Abstract
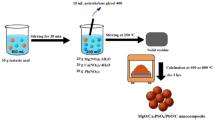
This study investigated the removal of toxic Malachite Green (MG) dye from aqueous solution using a nanocomposite of TiO₂ loaded on functionalized multi-walled carbon nanotubes (TiO₂/f-MWCNT). The nanocomposite was synthesized via a hydrothermal method and characterized using XRD, FESEM, FTIR, BET, and EDS. The optimal adsorption conditions were determined as pH = 8, adsorbent dose of 0.005 g, equilibrium time of 10 min, and temperature of 293 K. Under these conditions, the maximum adsorption capacity reached 38.61 mg/g with a removal efficiency exceeding 95%. Kinetic data showed an excellent fit to the pseudo-second-order model (R2 = 0.99), indicating a chemisorption-controlled process. The equilibrium adsorption behavior was well described by the Langmuir-2 isotherm model (R2 = 0.99), confirming monolayer formation on a homogeneous surface. Additionally, the Freundlich, Temkin, and Elovich models also showed favorable fitting (R2 ≥ 0.98), suggesting a combined physical–chemical adsorption mechanism on a heterogeneous surface. The adsorbent was effectively regenerated with a 1 M NaOH solution, achieving 99% recovery. These results demonstrate the rapid and efficient performance of TiO₂/f-MWCNT as a promising adsorbent for the treatment of wastewater containing cationic dyes.
Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper [and its supplementary information files], a request for more detailed data should be send to the corresponding authors with the permission of all authors.
References
Boochakiat, S., Inceesungvorn, B., Nattestad, A., Kim, J. H. & Chen, J. J. I. C. Synergistic performance of bi2moo6/graphene oxide on carbon cloth in photoelectrocatalytic malachite green removal. Inorg. Chem. 174, 113913 (2025).
Bai, H. et al. Technology P Adsorption-desorption behavior of malachite green on aged microplastics in seawater environment. Sep. Purif. Technol. 354, 128991 (2025).
Ali, R. et al. A novel red emissive glutathione-capped carbon dots embedded within molecularly-imprinted polymers for adsorption and fluorescent sensing of malachite green in food samples. Microchem. J. 212, 113376 (2025).
Samadi A., & Zhao, S. Polyaniline-based adsorbents and photocatalysts for the elimination of toxic heavy metals. Advanced materials for a sustainable environment. Adv. Mater. Sustain. Environ. (pp 101–122) (CRC Press, 2022).
Dilek, ŞA., Hüseyin, E., Zeynep, M. Ş, El Noureddine, M. & Valbonë, M. Preparation of polyacrylamide titanium dioxide hybrid nanocomposite by direct polymerization and its applicability in removing crystal violet from aqueous solution. J. Polym. Environ. 32, 573–587 (2023).
Xu, H. et al. Recent advances in tio 2-based photocatalysis. J. Mater. Chem. A. 2(32), 12642–12661 (2014).
Guo, J., Zhou, T., Guo, H., Ge, C. & Lu, J. Fabrics application of nano-TiO2@ adsorbent composites in the treatment of dye wastewater: A review. J. Eng. Fibers Fabr. 20, 15589250251329450 (2025).
Yue, Y., Yue, X., Tang, X., Han, L., Wang, J., Wang, S. & Du, C. Synergistic adsorption and photocatalysis study of tio2 and activated carbon composite. Heliyon, 10(10) (2024).
Ahamad, Z., Mashkoor, F., Nasar, A. & Jeong, C. Multi-walled carbon nanotubes/TiO2/chitosan nanocomposite for efficient removal of malachite green dye from aqueous system: A comprehensive experimental and theoretical investigation. Int. J. Biol. Macromol. 295, 139461 (2025).
Khan, J., Din, I. U., Alharthi, A. I., Alotaibi, M. A., Nasir, Q. & Thebo, K. The opportunities and challenges of using nanomaterials as photocatalysts for the degradation of environmental pollutants. Comments Inorg. Chem. 1–46 (2025).
Pajootan, E., Rahimdokht, M. & Arami, M. chemistry e Carbon and cnt fabricated carbon substrates for tio2 nanoparticles immobilization with industrial perspective of continuous photocatalytic elimination of dye molecules. J. Ind. Eng. Chem. 55, 149–163 (2017).
Abega, A. V. et al. M Easy and convenient synthesis of CNT/TiO2 nanohybrid by in-surface oxidation of Ti3+ ions and application in the photocatalytic degradation of organic contaminants in water. Synth. Met. 251, 1–14 (2019).
Ghaedi, M., Kokhdan, S. N., Sahraei, R. & Daneshfar, A. Chemistry E palladium, silver, and zinc oxide nanoparticles loaded on activated carbon as adsorbent for removal of bromophenol red from aqueous solution. J. Ind. Eng. Chem. 19(4), 1209–1217 (2013).
