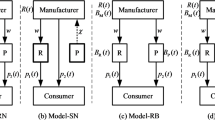
Abstract
Counterfeit and substandard pharmaceuticals represent a critical global health crisis, with the World Health Organisation (WHO) reporting that falsified medicines comprise 10% of the global pharmaceutical trade, constituting one of the fastest-growing grey economies worldwide. This illicit market affects all regions, including high-income countries, causing devastating health and economic consequences that contribute to increased mortality and morbidity rates. The problem is particularly severe in developing nations with inadequate regulatory systems. Mobile health (mHealth) technologies have emerged as promising solutions for enhancing pharmaceutical supply chain integrity. However, comprehensive frameworks that integrate multiple authentication mechanisms remain limited in addressing the growing counterfeit drug crisis. The primary objective of this study is to design and implement a novel mobile health framework that integrates innovative packaging technology and computer vision to enhance pharmaceutical integrity and patient safety. This innovative approach enables consumers to authenticate genuine drugs and detect counterfeit/spurious products through advanced Quick Response (QR) code verification systems and artificial intelligence- powered tablet recognition capabilities. The proposed system utilises individual strip QR codes that enable real-time scanning at the point of sale to verify drug authenticity, directly addressing critical gaps identified in current pharmaceutical authentication methods. To overcome the practical challenge of expiration date loss when medication strips are cut at pharmacies, we developed a computer vision-based Artificial Intelligence (AI) model that automatically recognises the number of tablets remaining in a strip and correlates this information with the unique Identifier (ID). This approach leverages recent advances in computer vision for pharmaceutical applications and automated packaging inspection technologies. Each medication strip is assigned to an individual customer at the time of purchase, with pharmacists recording detailed customer information to ensure comprehensive tracking and accountability throughout the pharmaceutical supply chain. The integrated mobile application creates a robust anti-counterfeiting ecosystem by combining secure QR code authentication with intelligent visual recognition capabilities. The computer vision model provides accurate tablet counting and strip identification, maintaining continuity of medication tracking even when packaging is modified during dispensing processes, thus significantly enhancing supply chain transparency. This dual-authentication approach builds consumer confidence in pharmaceutical authenticity while directly addressing critical vulnerabilities identified in current regulatory frameworks. This comprehensive mobile health solution provides a scalable, evidence-based approach to pharmaceutical authentication that can be readily implemented across diverse healthcare systems globally, offering substantial potential for reducing the circulation of falsified medicines and improving patient safety outcomes in the ongoing battle against the pandemic of counterfeit pharmaceuticals. To overcome the practical challenge of expiration date loss when medication strips are cut at pharmacies, we developed a computer vision-based Artificial Intelligence (AI) model that automatically recognises the number of tablets remaining in a strip and correlates this information with the unique Identifier (ID). This approach leverages recent advances in computer vision for pharmaceutical applications and automated packaging inspection technologies. Each medication strip is assigned to an individual customer at the time of purchase, with pharmacists recording detailed customer information to ensure comprehensive tracking and accountability throughout the pharmaceutical supply chain. The integrated mobile application creates a robust anti-counterfeiting ecosystem by combining secure QR code authentication with intelligent visual recognition capabilities. The computer vision model provides accurate tablet counting and strip identification, maintaining continuity of medication tracking even when packaging is modified during dispensing processes, thus significantly enhancing supply chain transparency. This dual-authentication approach builds consumer confidence in pharmaceutical authenticity while directly addressing critical vulnerabilities identified in current regulatory frameworks. This comprehensive mobile health solution provides a scalable, evidence-based approach to pharmaceutical authentication that can be readily implemented across diverse healthcare systems globally, offering substantial potential for reducing the circulation of falsified medicines and improving patient safety outcomes in the ongoing battle against the pandemic of counterfeit pharmaceuticals.
Similar content being viewed by others
Data availability
The dataset generated and analysed during the current study is available in the dataset_project repository, https://github.com/sanjay2422-dot/dataset_project.The dataset analysed during the current study is available in the Ultralytics repository, https://github.com/ultralytics/ultralytics/blob/main/ultralytics/cfg/datasets/medical-pills.yaml.
References
World Health Organization. WHO Global Surveillance and Monitoring System for substandard and falsified medical products. Geneva: World Health Organization. (2017). https://www.who.int/publications/i/item/9789240097513
Ozawa, S. et al. Prevalence and estimated economic burden of substandard and falsified medicines in low- and middle-income countries: A systematic review and meta-analysis. JAMA Netw. Open 1(4), e181662. https://doi.org/10.1001/jamanetworkopen.2018.1662 (2018).
