces are dynamically represented during arm movement, a question especially relevant for blind individuals, who rely more on nonvisual cues when interacting with sonified objects.
In this study, we addressed these questions across two experiments. Experiment 1 tested whether the well-known facilitation of tactile perception by nearby sounds154 is modulated by active arm movements directed toward the sound source. Experiment 2 examined whether these sensorimotor effects differ for approaching vs. receding sounds42,43,55,56 and controlled for expectancy effects57,58. Both experiments assessed whether audio-tactile facilitation depends on the relationship between the moving effector and the tactile stimulation site (hand vs. chest)21, helping to determine whether the effects are peripheral or central in origin. Based on previous work49,50,51, we also tested whether arm movement modulates tactile detection even when sounds occur at far distances, consistent with the dynamic, hand-centered coding of PPS38.
Understanding how movement influences multisensory (audio-tactile) processing is crucial for elucidating how sensory modalities are weighted during action. This study contributes to our understanding of multisensory processing in dynamic contexts by employing a motor related experimental paradigm designed to approximate everyday naturalistic interactions with the environment. Beyond theoretical implications, these findings have practical relevance for rehabilitation, assistive haptic technologies, and action-guided systems for blind individuals, whose tactile processing during movement differs markedly from that of sighted people59. Such insights are also valuable for designing training, learning, and immersive technologies, as modern environments increasingly rely on multisensory and interactive systems.
Methods
Participants
The experimental protocol was approved by the ethics committee of CET Area 5 Lazio (Prot. N. 7183) and was conducted in agreement with the 1964 Declaration of Helsinki. Canzoneri et al.42 had a sample of 17 participants. Kuroda & Teramoto49,50 performed an a priori power analyses and recruited 24 and 23 participants in 2 × 5 and 3 × 3 within-subject designs, respectively. Our hypothesized effect of interest concerned a 2 × 2 × 2 × 5 interaction between Body part (trunk vs. hand), Hand status (motor vs. static), Sound direction (looming vs. receding), and Sound Distance (D1 vs. D2 vs. D3 vs. D3 vs. D5), and has more conditions (i.e., 40) than previous studies. We thus adopted a sample size of 31 participants (19 females, mean age = 27 years, range 22–37) in Experiment 1 which is slightly larger than those of previous studies (n = 25) to be able to describe a small effect size (Cohen’s f = 0.1)60 with α error probability = 0.05 and power [1-β error probability] = 0.80. Since in the analysis of Experiment 1 we reduced the number of conditions from 40 to 12 and we found the significant main effect of Sound Intensity Distance (on 3 conditions) with an effect size of 0.22, in Experiment 2 we aimed at finding the same effect size of 0.22 (with α error probability = 0.05 and power [1-β error probability] = 0.80) but on 5 conditions (Sound Distance) for which the number of participants turned to be 24 (14 females, mean age = 26.5 years, range 22–32). None of them had psychiatric issues or neurological conditions and all of them were right-handed. All participants reported normal touch and hearing and all of them gave their informed consent to take part in these studies.
Stimuli and apparatus
Acoustic stimuli
In Experiment 1 and 2, auditory stimuli were emitted by two speakers positioned along the sagittal plane in front of the participant, 100 cm apart from each other. The speaker closest to the participant was placed 10 cm from the participant’s hand resting on the table. Each stimulus consisted of a 3000 ms (Experiment 1) and 4000 ms (Experiment 2) pink noise sound. We decided to make the sound longer in Experiment 2 to reduce any possible confounds in RTs of motor conditions due to tactile stimulus at T1 being presented too early which may find participants not yet in movement (more clarification in the next section). Pink noise samples at 44.1 kHz were generated using MATLAB (2022b) and Audacity 2.4.1. Following the procedure of Canzoneri et al.42, we generated two stimuli that were combined in the two near and far speakers in each trial in order to obtain looming and receding sounds; one stimulus had an exponential increase in acoustic intensity from 55 to 70 dB Sound Pressure Level (SPL), measured with an audiometer at the subjects’ ears, while the other stimulus had an exponential decrease from 70 to 55 dB. For looming sounds, the far speaker started at maximum intensity and decreased to silence, while the near speaker started at minimum intensity and increased to maximum. This process was reversed for receding sounds. Thus, looming sounds conveyed the impression (see Supplementary Fig. 1) of a source moving towards the participant’s body, while receding sounds suggested a source moving away from the participant (Fig. 1a).
Tactile stimuli
The electro cutaneous stimuli were produced by a Constant Current Stimulator (Digitimer, model 144 DS7A, United Kingdom, Welwyn) and a remote electrode selector was used to activate the desired electrode(s) (Digitimer, model D188, United Kingdom, Welwyn). Two electrodes were placed next to each other, both on the surface of participant’s radial aspect of the right index finger or on the centre of the participant’s chest. These two sites are distal and proximal, respectively, with respect to the limb used for the experimental movement (see below) and may provide information on the role of peripheral and central mechanisms of sensory suppression21. The electrical stimulus used was a single, constant voltage rectangular monophasic pulse with 100µs duration for the trunk and 200µs for the finger. Before the experiment, the intensity of the tactile stimulus was calibrated for each individual via an ascending method of limit. This calibration involved setting the stimulator to its minimum intensity and then progressively increasing it until the person confirmed that they could distinctly feel the stimulation over their finger and over their chest separately. Subsequently, participants underwent a series of 10 stimuli and were required to report when they felt the tactile stimulus. If the participant did not perform this task accurately detecting 10 out of 10 stimulations, the intensity was increased in steps of 5–10 mA, and the procedure was repeated. In line with previous studies42,50,51, the final intensity was set to be clearly detectable yet never painful. Because the study employed a within-subject design, these individually tailored thresholds do not systematically bias RTs across conditions. Moreover, before the start of each block of trials participants’ thresholds were consistently reevaluated performing the initial assessment. Additionally, if participants perceived weaker stimulation during the middle of a block due to habituation, they were encouraged to report it. In such cases, the task was paused and the threshold was reassessed, as in previous studies42,43,50. Since the experimental conditions were counterbalanced across participants, we expect no potential influence of threshold re-evaluation on the pattern of results.
In Experiment 1, during audio-tactile blocks participants received a tactile stimulation at one of five different temporal delays (T1 at 300 ms, T2 at 800 ms, T3 at 1500 ms, T4 at 2200 ms and T5 at 2700 ms) from the sound onset, as depicted in Fig. 1b. Conversely, during unisensory blocks, they received a tactile stimulation only at two different temporal delays (T1 at 300 ms and T5 at 2700 ms) from the trial initiation as in Canzoneri et al.41. In each block of trials, tactile stimuli were administered only to the trunk or only to the right index finger.
In Experiment 2, the duration of the auditory stimuli was increased but the same intervals between the five temporal delays were maintained from Experiment 1 resulting in tactile stimulations occurring at the following timings from the onset of the sound: T1 at 800 ms, T2 at 1300 ms, T3 at 2000 ms, T4 at 2700 ms, and T5 at 3200 ms (see Fig. 1b). This change made the first tactile stimulus arrive after 800 ms from sound onset instead of 300 ms as in Experiment 1 thus reducing the challenge of perceiving the stimulus at all. Moreover, differently to Experiment 1, in Experiment 2 the unisensory baseline condition included all the 5 temporal delays at which the tactile stimulation occurred during the audio-tactile condition. Also in Experiment 2, in each block of trials tactile stimuli where either delivered to the trunk or to the finger.