Ghaedi, M. et al. Synthesis and characterization of zinc sulfide nanoparticles loaded on activated carbon for the removal of methylene blue. Environ. Prog. Sustain. Energy 32(3), 535–542 (2013).
Khang, C. N., Minh Phan, N. & Van Minh, N. Enhanced photocatalytic activity of nanohybrids TiO2/CNTs materials. Mater. Lett. 165, 247–251 (2016).
Anshu, K. S. et al. A comparative study of band gap engineered in-situ and ex-situ MWCNTs/TiO2 heterostructures for their enhanced photocatalytic activity under visible light. Inorg. Chem. Commun. 150, 110540 (2023).
Abreu-Jaureguí, C., Sepúlveda-Escribano, A. & Silvestre-Albero, J. Improved photocatalytic performance of TiO2/carbon photocatalysts: Role of carbon additive. Environ. Res. 251, 118672 (2024).
Zhang, W., Zhang, X., Zhang, L., Chen, G. J. S. & Chemical, A. B Fabrication of carbon nanotube-nickel nanoparticle hybrid paste electrodes for electrochemical sensing of carbohydrates. Sens. Actuators B Chem 192, 459–466 (2014).
Dresselhaus, M. S., Dresselhaus, G., Saito, R. & Jorio, A. Raman spectroscopy of carbon nanotubes. Phys Rep 409(2), 47–99 (2005).
Reyhani, A., Mortazavi, S., Moshfegh, A., Golikand, A. N. & Amiri, M. J. Enhanced electrochemical hydrogen storage by catalytic fe-doped multi-walled carbon nanotubes synthesized by thermal chemical vapor deposition. J. Power Sour. 188(2), 404–410 (2009).
Leofanti, G., Padovan, M., Tozzola, G. & Venturelli, B. J. C. t Surface area and pore texture of catalysts. Catal. Today 41(1–3), 207–219 (1998).
Das, E., Rabha, S., Talukdar, K., Goswami, M. & Devi, A. Propensity of a low-cost adsorbent derived from agricultural wastes to interact with cationic dyes in aqueous solutions. Environ. Monit. Assess. 195, 1044 (2023).
Zhang, Y. et al. Technology P Heterogeneous fenton-like reaction followed by gac filtration improved removal efficiency of nom and dbps without adjusting PH. Sep. Purif. Technol. 260, 118234 (2021).
Mashkoor, F., Nasar, A. & Inamuddin.,. Carbon nanotube-based adsorbents for the removal of dyes from waters: A review. Environ. Chem. Lett. 18, 605–629 (2020).
Kang, L., Mucci, M. & Lürling, M. Influence of temperature and ph on phosphate removal efficiency of different sorbents used in lake restoration. Sci. Total Environ. 812, 151489 (2022).
Kannan, N. & Sundaram, M. M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dyes Pigm. 51(1), 25–40 (2001).
Weber, W. J. Jr. & Morris, J. C. Kinetics of adsorption on carbon from solution. J Sanit. Eng. Div. 89(2), 31–59 (1963).
Chien, S. & Clayton, W. Application of elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 44(2), 265–268 (1980).
Taffarel, S. R. & Rubio, J. Adsorption of sodium dodecyl benzene sulfonate from aqueous solution using a modified natural zeolite with CTAB. Miner. Eng. 23(10), 771–779 (2010).
Saadi, Z., Fazaeli, R., Vafajoo, L. & Naser, I. Adsorptive removal of apramycin antibiotic from aqueous solutions using tween 80-and triton x–100 modified clinoptilolite: Experimental and fixed-bed modeling investigations. Int. J. Environ. Health Res. 30(5), 558–583 (2020).
Ho, Y.-S. & McKay, G. J. A. S. Application of kinetic models to the sorption of copper (ii) on to peat. Adsorpt. Sci. Technol. 20(8), 797–815 (2002).
Edet, U. A. & Ifelebuegu, A. O. J. Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. Processes 8(6), 665 (2020).
Acharya, A., Jeppu, G., Raju, G. C. & Prabhu, B. J. Development of a multicomponent adsorption isotherm equation and its validation by modeling. Langmuir 39(49), 17862–17878 (2023).
Freundlich, H. Über die adsorption in lösungen. Z Phys. Chem. 57(1), 385–470 (1907).
Tempkin, M. & Pyzhev, V. J. A. P. C. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Physiochim. URSS 12(1), 327 (1940).
Zeynep, M. S. & Hasan, A. Influential biosorption of lead ions from aqueous solution using sand leek (Allium scorodoprasum L.) biomass: Kinetic and isotherm study. Biomass Convers. Biorefinery 15, 8809–8819 (2025).