Ferrario, A., Orubu, E. S. F., Adeyeye, M. C., Zaman, M. H. & Wirtz, V. J. The need for comprehensive and multidisciplinary training in substandard and falsified medicines for pharmacists. BMJ Global Health 4(4), e001681. https://doi.org/10.1136/bmjgh-2019-001681 (2019).
Baines, D. et al. A scoping review of the quality and the design of evaluations of mobile health, telehealth, smart pump and monitoring technologies performed in a pharmacy-related setting. Front. Pharmacol. 9, 678. https://doi.org/10.3389/fphar.2018.00678 (2018).
El-Jardali, F. et al. Interventions to combat or prevent drug counterfeiting: a systematic review. BMJ Open 5(3), e006290. https://doi.org/10.1136/bmjopen-2014-006290 (2015).
Hui, T. K. L., Mohammed, B., Donyai, P., McCrindle, R. & Sherratt, R. S. Enhancing pharmaceutical packaging through a technology ecosystem to facilitate the reuse of medicines and reduce medicinal waste. Pharmacy (Basel) 8(2), 58. https://doi.org/10.3390/pharmacy8020058 (2020).
Koponen, J., Haataja, K. & Toivanen, P. Recent advancements in machine vision methods for product code recognition: A systematic review. F1000Research 11, 1099. https://doi.org/10.12688/f1000research.124796.1 (2022).
Tan, L., Huangfu, T., Wu, L. & Chen, W. Comparison of RetinaNet, SSD, and YOLO v3 for real-time pill identification. BMC Med. Inform. Decis. Mak. 21(1), 324. https://doi.org/10.1186/s12911-021-01691-8 (2021).
Freiermuth, E., Kohler, D., Hofstetter, A., Thun, J. & Juhnke, M. Detection and quantification of visual tablet surface defects by combining convolutional neural network-based object detection and deterministic computer vision approaches. J. Pharm. BioTech Ind. 2(2), 9. https://doi.org/10.3390/jpbi2020009 (2025).
Farkas, D., Madarász, L., Nagy, Z. K., Antal, I. & Kállai-Szabó, N. Image analysis: A versatile tool in the manufacturing and quality control of pharmaceutical dosage forms. Pharmaceutics 13(5), 685. https://doi.org/10.3390/pharmaceutics13050685 (2021).
Vijayakumar, A. et al. Real-time visual intelligence for defect detection in pharmaceutical packaging. Sci. Rep. 14(1), 18811. https://doi.org/10.1038/s41598-024-69701-z (2024).
Zheng, Y. et al. Designing human-centered ai to prevent medication dispensing errors: Focus group study with pharmacists. JMIR Form. Res. 7, e51921. https://doi.org/10.2196/51921 (2023).
Holtkötter, J. et al. Development and validation of a digital image processing-based pill detection tool for an oral medication self-monitoring system. Sensors (Basel) 22(8), 2958. https://doi.org/10.3390/s22082958 (2022).
Agius, S. et al. Leveraging digital technologies to enhance patient safety. Health Technol. 15, 1053–1063. https://doi.org/10.1007/s12553-025-01001-6 (2025).
Kushwaha, V., Agrawal, P., Sahu, A. & Gupta, P. Spurious drugs: A brief overview. World J. Pharm. Res. 12(2), 564–574 (2023).
Lima, M. B. A. & Yonamine, M. Counterfeit medicines: relevance, consequences and strategies to combat the global crisis. Brazilian J. Pharm. Sci. 59, e20402. https://doi.org/10.1590/s2175-97902023e20402 (2023).
Khan, A. N. & Khar, R. K. Current scenario of spurious and substandard medicines in India: A systematic review. Indian J Pharm Sci. 77(1), 2–7. https://doi.org/10.4103/0250-474X.151550 (2015).
Das, S. & Bhakta, T. A survey to the extent of spurious drugs in the market of India. Res. J. Pharmacognosy Phytochemistry. 10 (3), 233. https://doi.org/10.5958/0975-4385.2018.00038.9 (2018).
Rahman, M. S. et al. The health consequences of falsified medicines: A study of the published literature. Trop Med Int Health. https://doi.org/10.1111/tmi.13161 (2018).
Imran, M. et al. Fatal intoxications due to administration of isosorbide tablets contaminated with pyrimethamine. J. Forensic Sci. 61 (5), 1382–1385. https://doi.org/10.1111/1556-4029.13125 (2016).