General procedure
In both Experiment 1 and 2, upon arrival at the laboratory, after having given their informed consent, participants were blindfolded and equipped with superficial electrodes on the chest and on the index finger of the right hand. They were informed that they would experience tactile stimuli over their chest or right index finger alone (Unisensory condition) or paired with auditory stimuli (the specific nature of which was not disclosed) (audio-tactile condition). Participants were instructed that the auditory stimuli were irrelevant to the task, emphasizing that their primary goal was to promptly and accurately respond to tactile stimuli by pressing a designated key on the keyboard with their left hand (Fig. 1a) as in44,49,50,51. The keyboard used was a logitech k120 usb-type with low-profile membrane keys with an effective polling rate of 10 ms and an average latency of 19.9 ms. Participants were made aware that some trials (catch trials) within the audio-tactile blocks would only involve auditory stimuli without any tactile stimulation. Blindfolded participants were instructed to hold a mouse with their right hand throughout the experiment, and to move it on a sagittal plane in front of them in the motor conditions of the experiment or remaining still in static conditions (Fig. 1c). Before starting the experimental sessions, participants were trained to move the mouse in a straightforward direction at a constant speed for the entire duration of the auditory stimulus, starting the movement once the sound began and stopping it once the sound was over, regardless of the sound direction (Fig. 1a and c). In both Experiment 1 and 2 participants were trained to reach a barrier placed 30 cm away on the table by the time the sound ceased (3 s for Experiment 1 and 4 s for Experiment 2). Thus, the average speed for Experiment 1 is 10 cm/s and for Experiment 2 is 7,5 cm/s. Although movements were standardized, this setup was designed to approximate everyday human-environment interactions, where upper limb movements serve as primary effectors for engaging with objects. Movement execution was tracked with a customized script on E-prime61 at 20 Hz. Each trial started when participants pressed the left key of the mouse in Experiment 1 and when they released a pedal in Experiment 2.
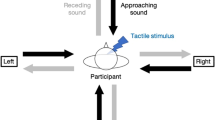
The image illustrates the experimental setups of the audio-tactile detection task of both Experiment 1 and 2. (a) It shows how looming and receding sounds are generated from the two speakers; moreover, the image represents how blindfolded participants respond to tactile stimuli using their left hand on a keyboard while holding a mouse with their right hand. (b) The timing of tactile stimulus delivery is represented in relation to the initiation of the dynamic sound of different length for Experiment 1 (3000 ms) and Experiment 2 (4000 ms). This setup ensures that the sound is perceived at varying distances from the participant’s body when the tactile stimulus is received. (c) The illustration depicts the four experimental conditions administered to participants for both Experiments 1 & 2. The terms “trunk” and “hand” indicate the location of the tactile stimulus, while “static” and “motor” specify whether the participant’s right hand was instructed to remain stationary or to move.
Design and statistical analysis
Design
The experimental design of Experiment 1 had four within-subject factors in the audio-tactile conditions: Body Part (trunk vs. hand), Hand Status (motor vs. static), Sound Direction (looming vs. receding), and Sound Distance (D1 vs. D2 vs. D3 vs. D3 vs. D5) (note: sound distances correspond to where the sound is spatially perceived when a tactile stimulus is delivered; thus T1 to T5 correspond to D1 to D5 for receding trials respectively, while the correspondence is flipped for looming sounds, that is, T1 to T5 correspond to D5 to D1). Throughout the paper, the terms “trunk” and “hand” designate the location of the tactile stimulus, while “static” and “motor” indicate whether the right hand is still or moves (note: the right hand is also the stimulated hand) (Fig. 1c). Each combination of the factors’ levels was tested with 15 trials, gathered into four blocks diversified for the body part stimulated and the hand status which were tested in separated blocks: trunk motor, trunk static, hand motor, and hand static. Within each block, both looming and receding auditory trials were included; thus, each block consisted of 15 trials for each of the 2 Sound Direction × 5 Sound Distance conditions + 16 catch trials, with a total of 166 trials (Table 1).
Additionally, four unisensory blocks were presented before and four after the audio-tactile blocks. The 8 unisensory blocks had three within-subject factors: Body Part (trunk vs. hand), Hand Status (motor vs. static), Temporal delay (T1 and T5) counterbalanced across participants. Each unisensory block comprised 6 trials of tactile stimulation at each of two different Temporal delays conditions (T1 and T5). In total, there were 8 unisensory blocks × 12 trials = 96 trials, and 4 audio-tactile blocks × 166 trials = 664 trials, resulting in a grand total of 760 trials. The presentation of blocks was randomized and fully counterbalanced across participants.
In Experiment 2 the number of trials per condition was diminished to 10 trials for each condition and the number of unisensory blocks was reduced to 4 with 5 temporal delays for the tactile stimulation (Table 1). Thus, the 4 audio-tactile blocks consisted of 110 trials [(2 Sound Direction x 5 Sound Distance) x 10 + 10 catch trials], and the 4 unisensory blocks included 50 trials (5 Temporal Delays x 10). In total, there were 4 unisensory blocks × 50 trials = 200 trials, and 4 audio-tactile blocks × 110 trials = 440 trials, resulting in a grand total of 640 trials. The presentation of blocks was randomized and fully counterbalanced across participants (Table 1).
Statistical analyses
Audio-tactile detection task
Statistical analyses for Experiments 1 and 2 were conducted using R (version 4.3.0). Reaction times (RTs) served as the primary measure to assess the influence of auditory stimuli and movement execution on tactile detection. RTs shorter than 100 ms or longer than 1000 ms were excluded56,62 and RTs exceeding ± 2 standard deviations from the individual mean within each experimental condition were also removed as outliers. This step excluded 5.5% of trials in Experiment 1 and 4.1% of trials in Experiment 2 (analysis with all outliers included were run as a double check and can be found in the Supplementary material, Supplementary Figs. 5–8). Furthermore, participants who failed to respond to 50% of tactile stimuli across the experiment were excluded, leaving 30 participants in Experiment 1 (1 excluded) and 24 participants in Experiment 2 (no participant was excluded).