Chu, K. H. & Hashim, M. A. Modeling of aqueous phase adsorption: Is it time to bid adieu to the harkins–jura isotherm?. J. Mol. Liq. 371, 121122 (2023).
de Vargas Brião, G., Hashim, M. A. & Chu, K. H. The sips isotherm equation: Often used and sometimes misused. Sep Sci Technol 58(5), 884–892 (2023).
Wu, F.-C., Liu, B.-L., Wu, K.-T. & Tseng, R.-L. A new linear form analysis of redlich–peterson isotherm equation for the adsorptions of dyes. Chem. Eng. J. 162(1), 21–27 (2010).
Nechifor, G., Pascu, D.-E., Pascu, M., Traistaru, G. A. & Albu, P. C. Comparative study of temkin and flory-huggins isotherms for adsorption of phosphate anion on membranes. Sci. Bull. Series B 77(2), 63–72 (2015).
Halsey, G. Physical adsorption on non-uniform surfaces. Agric. Eng. 16(10), 931–937 (1948).
Henderson, S. A basic concept of equilibrium moisture. Agric. Eng. (1952).
Başar, C. A. Applicability of the various adsorption models of three dyes adsorption onto activated carbon prepared waste apricot. J. Hazard Mater. 135(1–3), 232–241 (2006).
Treeamnuk T., Pengprakhon T. & Treeamnuk K. Effect of temperature on moisture sorption isotherm characteristics of Thai jasmine paddy (Khao Dawk Mali 105) Author links open overlay panel. LWT 210, 116871 (2024).
Hamdaoui, O. & Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part i. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard Mater. 147(1–2), 381–394 (2007).
Farouq, R. & Yousef, N. Equilibrium and kinetics studies of adsorption of copper (ii) ions on natural biosorbent. Int. J. Chem. Eng. Appl. 6(5), 319 (2015).
Mohammadzadeh, F., Golshan, M., Haddadi-Asl, V. & Salami-Kalajahi, M. Adsorption kinetics of methylene blue from wastewater using pH-sensitive starch-based hydrogels. Sci. Rep. 13, 11900 (2023).
Ahamad, Z., Mashkoor, F., Nasar, A. & Jeong, C. Multi-walled carbon nanotubes/TiO₂/chitosan nanocomposite for efficient removal of malachite green dye from aqueous system: A comprehensive experimental and theoretical investigation. Int. J. Biol. Macromol. 295, 139461 (2025).
Ghaedi, M., Kokhdan, S. N., Sahraei, R. & Daneshfar, A. Palladium, silver, and zinc oxide nanoparticles loaded on activated carbon as adsorbent for removal of bromophenol red from aqueous solution. J. Ind. Eng. Chem 19(4), 1209–1217 (2013).
Ghaedi, M., Ansari, A., Bahari, F., Ghaedi, A. M. & Vafaei, A. A hybrid artificial neural network and particle swarm optimization for prediction of removal of hazardous dye brilliant green from aqueous solution using zinc sulfide nanoparticle loaded on activated carbon. Spectrochim. Acta A Mol. Biomol. Spectrosc. 137, 1004–1015 (2015).
Kumar, R. & Ahmad, R. Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (TGW). Desalination 265(1–3), 112–118 (2011).
Liu, T. et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B Biointerfaces 90, 197–203 (2012).
Chatterjee, S., Lee, D. S., Lee, M. W. & Woo, S. H. Enhanced adsorption of congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyl trimethyl ammonium bromide. Bioresour. Technol. 100(11), 2803–2809 (2009).
Bhaumik, M., Leswifi, T. Y., Maity, A., Srinivasu, V. V. & Onyango, M. S. Removal of fluoride from aqueous solution by polypyrrole/Fe₃O₄ magnetic nanocomposite. J. Hazard. Mater. 186(1), 150–159 (2011).
Acknowledgements
The authors thanks Behbahan Khatam Alanbia university of technology for financial support (Grant numbers, 3.2.8124).
Author information
Authors and Affiliations
Contributions
Fereshteh Jomardani: Investigation, Methodology, Formal analysis. Reza shakeri: Supervision, Data curation, Investigation, Writing—review & editing. Raziyeh Akbarzadeh: Supervision, Software, Formal analysis, Methodology, Writing—review & editing, Writing—original draft, Project administration, Investigation, Data curation, Validation, Resources, Visualization, Funding acquisition. Syamak Nasiri Kokhdan: Project administration, Software, Writing—review & editing, Visualization, Conceptualization, Data curation, Visualization, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jomardani, F., shakeri, R., Akbarzadeh, R. et al. Adsorption kinetics and isotherms of malachite green removal from aqueous solution using TiO2 loaded on f-MWCNTs nanocomposite. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38582-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38582-9