Vardanyan, R. S. & Hruby, V. J. Antimicrobial Drugs. In Synthesis of Essential Drugs (eds Vardanyan, R. S. & Hruby, V. J.) 499–523 (Elsevier, 2006). https://doi.org/10.1016/B978-044452166-8/50033-9.
Fazaludeen Koya, S. et al. Antibiotic consumption in India: geographical variations and temporal changes between 2011 and 2019. JAC Antimicrob. Resist. 4(5), dlac112. https://doi.org/10.1093/jacamr/dlac112 (2022).
Malasari, N. & Sudrajat, J. Expired medication detection information system using the first expired first out method: case study of one pharmacy in Bandung. J. Comput. Bisnis. 17(2), 97–103 (2024).
Tomar, I. & Tomar, B. A Smart Expiry Date Reminder System for Medicines. In 2021 International Conference on Industrial Electronics Research and Applications (ICIERA), 1–6. (IEEE, New Delhi, India, 2021). https://doi.org/10.1109/ICIERA53202.2021.9726728
Namasivayam, V. et al. Lessons learned from redesigning public health medicines supply chain model in Uttar Pradesh, India. Front. Public Health 13, 1588227. https://doi.org/10.3389/fpubh.2025.1588227 (2025).
Loeffler, S., Roth, R. E., Goring, S. & Myrbo, A. Mobile UX design: learning from the flyover country mobile app. J. Maps. 17 (2), 39–50. https://doi.org/10.1080/17445647.2020.1867247 (2021).
Lai, H.-C. et al. Colloquium. Br. J. Educ. Technol. 44, E57–E62. https://doi.org/10.1111/j.1467-8535.2012.01343.x (2013).
Belcher, H. M. E. & Shinitzky, H. E. Substance abuse in children: Prediction, protection, and prevention. Arch. Pediatr. Adolesc. Med. 152(10), 952–960 (1998).
Hassan, E. et al. Medical Prescription Recognition using Machine Learning. In IEEE 11th Annual Computing and Communication Workshop and Conference (CCWC), 973–979 (IEEE, NV, USA, 2021). https://doi.org/10.1109/CCWC51732.2021.9376141
Mamoun, R., Nasor, M. & Abulikailik, S. H. Design and development of mobile healthcare application prototype using Flutter. In 2020 International Conference on Computer, Control, Electrical, and Electronics Engineering (ICCCEEE), 1–6. (IEEE, Morocco, 2021). https://doi.org/10.1109/ICCCEEE49695.2021.9429595
Ultralytics Medical Pills Dataset – Object Detection. Ultralytics Documentation. (2023). https://docs.ultralytics.com/datasets/detect/medical-pills/
Jocher, G. et al. YOLOv5. Ultralytics. (2020). https://github.com/ultralytics/yolov5
Ultralytics. YOLOv8. Ultralytics. (2023). https://github.com/ultralytics/ultralytics
Brownlee, J., Supervised & Unsupervised Machine Learning Algorithms. and. Machine Learning Mastery; (2016). https://machinelearningmastery.com/supervised-and-unsupervised-machine-learning-algorithms/
Esteva, A. et al. A guide to deep learning in healthcare. Nat. Med. 25, 24–29. https://doi.org/10.1038/s41591-018-0316-z (2019).
Goodfellow, I., Bengio, Y. & Courville, A. Deep Learning (MIT Press, 2016).
Dassault Systèmes. SolidWorks (Education Version 2022). [Computer software]. (2022). Available from: https://www.solidworks.com/
Funding
Open access funding provided by Vellore Institute of Technology. This research received no external financial or non-financial support from any funding agency in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
H.K. - Core Ideation, Writing – review & editing, Writing – original draft, Visualisation, Validation, Methodology, Investigation, Formal analysis, Data curation, ConceptualisationV.P. - Ideation, Writing – review & editing, Writing – original draft, Visualisation, Validation, Methodology, AI Training, Formal analysis, Data curation, ConceptualisationP.S.K. - Ideation, Writing – review & editing, Writing – original draft, Visualisation, Validation, Methodology, Application Development, Formal analysis, Data curation, ConceptualisationS.D. - Ideation, Writing – review & editing, Writing – original draft, Visualisation, Validation, Methodology, AI Training, CAD Model Development, Formal analysis, Data curation, ConceptualisationR.V.- Review & Editing, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Conceptualisation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
K, H., Parikh, V., K, P.S. et al. A framework for enhancing pharmaceutical integrity and patient safety: novel mobile health solution integrating smart packaging and computer vision. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38215-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38215-1