Consistent with previous findings57,58,63, our data showed an expectancy effect for both looming and receding sound conditions. Specifically, RTs systematically decreased at longer temporal intervals between trial onset and tactile stimulation (Supplementary Fig. 2). As demonstrated by Kandula et al.57, such expectancy effects arise due to a hazard function, defined as the conditional probability of an event occurring as time progresses, accounting for temporal uncertainty in perception64. Thus, although facilitatory multisensory audio-tactile effects have been previously computed by subtracting the RT mean value of the fastest condition between T1 and T5 of the unisensory baseline conditions to the multisensory RTs42,43, a consistent approach to remove expectancy effects from the RTs is to perform a subtraction between two conditions that account for the same expectancy but differ for the dimension(s) that is under investigation, i.e., the spatial location of sounds and their direction57,65. Therefore, to address potential confounds and isolate the effects of sound distance (near vs. far) on tactile processing, we adopted the method described in Noel et al.58. This approach involves subtracting RTs across temporally-, and thus expectancy, matched looming and receding conditions, always placing the closer sound condition as the first term of the subtraction [e.g., (looming T5/D1 - receding T5/D5); (looming T4/D2 - receding T4/D4); (receding T2/D2 - looming T2/D4); (receding T1/D1 - looming T1/D5)]. For the third interval (T3), half of the data were calculated as (looming T3/D3 - receding T3/D3) and the other half as (receding T3/D3 - looming T3/D3). These five intervals describe RTs as a function of the distance and direction (not the expectancy) of the sound and are symmetrical around the central one in terms of the direction of the sound, i.e., the first two interval index approaching sounds and the last two capture receding ones. Then, to isolate the effect of sound distance, we averaged expectancy-corrected RTs across pairs of looming and receding conditions involving the same spatial distance difference (i.e., the first D1 - D5 and fifth D5 - D1; the second D2 - D4 and the fourth D4 - D2). This ensured that the resulting index reflected only the impact of sound distance, not that of sound direction. At the end of this step, the five original intervals were thus reduced to three conditions, each representing a specific level of sound distance contrast: (1) strong intensity difference, comparing a very near sound (D1) to a far one (D5); (2) medium intensity difference, comparing a moderately near sound (D2) to a far one (D4); and (3) no intensity difference, using balanced intermediate distances (D3), where the auditory intensity was constant. Negative values of the index for medium and strong intensity differences indicate tactile facilitation for close compared to far sounds (i.e., the second term of the subtraction generate longer RTs compared to the first one), while a value of zero reflects no facilitation of tactile processing. At the same time, the index should assume the value zero if the two terms of the subtraction have the same sound intensity as in the no intensity difference case.
Expectancy-corrected RT were analysed with a generalized linear mixed-effects model (GLMM) in R (lmerTest package66, with the most complex structure that did not give singularities that had the following form:
Following the GLMM analysis, ANOVAs were conducted to examine main and interaction effects. When appropriate Tukey-corrected post-hoc pairwise contrasts were performed using the emmeans R package67. The following statistical analysis was performed also for Experiment 2 and for the mouse tracking investigation.
In Experiment 2, differently from Experiment 1, unisensory baseline trials were included for all five temporal delays from sound onset and could thus be used to eliminate expectancy-related effects (Supplementary Fig. 2). Multisensory audio-tactile and unisensory tactile trials, however, were conducted in separated blocks, with only audio-tactile blocks containing catch trials which abolished the facilitation for multisensory trials compared to unisensory ones (Supplementary Fig. 4). Thus, to create expectancy-corrected RT, unisensory RTs of each of the five time intervals were subtracted from those of the corresponding audio-tactile condition separately for looming and receding conditions. Expectancy-corrected RTs were analyzed using a generalized linear mixed-effects model modelled with the most complex structure that did not give singularities, i.e., Body Part (trunk vs. hand), Hand Status (motor vs. static), Sound Direction (looming vs. receding), and Sound Distance (D1 vs. D2 vs. D3 vs. D3 vs. D5) and their interaction as fixed effects, and random intercepts and slopes for Hand Status and Body Part and their interaction for each subject. The model had the following structure:
Linear fitting for experiment 2
To evaluate the spatial effect of sound distance on tactile processing between near and far spaces as a function of looming and receding directions, in Experiment 2 we adopted the approach used in Bertoni et al.68. Expectancy-corrected RTs at the five spatial intervals (D1 - D5) for each experimental condition (hand/trunk, static/motor, looming/receding) were fitted with a linear function (Eq. 1):
Here, a represents the intercept, while b denotes the slope of the function. The slope serves as a quantitative measure of the effect of sound proportional distance and looming/receding direction on tactile processing69,70. Higher slope values reflect a stronger modulation of multisensory processing based on the spatial position of the sound and its looming/receding direction, indicating a more pronounced differentiation between the near space (PPS) and the far space (EPS).
Mouse tracking
To assess whether participants moved constantly throughout the trial in all motor conditions in both Experiment 1 and Experiment 2, their hand movements were monitored by tracking the vertical coordinates of the mouse pointer along the median axis of the display (in pixels). This was achieved using a customized E-Prime script. The collected data underwent pre-processing cleaning step based on the same criteria used for the reaction time (RT) analysis. Additionally, trials were excluded if the tracking system reported an error (< 1% of trials) and if participants didn’t start the movement. Cleaned trials were normalized on a trial-by-trial basis. Normalization ensured that each trial’s coordinates were scaled relative to its own minimum and maximum values (i.e., from 0 to 1). Only the coordinates corresponding to the moment of when the tactile stimulus was delivered were extracted, allowing for the determination of the hand’s position at the time of tactile stimulation. These normalized coordinates were modelled with a mixed model having Body Part (trunk vs. hand), Sound Direction (looming vs. receding), Temporal Delay (T1-T5), and their interaction as fixed effects, and random intercepts and slopes for Sound Direction and Body Part for each subject. The model had the following structure:
Results
Experiment 1
The 2 Body Part (hand vs. trunk) × 2 Hand Status (motor vs. static) × 3 Sound Intensity Difference (strong vs. medium vs. no difference) mixed-model on expectancy-corrected (i.e., looming - receding) RTs showed a significant main effect of Hand Status (F(1, 290) = 6.17, p = 0.01, \(\:{\eta\:}_{p}^{2}\) = 0.02) indicating that tactile RTs were faster during static compared to motor conditions (Fig. 2a). Also, the main effect of Sound Intensity Difference was significant (F(2, 290) = 7.09, p = 0.0009, \(\:{\eta\:}_{p}^{2}\) = 0.05), indicating that participants responded more quickly when the sound intensity difference was medium compared to when it was absent (p = 0.0006) while no difference appeared between medium and strong intensity difference (p = 0.10) nor between strong and no intensity difference (p = 0.19) (Fig. 2b). The lack of a stronger facilitation for the strong intensity difference condition may be due to the fact that the RT subtraction terms included early and late tactile time intervals, which could have occurred very early or very close to sound end relative to the trial onset, when participants might have been surprised and/or less reactive (see Discussion). Supplementary analyses including all outlier trials showed the same pattern for the Hand Status factor, although the effect did not reach significance (Supplementary Fig. 5a). For Sound Intensity Difference, the same trends and statistical significance were replicated, and the contrast between strong and no intensity difference also reached significance (Supplementary Fig. 5b). Together, these findings suggest that extreme RT values, likely reflecting anticipation or distraction, can obscure subtle intersensory effects.
Experiment 1 results. The graph illustrates the significant main effect of Hand Status (a) and of Sound Intensity Difference (b) from the mixed-model ANOVA. (note: “ID” stands for “intensity difference”). Error bars represent the standard error of the mean (S.E.M.) (‘***’ p < 0.001, ‘**’ p < 0.01, ‘*’ p < 0.05).
Experiment 2
RTs analysis
The 2 Body Part (hand vs. trunk) × 2 Hand Status (motor vs. static) × 2 Sound Direction (looming vs. receding) x 5 Sound Distance (D1 vs. D2 vs. D3 vs. D4 vs. D5) mixed-model showed a significant main effect of Sound Distance (F(4, 828) = 2.58, p = 0.04, \(\:{\eta\:}_{p}^{2}\) = 0.01), but no post-hoc comparison turned out to be significant (all ps > 0.05) (Fig. 3a). Crucially, a significant triple interaction between Hand Status, Sound Distance and Sound Direction was found (F(4, 828) = 3.84, p = 0.004, \(\:{\eta\:}_{p}^{2}\) = 0.02). Post-hoc comparisons indicated that only during looming static conditions, participants were faster in responding to a tactile stimulus when the sound was perceived at close distances (D1 and D2) compared to far ones (D1 v s D5, p = 0.003; D2 vs. D5, p = 0.0024) (Fig. 3b). Further supplementary analyses including all outlier trials support the present findings (Supplementary Fig. 7).
These results are consistent with the findings of Experiment 1 and suggest that participants exhibited faster tactile stimulus detection only when being static and perceiving a close looming sound compared to when perceiving a far looming sound. This replicates the earlier effect of spatial proximity on reaction times under static conditions. Importantly, the current findings also extend this effect by showing that the facilitation of tactile detection by close versus far sounds occurs specifically in the context of looming sounds but not receding sounds. Moreover, this differentiation was not observed under motor conditions, implying that movement attenuates the influence of sound distance on RTs.
Linear fitting of RTs
We applied Eq. 1 to model the relationship between normalized expectancy-corrected tactile RTs and the distance of a perceived sound for each participant in Experiment 2. We extracted and analyzed the slope of the linear fit using a generalized linear mixed-effects model to compare how hand movement affect intersensory effects in near/far space during looming and receding sound presentation compared to static conditions. Thus, an ANOVA was conducted to assess the impact of the factors: 2 Body Part (hand vs. trunk) × 2 Hand Status (motor vs. static) x 2 Sound Direction (looming vs. receding) on the dependent variable.
The analysis revealed a significant Hand Status x Sound Direction interaction (F(1, 184) = 7.79, p = 0.005, \(\:{\eta\:}_{p}^{2}\) = 0.04), indicating that in the static condition, looming sounds generated a steeper slope compared to the motor condition (p = 0.023), and that in the static condition looming sounds generated steeper slopes compared to receding sounds (p = 0.011), i.e., looming sounds had a strong spatial-dependent facilitatory effect on RTs. No difference was found during the presentation of receding sounds between static and motor conditions (p = 0.7) (Fig. 3c and d).
Experiment 2 results. (a) The graph illustrates the significant main effect of Hand Status from the mixed-model ANOVA. (b) The graph illustrates the significant interaction between Hand Status, Sound Distance and Sound Direction from the mixed-model ANOVA. Significant post-hoc results are represented in the figure, showing faster RTs when a looming sound was near (D1 and D2) compared to when it was far (D5), but only in the static condition. (c & d) The graphs illustrate the slope steepness of the linear model across experimental conditions, highlighting the significant interaction between Sound Direction and Hand Status revealed by the mixed-model ANOVA. Error bars represent the standard error of the mean (S.E.M.) (‘***’ p < 0.001, ‘**’ p < 0.01, ‘*’ p < 0.05).
Mouse tracking analysis of experiment 1 & 2
In Experiment 1, a 2 (Body Part: hand vs. trunk) × 2 (Sound Direction: looming vs. receding) × 5 (Temporal Delay: T1 vs. T2 vs. T3 vs. T4 vs. T5) mixed-model analysis on normalized mouse coordinates revealed a significant main effect of Sound Direction (F(1, 27.7) = 63.57, p < 0.001, \(\:{\eta\:}_{p}^{2}\) = 0.52) indicating that participants reached farther distances during the receding condition compared to the looming condition (Fig. 4a). There was also a significant main effect of Temporal Delay (F(4, 7244.2) = 55485, p < 0.001, \(\:{\eta\:}_{p}^{2}\) = 0.97), indicating consistent differences in the distances reached across different tactile stimulation timings (all ps < 0.001; Fig. 4b). Additionally, a significant double interaction between Sound Direction and Temporal Delay was found (F(4, 7243.6) = 17.1, p < 0.001, \(\:{\eta\:}_{p}^{2}\) = 0.01). Post-hoc comparisons indicated that participants reached farther distances during receding sounds compared to looming sounds only at intermediate tactile stimulation timings (T2, T3, and T4; p < 0.001; Fig. 4c). A significant interaction between Body Part and Temporal Delay was also observed (F(4, 7243.2) = 4.37, p < 0.001, \(\:{\eta\:}_{p}^{2}\) = 0.002) though post-hoc tests revealed no significant differences between hand and trunk conditions across temporal delays (T1, p = 1; T2, p = 0.6; T3, p = 0.52; T4, p = 0.13; T5, p = 1).
In Experiment 2, the same mixed-model analysis similarly showed a significant main effect of Sound Direction (F(1, 21.9) = 22.21, p < 0.001, \(\:{\eta\:}_{p}^{2}\) = 0.50) with participants again reaching farther distances during receding sounds compared to looming sounds (Fig. 4e). Also, the main effect of Temporal Delay was significant (F(4, 4334.7) = 14477, p < 0.001, \(\:{\eta\:}_{p}^{2}\) = 0.93), indicating consistent differences in the distances reached across different tactile stimulation timings (all ps < 0.001; Fig. 4f). Additionally, a significant double interaction between Sound Direction and Temporal Delay was found (F(4, 4335.2) = 3.2, p = 0.01, \(\:{\eta\:}_{p}^{2}\) = 0.003). Post-hoc comparisons showed that participants reached significantly farther distances during receding versus looming sounds only at intermediate stimulation timings (T3 and T4; p < 0.001; Fig. 4g).
The image shows the results from the mouse tracking analysis of both Experiment 1 and 2. (a) The graph illustrates the significant main effect of Sound Direction from the mixed-model ANOVA of Experiment 1. (b) The graph illustrates the significant main effect of Temporal Delay from the mixed-model ANOVA of Experiment 1. (c) The graph illustrates the significant interaction effect between Sound Direction and Temporal Delay from the mixed-model ANOVA of Experiment 1. Significant post-hoc comparisons are represented. (d) The graph illustrates the significant interaction effect between Body Part and Temporal Delay from the mixed-model ANOVA of Experiment (1) (e) The graph illustrates the significant main effect of Sound Direction from the mixed-model ANOVA of Experiment (2) (f) The graph illustrates the significant main effect of Temporal Delay from the mixed-model ANOVA of Experiment 2. (g) The graph illustrates the significant interaction effect between Sound Direction and Temporal Delay from the mixed-model ANOVA of Experiment 2. Significant post-hoc comparisons are represented.
Discussion
In the present experiments we investigated whether audio-tactile intersensory effects associated to looming and receding sounds in near and far space relative to the body (trunk) and the hand of an individual are modulated differently during arm movements and when at rest. We aimed at exploring whether intersensory effects are modulated only when occurring near the moving body part (arm-hand) (i.e., whether they are somatotopic) or whether movement execution exerts a general influence on intersensory processing that changes the impact of dynamic sounds on tactile perception also at non-moving body parts. Previous studies have indeed examined the effects of active body movements on visuo-tactile effects and PPS representation by implementing movements of isolated body parts such as the hand46,71,72, or full body transport movements49,50,51. These investigations collectively have suggested that motor activity can broadly reshape intersensory processing and PPS boundaries. Building upon this notion, our approach offers novel insights into the flexibility and dynamic nature of the peripersonal space (PPS), specifically the peritrunk space (PTS) and the perihand space (PHS), in relation to the role of motor execution in shaping the way sensory cues around the body are processed and integrated.
In two experiments we successfully replicated the finding that sound proximity enhances tactile detection speed42,43 by also controlling for expectancy effects (Experiment 2)57,58,64. Specifically, participants exhibited faster tactile responses when a sound approached a static body part42,56, an effect observed for both the hand and the trunk43.
A previous study showed that the PHS is linked to the position of the hand in space38 which would predict that during arm movement the spatial extend of tactile facilitation for sound passing near the hand should show a shift along the position of the hand. Our study seems to suggest that this is not the case and that arm and hand movements reduce the facilitatory effect of looming sounds on tactile processing at the moving hand and at trunk. Indeed, our results revealed that when participants performed an arm and hand movement, space dependent audio-tactile effects were attenuated (Fig. 2a) compared to when being still in Experiment 1. We further confirm this effect in Experiment 2 finding no significant difference in tactile reactivity between near and far looming sound locations in the motor condition (Fig. 3b and c). Importantly, this reduction of the intersensory effect was found across static and moving body parts, suggesting that arms movement exerted a central influence on multisensory processing rather than a peripheral one as they were not confined to the moving effector13,23,24. This interpretation aligns with findings from Casado-Palacios et al.59, who showed that active, but not passive, movement impair tactile processing in blind individuals, underscoring the critical role of vision in mediating intersensory effects during movement.
Concerning the somatotopy-independent effects of movement on audio-tactile integration, our results are supported by previous evidence provided by Hao et al.73, who demonstrated that active movement of one body part (e.g., the right index finger) can influence sensory processing at a distant, stationary body part (e.g., the left index finger). Specifically, Hao et al.74 found that actively moving the right finger, as opposed to passive movements induced externally, modified the temporal processing of audio-tactile stimuli presented to the stationary left finger. This finding suggests that movement-related effects on sensory integration are not confined to the moving effector, but rather reflect a broader, centrally mediated influence on sensory perception and multisensory integration. In line with Hao et al.73, the present results indicate that the reduced audio-tactile intersensory effects during movement execution cannot simply be attributed to peripheral gating mechanisms as: (1) the speed of the performed movement was below the threshold for activating gating mechanisms as described by Cysbuska et al.28; (2) the reduction of the intersensory effects was found for tactile stimuli delivered both at the moving limb and also on the static chest of participants. Thus, our results suggest that movement execution has a specific effect on multisensory integration that cannot be explained by movement-related, limb-specific, gating of tactile processing. This is further supported by an additional analysis on unisensory motor conditions in the Supplementary material (Supplementary Fig. 4) which revealed no interaction effect between Hand Status and Body Part. This interpretation aligns with broader evidence indicating that movement execution typically modulates sensory perception across different modalities, often via central mechanisms and predictive processing. For instance, besides affecting tactile processing, movement execution is also known to reduce auditory perception74 and modulate visual perception75,76 according to coherent predictions about stimulus features related to the executed movement. Such predictive modulations underlie the phenomenon of sensory attenuation for self-generated stimuli, which has been documented extensively across sensory modalities including auditory74, visual77, and vestibular domains78, as well as across various species79,80. Therefore, our findings on audio-tactile intersensory modulation during arm movement might reflect a generalized central mechanism of sensory prediction and integration that goes beyond somatosensory-specific gating.
Notably, the differential effects between static and movement conditions were consistently replicated across both experiments, despite employing different expectancy correction methods for RTs and conducting additional analyses based on slope values derived from a linear model (Fig. 3c and d). This consistency supports two main conclusions. First, minor variations in the experimental setups, such as differences in sound duration and the positioning of the release button, did not significantly influence the observed results. Second, irrespective of whether we corrected raw RTs using the Sound Intensity Difference index in Experiment 1 or the unisensory RTs in Experiment 2, we consistently found a distinction between motor and static conditions depending on sound distances. This finding further validates that both methods effectively controlled for temporal expectancy effects, allowing us to isolate and evaluate the specific influences of sound distance and motor execution on tactile processing.
However, in Experiment 1, although we observed a significant difference between the no-intensity-difference condition and the medium-intensity-difference condition, we had anticipated a stronger effect when comparing the no-intensity condition with the strong-intensity-difference condition (Fig. 2b). We interpret this unexpected result as potentially due to participants being at the very beginning (D1) or end (D5) of the sound. Furthermore, in the motor condition, these intervals also implied that participants either just started their movement or were approaching their end (Fig. 4b and c) which may have introduced greater RTs variability. Interestingly, this effect is not evident in Experiment 2, despite the use of a different RT index. A possible explanation is that the timing of the tactile stimulation, beginning slightly after movement onset and ending slightly before movement offset (Fig. 1b), may have missed the peak periods when such gating effects are most pronounced.
Overall, these findings confirm and contribute to describe the interplay between multisensory processing and motor activity and their potential role in the dynamic and flexible shaping of PPS. Specifically, this study demonstrates that the classic near-far space multisensory processing difference, typically studied in static conditions, are altered when individuals engage in upper limb movements. Moreover, our results indicate that, under these movement conditions, the influence of motor commands on sensory processing and integration is not confined to the moving body part but extends to audio-tactile intersensory effects and multisensory processing at different body parts. This is relevant in terms of the understanding of whether central or peripheral factors underpin the modulation of sensory processing and intersensory integration13,23,24.
Although not the primary focus of this study, the mouse-tracking analysis served two important purposes. First, it confirmed that participants’ hand movements progressively covered a greater distance along the 30 cm path as the temporal delay from sound onset increased in both Experiment 1 and Experiment 2 (Fig. 4b and f). Second, the data revealed that participants tended to reach farther, i.e., moved faster, when exposed to receding sounds compared to looming sounds (Fig. 4a, c, e and g). This finding is consistent with prior literature81, which suggests that looming stimuli are typically perceived as lasting longer than receding ones. Such a perceptual bias could have caused participants to slow down slightly when performing their movements with looming sounds compared to receding ones. Furthermore, it is plausible that looming sounds, known to elicit defensive responses2,3, led participants to adopt more cautious movement strategies. This is further supported by analyses on movement speed and acceleration that can be found in the Supplementary material (Supplementary Figs. 9–11). Conversely, the alignment between the direction of receding sounds and the natural outward extension of the arm may have facilitated quicker movements. However, these interpretations remain speculative and warrant further investigation to more precisely determine the underlying mechanisms driving this effect.
Limitations
Some limitations of the present studies may be acknowledged. First, some methodological decisions prevented finding a clear multisensory facilitation compared to unisensory tactile processing. In Experiment 1 and 2, indeed, we had unisensory tactile and audio-tactile conditions in different blocks which has induced strong expectancy effects reducing the difference between these two experimental conditions. Future studies should address this issue by using intermixed unisensory and multisensory trials. Second, the tactile and auditory stimuli used in the present studies are unrelated to the movement that was performed. It has been shown that tactile events that are functionally related to the performed movement10,11,12, as well as sounds that are related to the movement50,51,52, may induce different modulating gating/facilitatory sensory effects. However, while we recognize that the auditory and tactile stimuli were not functionally related to the executed movement, we believe that our set-up points to an ecologically valid situations as it allows studying intersensory effect during arm action execution, which is a typical situation in everyday life. Future studies should establish whether the effects described in the present studies extend to tactile and/on sound stimuli that bear an ecological relation with the movements performed. Finally, RT analysis alone is insufficient to draw any conclusion concerning the neural systems and the cognitive mechanisms supporting the found effects. Further studies using techniques such as EEG are necessary. Specifically, future work could investigate early tactile sensory components (e.g., N80, N140) and later, more attention-related components (e.g., P300) to distinguish whether these effects are primarily sensory or attentional in nature.
Conclusions
In summary, the present results confirm the presence of facilitatory intersensory audio-tactile effects of looming sounds in static conditions for tactile stimuli at limb and trunk. We expand current understanding of audio-tactile effects by showing that upper limb (arm-hand) movements reduce intersensory effects at both the moving (arm-hand) and non-moving (trunk) body sites hinting to a role for central mechanisms of sensorimotor integration rather than peripheral gating ones.
Data availability
The original contributions presented in the study are included in the article/supplementary material. Datasets can be found at the following repository link: https://osf.io/vx4zf/overview?view_only=71260a2c3e0548df8a38d718f9ef364e.
References
Serino, A. Peripersonal space (PPS) as a multisensory interface between the individual and the environment, defining the space of the self. Neurosci. Biobehavioral Reviews. 99, 138–159. https://doi.org/10.1016/j.neubiorev.2019.01.016 (2019).
Graziano, M. S. A. & Cooke, D. F. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia 44 (6), 845–859. https://doi.org/10.1016/j.neuropsychologia.2005.09.009 (2006).
Bufacchi, R. J. & Iannetti, G. D. An action field theory of peripersonal space. Trends Cogn. Sci. 22 (12), 1076–1090. https://doi.org/10.1016/j.tics.2018.09.004 (2018).
Zagha, E., Casale, A. E., Sachdev, R. N. S., McGinley, M. J. & McCormick, D. A. Motor cortex feedback influences sensory processing by modulating network state. Neuron 79 (3), 567–578. https://doi.org/10.1016/j.neuron.2013.06.008 (2013).
Schroeder, C. E., Wilson, D. A., Radman, T., Scharfman, H. & Lakatos, P. Dynamics of active sensing and perceptual selection. Curr. Opin. Neurobiol. 20 (2), 172–176. https://doi.org/10.1016/j.conb.2010.02.010 (2010).
Creem-Regehr, S. H. & Kunz, B. R. Perception and action. Wires Cogn. Sci. 1 (6), 800–810. https://doi.org/10.1002/wcs.82 (2010). Portico.
Hommel, B., Müsseler, J., Aschersleben, G. & Prinz, W. The theory of event coding (TEC): A framework for perception and action planning. Behav. Brain Sci. 24 (5), 849–878. https://doi.org/10.1017/s0140525x01000103 (2001).
Smeets, J. B. J. & Brenner, E. Perception and action are inseparable. Ecol. Psychol. 13 (2), 163–166. https://doi.org/10.1207/s15326969eco1302_8 (2001).
Friston, K. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11 (2), 127–138. https://doi.org/10.1038/nrn2787 (2010).
Juravle, G., McGlone, F. & Spence, C. Context-dependent changes in tactile perception during movement execution. Front. Psychol. https://doi.org/10.3389/fpsyg.2013.00913 (2013). 4.
van Ede, F., van Doren, T. I., Damhuis, J., de Lange, F. P. & Maris, E. Movement Preparation improves touch perception without awareness. Cognition 137, 189–195. https://doi.org/10.1016/j.cognition.2015.01.009 (2015).
Thomas, E. R., Yon, D., de Lange, F. P. & Press, C. Action Enhances Predicted Touch Psychol. Sci., 33(1), 48–59. https://doi.org/10.1177/09567976211017505 (2021).
Rushton, D. N., Rothwell, J. C. & Craggs, M. D. Gating of somatosensory evoked potentials during different kinds of movement in man. Brain 104, 465–491. https://doi.org/10.1093/brain/104.3.465 (1981).
Juravle, G., Binsted, G. & Spence, C. Tactile suppression in goal-directed movement. Psychon. Bull. Rev. 24 (4), 1060–1076. https://doi.org/10.3758/s13423-016-1203-6 (2016).
Colino, F. L., Buckingham, G., Cheng, D. T., van Donkelaar, P. & Binsted, G. Tactile gating in a reaching and grasping task. Physiological Rep. 2 (3), e00267. https://doi.org/10.1002/phy2.267 (2014). Portico.
Arikan, B. E., Voudouris, D., Straube, B. & Fiehler, K. Distinct role of central predictive mechanisms in tactile suppression. (2023). https://doi.org/10.2139/ssrn.4622696
Williams, S. R. & Chapman, C. E. Time course and magnitude of Movement-Related gating of tactile detection in Humans. II. Effects of stimulus intensity. J. Neurophysiol. 84 (2), 863–875. https://doi.org/10.1152/jn.2000.84.2.863 (2000).
Lei, Y., Ozdemir, R. A. & Perez, M. A. Gating of sensory input at subcortical and cortical levels during grasping in humans. J. Neurosci. 38 (33), 7237–7247. https://doi.org/10.1523/jneurosci.0545-18.2018 (2018).
Kilteni, K. & Ehrsson, H. H. Predictive Attenuation of touch and tactile gating are distinct perceptual phenomena. IScience 25 (4), 104077. https://doi.org/10.1016/j.isci.2022.104077 (2022).
Fuehrer, E., Voudouris, D., Lezkan, A., Drewing, K. & Fiehler, K. Tactile suppression stems from specific sensorimotor predictions. Proc. Natl. Acad. Sci. 119 (20). https://doi.org/10.1073/pnas.2118445119 (2022).
Williams, S. R., Shenasa, J. & Chapman, C. E. Time course and magnitude of Movement-Related gating of tactile detection in Humans. I. Importance of stimulus location. J. Neurophysiol. 79 (2), 947–963. https://doi.org/10.1152/jn.1998.79.2.947 (1998).
Kilteni, K., Cullen, K., Schneider, D. M. & Schwarz, C. Suppressing sensation during action across species and sensory modalities: predictive and nonpredictive mechanisms of sensory modulation. J. Neurosci. 45 (46), e1351252025. https://doi.org/10.1523/jneurosci.1351-25.2025 (2025).
Seki, K. & Fetz, E. E. Gating of sensory input at spinal and cortical levels during Preparation and execution of voluntary movement. J. Neurosci. 32, 890–902. https://doi.org/10.1523/JNEUROSCI.4958-11.2012 (2012).
Staines, W. R., Brooke, J. D., Angerilli, P. A. & McIlroy, W. E. Generalisability of sensory gating during passive movement of the legs. Brain Res. 801, 125–129. https://doi.org/10.1016/S0006-8993(98)00553-8 (1998).
Jones, S. J., Hal Malenka onen, J. P. & Shawkat, F. Centrifugal and centripetal mechanisms involved in the ‘gating’ of cortical SEPs during movement. Electroencephalogr. Clin. Neurophysiol. 74 (89), 36–45. https://doi.org/10.1016/0168-5597 (1989).
Chapman, C. E. Constancy in the somatosensory system: central neural mechanisms underlying the appreciation of texture during active touch. Neural Aspects Tactile Sensation. 275–298. https://doi.org/10.1016/s0166-4115(98)80070-8 (1998).
Schmidt, R. F., Schady, W. J. L. & Torebjörk, H. E. Gating of tactile input from the hand. Exp. Brain Res. 79 (1), 97–102. https://doi.org/10.1007/bf00228877 (1990).
Cybulska-Klosowicz, A., Meftah, E. M., Raby, M., Lemieux, M. L. & Chapman, C. E. A critical speed for gating of tactile detection during voluntary movement. Exp. Brain Res. 210 (2), 291–301. https://doi.org/10.1007/s00221-011-2632-0 (2011).
Casado-Palacios, M., Tonelli, A., Campus, C. & Gori, M. Movement-related tactile gating in blindness. Sci. Rep. 13 (1). https://doi.org/10.1038/s41598-023-43526-8 (2023).
Voudouris, D. & Fiehler, K. The role of grasping demands on tactile suppression. Hum. Mov. Sci. 83, 102957. https://doi.org/10.1016/j.humov.2022.102957 (2022).
Voudouris, D., Broda, M. D. & Fiehler, K. Anticipatory grasping control modulates somatosensory perception. J. Vis. 19 (5), 4. https://doi.org/10.1167/19.5.4 (2019).
Voudouris, D. & Fiehler, K. Dynamic Temporal modulation of somatosensory processing during reaching. Sci. Rep. 11 (1). https://doi.org/10.1038/s41598-021-81156-0 (2021).
Manzone, D. M., Inglis, J. T., Franks, I. M. & Chua, R. Relevance-dependent modulation of tactile suppression during active, passive and pantomime reach-to-grasp movements. Behav. Brain. Res. 339, 93–105. https://doi.org/10.1016/j.bbr.2017.11.024 (2018).
Fogassi, L. & Gallese, V. Action as a Binding Key to Multisensory Integration. The Handbook of Multisensory Processes, 425–442. (2004). https://doi.org/10.7551/mitpress/3422.003.0032
Calvert, G. A., Spence, C. & Stein, B. E. (eds). The Handbook of Multisensory Processes. (2004). https://doi.org/10.7551/mitpress/3422.001.0001
Sutter, C., Drewing, K. & Müsseler, J. Multisensory integration in action control. Front. Psychol. https://doi.org/10.3389/fpsyg.2014.00544 (2014). 5.
Basile, G. A. et al. Neuroanatomical correlates of peripersonal space: bridging the gap between perception, action, emotion and social cognition. Brain Struct. Function. 229 (5), 1047–1072. https://doi.org/10.1007/s00429-024-02781-9 (2024).
Zanini, A. et al. Peripersonal and reaching space differ: evidence from their Spatial extent and multisensory facilitation pattern. Psychon. Bull. Rev. 28 (6), 1894–1905. https://doi.org/10.3758/s13423-021-01942-9 (2021).
Cléry, J. et al. The prediction of impact of a looming stimulus onto the body is subserved by multisensory integration mechanisms. J. Neurosci. 37 (44), 10656–10670. https://doi.org/10.1523/jneurosci.0610-17.2017 (2017).
Cléry, J., Guipponi, O., Odouard, S., Wardak, C. & Ben Hamed, S. Impact prediction by looming visual stimuli enhances tactile detection. J. Neurosci. 35 (10), 4179–4189. https://doi.org/10.1523/jneurosci.3031-14.2015 (2015).
Kandula, M., Hofman, D. & Dijkerman, H. C. Visuo-tactile interactions are dependent on the predictive value of the visual stimulus. Neuropsychologia 70, 358–366. https://doi.org/10.1016/j.neuropsychologia.2014.12.008 (2015).
Canzoneri, E., Magosso, E. & Serino, A. Dynamic sounds capture the boundaries of peripersonal space representation in humans. PLoS ONE. 7 (9), e44306. https://doi.org/10.1371/journal.pone.0044306 (2012).
Serino, A. et al. Body part-centered and full body-centered peripersonal space representations. Sci. Rep. 5 (1). https://doi.org/10.1038/srep18603 (2015).
Hobeika, L., Taffou, M., Carpentier, T., Warusfel, O. & Viaud-Delmon, I. Capturing the dynamics of peripersonal space by integrating expectancy effects and sound propagation properties. J. Neurosci. Methods. 332, 108534. https://doi.org/10.1016/j.jneumeth.2019.108534 (2020).
Finisguerra, A., Canzoneri, E., Serino, A., Pozzo, T. & Bassolino, M. Moving sounds within the peripersonal space modulate the motor system. Neuropsychologia 70, 421–428. https://doi.org/10.1016/j.neuropsychologia.2014.09.043 (2015).
Brozzoli, C., Pavani, F., Urquizar, C., Cardinali, L. & Farnè, A. Grasping actions remap peripersonal space. NeuroReport 20 (10), 913–917. https://doi.org/10.1097/wnr.0b013e32832c0b9b (2009).
Patané, I. et al. Action planning modulates peripersonal space. J. Cogn. Neurosci. 31 (8), 1141–1154. https://doi.org/10.1162/jocn_a_01349 (2019).
Lohmann, J., Belardinelli, A. & Butz, M. V. Hands ahead in Mind and motion: active inference in peripersonal hand space. Vision 3 (2), 15. https://doi.org/10.3390/vision3020015 (2019).
Kuroda, N. & Teramoto, W. Motor information contributes to visuotactile interaction in trunk-centered peripersonal space during a pedaling situation. Exp. Brain Res. 243 (1). https://doi.org/10.1007/s00221-024-06975-9 (2024).
Kuroda, N. & Teramoto, W. Contribution of motor and proprioceptive information to visuotactile interaction in peripersonal space during bike riding. Exp. Brain Res. 240 (2), 491–501. https://doi.org/10.1007/s00221-021-06269-4 (2021).
Noel, J. P. et al. Full body action remapping of peripersonal space: the case of walking. Neuropsychologia 70, 375–384. https://doi.org/10.1016/j.neuropsychologia.2014.08.030 (2015).
Amemiya, T., Ikei, Y. & Kitazaki, M. Remapping peripersonal space by using Foot-Sole vibrations without any body movement. Psychol. Sci. 30 (10), 1522–1532. https://doi.org/10.1177/0956797619869337 (2019).
Bahadori, M. & Cesari, P. Affective sounds entering the peripersonal space influence the whole-body action Preparation. Neuropsychologia 159, 107917. https://doi.org/10.1016/j.neuropsychologia.2021.107917 (2021).
Làdavas, E. & Serino, A. Action-dependent plasticity in peripersonal space representations. Cognit. Neuropsychol. 25 (7–8), 1099–1113. https://doi.org/10.1080/02643290802359113 (2008).
Conrad, V. et al. Naturalistic stimulus structure determines the integration of audiovisual looming signals in binocular rivalry. PLoS ONE. 8 (8), e70710. https://doi.org/10.1371/journal.pone.0070710 (2013).
Matsuda, Y., Sugimoto, M., Inami, M. & Kitazaki, M. Peripersonal space in the front, rear, left and right directions for audio-tactile multisensory integration. Sci. Rep. 11 (1). https://doi.org/10.1038/s41598-021-90784-5 (2021).
Kandula, M., Van der Stoep, N., Hofman, D. & Dijkerman, H. C. On the contribution of overt tactile expectations to visuo-tactile interactions within the peripersonal space. Exp. Brain Res. 235 (8), 2511–2522. https://doi.org/10.1007/s00221-017-4965-9 (2017).
Noel, J. P. et al. Visual-Tactile Spatial multisensory interaction in adults with autism and schizophrenia. Front. Psychiatry. 11 https://doi.org/10.3389/fpsyt.2020.578401 (2020).
Casado-Palacios, M., Tonelli, A., Campus, C. & Gori, M. Cross-Modal interactions and Movement-Related tactile gating: the role of vision. Brain Sci. 15 (3), 288. https://doi.org/10.3390/brainsci15030288 (2025).
Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1 (3), 98–101. https://doi.org/10.1111/1467-8721.ep10768783 (1992).
Schneider, W., Eschman, A. & Zuccolotto, A. E-Prime (Version 2.0). [Computer Software and manual] (Psychology Software Tools Inc, 2002).
Petrizzo, I., Mikellidou, K., Avraam, S., Avraamides, M. & Arrighi, R. Reshaping the peripersonal space in virtual reality. Sci. Rep. 14 (1). https://doi.org/10.1038/s41598-024-52383-y (2024).
Spaccasassi, C., Romano, D. & Maravita, A. Everything is worth when it is close to my body: how Spatial proximity and stimulus Valence affect visuo-tactile integration. Acta. Psychol. 192, 42–51. https://doi.org/10.1016/j.actpsy.2018.10.013 (2019).
Janssen, P. & Shadlen, M. N. A representation of the hazard rate of elapsed time in macaque area LIP. Nat. Neurosci. 8 (2), 234–241. https://doi.org/10.1038/nn1386 (2005).
Holmes, N. P., Martin, D., Mitchell, W., Noorani, Z. & Thorne, A. Do sounds near the hand facilitate tactile reaction times? Four experiments and a meta-analysis provide mixed support and suggest a small effect size. Exp. Brain Res. 238 (4), 995–1009. https://doi.org/10.1007/s00221-020-05771-5 (2020).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. LmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82 (13), 1–26. https://doi.org/10.18637/jss.v082.i13 (2017).
Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.11.1-00001, (2025). https://rvlenth.github.io/emmeans/
Bertoni, T., Paladino, M. P., Pellencin, E., Serino, S. & Serino, A. Space for power: feeling powerful over others’ behavior affects peri-personal space representation. Exp. Brain Res. 241 (11–12), 2779–2793. https://doi.org/10.1007/s00221-023-06719-1 (2023).
de Haan, A. M., Smit, M., Van der Stigchel, S. & Dijkerman, H. C. Approaching threat modulates visuotactile interactions in peripersonal space. Exp. Brain Res. 234 (7), 1875–1884. https://doi.org/10.1007/s00221-016-4571-2 (2016).
Noel, J. P., Serino, A. & Wallace, M. T. Increased neural strength and reliability to audiovisual stimuli at the boundary of peripersonal space. J. Cogn. Neurosci. 31 (8), 1155–1172. https://doi.org/10.1162/jocn_a_01334 (2019).
Maravita, A., Spence, C., Kennett, S. & Driver, J. Tool-use changes multimodal Spatial interactions between vision and touch in normal humans. Cognition 83 (2), B25–B34. https://doi.org/10.1016/s0010-0277(02)00003-3 (2002).
Chapman, C. E. & Beauchamp, E. Differential controls over tactile detection in humans by motor commands and peripheral reafference. J. Neurophysiol. 96 (3), 1664–1675. https://doi.org/10.1152/jn.00214.2006 (2006).
Hao, Q., Ora, H., Ogawa, K., Ogata, T. & Miyake, Y. Voluntary movement affects simultaneous perception of auditory and tactile stimuli presented to a non-moving body part. Sci. Rep. 6 (1). https://doi.org/10.1038/srep33336 (2016).
Thomas, L. E. Action experience drives Visual-Processing biases near the hands. Psychol. Sci. 28 (1), 124–131. https://doi.org/10.1177/0956797616678189 (2016).
Thomas, L. E. Grasp posture alters visual processing biases near the hands. Psychol. Sci. 26 (5), 625–632. https://doi.org/10.1177/0956797615571418 (2015).
Schröger, E., Marzecová, A. & SanMiguel, I. Attention and prediction in human audition: a lesson from cognitive psychophysiology. Eur. J. Neurosci. 41 (5), 641–664. https://doi.org/10.1111/ejn.12816 (2015). Portico.
Gremmler, S. & Lappe, M. Saccadic suppression during voluntary versus reactive saccades. J. Vis. 17, 8. https://doi.org/10.1167/17.8.8 (2017).
Angelaki, D. E. & Cullen, K. E. Vestibular system: the many facets of a multimodal sense. Annu. Rev. Neurosci. 31, 125–150. https://doi.org/10.1146/annurev.neuro.31.060407.125555 (2008).
Crapse, T. B. & Sommer, M. A. Corollary discharge across the animal Kingdom. Nat. Rev. Neurosci. 9, 587–600. https://doi.org/10.1038/nrn2457 (2008).
Schneider, D. M., Sundararajan, J. & Mooney, R. A cortical filter that learns to suppress the acoustic consequences of movement. Nature 561, 391–395. https://doi.org/10.1038/s41586-018-0520-5 (2018).
Grassi, M. Sex difference in subjective duration of looming and receding sounds. Perception 39 (10), 1424–1426. https://doi.org/10.1068/p6810 (2010).
Funding
MC was funded by Sapienza University grant (n. RG123188B4631694). PL was funded by Ernst &Young, the Italian Ministry of University MUR by the PNRR.
Author information
Authors and Affiliations
Contributions
P.L. and M.C. designed the experiment. P.L. performed the study and analysed the data. N.S. collected the data. P.L. and M.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Piero, L., Nafiseh, S. & Matteo, C. Somatotopy-independent reduction of audio-tactile intersensory facilitation for looming sounds within the peripersonal space during arm movements execution. Sci Rep 16, 7133 (2026). https://doi.org/10.1038/s41598-026-36796-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-026-36796-5