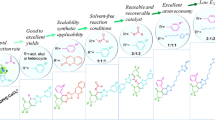
Abstract
In this study, a novel heterogeneous catalyst with the selected formula of RHA/TiO2-[bip]-NH2+NO3− was successfully synthesized and characterized, using of various techniques, including FT-IR, ¹H NMR, ¹³C NMR, TEM, XRD, XPS, TGA, EDX, and SEM mapping. The catalytic performance of this material was then evaluated in the synthesis of 1,8-dioxo-decahydroacridine and 2,3-dihydroquinazolin-4(1H)-one derivatives. The products were formed in very short reaction times under solvent-free conditions with excellent yields. In addition, the reusability of the catalyst was investigated, confirming its stability for at least five consecutive runs and also its ability to use in green and sustainable synthetic processes.
Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Shen, X., Hong, G. & Wang, L. Recent advances in green multi-component reactions for heterocyclic compound construction. Org. Biomol. Chem. 23, 2059–2078 (2025).
Tandi, M., Sharma, V., Gopal, B. & Sundriyal, S. Multicomponent reactions (MCRs) yielding medicinally relevant rings: a recent update and chemical space analysis of the scaffolds. RSC Adv. 15, 1447–1489 (2025).
Mohlala, R. L., Rashamuse, T. J. & Coyanis, E. M. Highlighting multicomponent reactions as an efficient and facile alternative route in the chemical synthesis of organic-based molecules: a tremendous growth in the past 5 years. Front Chem 12, (2024).
Zhang, X., Lu, X., Zhang, P., Dai, M. & Liang, T. Recent advances in the multicomponent reactions of Indoles. Eur. J. Org. Chem. 28, e202401446 (2025).
Carvalho, M. et al. Enhancing efficiency and sustainability: unleashing the potential of continuous flow in multicomponent reactions. ChemSusChem 18, e202401840 (2025).
Ingale, A. A., Kagne, R. P., Ghanwat, V. B. & Sargar, A. M. ZrO2 as an efficient heterogeneous catalyst for the synthesis of 2-benzylidene malononitrile derivatives via Knoevenagel condensation. Interactions 246, 94 (2025).
Ingale, A. A., Kagne, R. P., Topkar, R. R., Kulal, S. R. & Sargar, A. M. LaCoO3/Co3O4 catalyzed synthesis of tetrahydro benzo[b]pyran derivatives and their in vitro, in Silico anticancer evaluation. J. Indian Chem. Soc. 102, 102230 (2025).
Chahar, M. et al. Recent advances in the synthesis of nitrogen-containing heterocyclic compounds via multicomponent reaction and their emerging biological applications: a review. J. Iran. Chem. Soc. 22, 1–33 (2025).
Liu, J. et al. Recent advances in the synthesis of nitrogen heterocycles via Rh(III)-catalyzed chelation-assisted C–H activation/annulation with Diazo compounds. Org. Chem. Front. 12, 3065–3106 (2025).
Nguyen, H. T., Nguyen, T. T., Doan, V. T. C., Nguyen, T. H. & Tran, M. H. Recent advances in metal-free catalysts for the synthesis of N-heterocyclic frameworks focusing on 5- and 6-membered rings: a review. RSC Adv. 15, 9676–9755 (2025).
Ha, H. J. Recent advances in synthesizing and utilizing nitrogen-containing heterocycles. Front Chem 11, (2023).
Maity, P. & Mitra, A. K. Ionic liquid-assisted approaches in the synthesis of nitrogen-containing heterocycles: A focus on 3- to 6-membered rings. J. Ion Liq. 5, 100146 (2025).
Wong, X. K. & Yeong, K. Y. A patent review on the current developments of Benzoxazoles in drug discovery. ChemMedChem 16, 3237–3262 (2021).
Faizan, M. et al. Hantzsch reaction: the important key for pyridine/dihydropyridine synthesis. Synth. Commun. 54, 1221–1244 (2024).
Lavanya, G. et al. Recent Advances in the Synthesis of Dihydropyridine and Their Corresponding Fused Systems via Multi-Component Hantzsch Reaction Using Catalytic Nanomaterials. ChemistrySelect 9, e202403664 (2024).
Alvim, H. G. O., Júnior, E. N. S. & Neto, B. A. D. What do we know about multicomponent reactions? Mechanisms and trends for the Biginelli, Hantzsch, Mannich, passerini and Ugi MCRs. RSC Adv. 4, 54282–54299 (2014).
Liu, G., Pan, R., Wei, Y. & Tao, L. The Hantzsch reaction in polymer chemistry: from synthetic methods to applications. Macromolecular Rapid Commun. 42, 2000459 (2021).
Badolato, M., Aiello, F. & Neamati, N. 2,3-Dihydroquinazolin-4(1H)-one as a privileged scaffold in drug design. RSC Adv. 8, 20894–20921 (2018).
Zhang, X., Pang, Q., Liu, D. & Zhang, G. Selective synthesis of 2,3-dihydroquinazolin-4(1H)-ones and their N1-substituted analogues via Pd(II)-catalyzed cascade annulation of o-aminobenzoic acids with CO, ammonium acetate and aldehydes. Tetrahedron 155, 133915 (2024).
Rijwan, Kumar, S. & Kumar, S. Green and sustainable approaches in the synthesis of pharmaceutically relevant Quinazolinones. Tetrahedron 172, 134437 (2025).
Deng, Z. et al. Quinazolinones as potential anticancer agents: synthesis and action mechanisms. Biomolecules 15, 210 (2025).
Pele, R. et al. Antioxidant and cytotoxic activity of new polyphenolic derivatives of Quinazolin-4(3H)-one: synthesis and in vitro activities evaluation. Pharmaceutics 15, 136 (2022).
Amarasekara, A. S. Acidic ionic liquids. Chem. Rev. 116, 6133–6183 (2016).
Sarkar, A. & Pandey, S. Applications of ionic liquids in green catalysis: A review of recent efforts. Curr Catal 10, 165–178 .
Zhu, M. Ionic-liquid/metal–organic-framework composites: synthesis and emerging sustainable applications. Inorg. Chem. Front. 12, 39–84 (2025).
Wang, P. & Wang, R. Ionic Liquid-Catalyzed CO2 conversion for valuable chemicals. Molecules 29, 3805 (2024).
Qi, Z. et al. Challenges and perspectives on using acidic ionic liquids for biodiesel production via reactive distillation. Green. Chem. 26, 7718–7731 (2024).
Salas, R. et al. Ionic liquids in polymer technology. Green. Chem. 27, 1620–1651 (2025).
Zhang, Q. et al. Recent advances in supported acid/base ionic liquids as catalysts for biodiesel production. Front. Chem. 10, 999607 (2022).
Lu, Q. et al. MCM-41 supported quaternary ammonium ionic liquids as an effective heterogeneous catalyst for CO2 cycloaddition reaction. J. Porous Mater. 31, 897–912 (2024).
Tavera-Méndez, C. L. et al. Self-Assembled supported ionic liquids. Chem. - Eur. J. 30, e202303673 (2024).
Ingale, A. A., Kagne, R. P. & Sargar, A. M. NiFe2O4@PPA-DABCO: A novel magnetically separable bifunctional nanocatalyst for the synthesis of 2,2´-(Arylmethylene) bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) derivatives. J. Nanopart. Res. 27, 77 (2025).
Mazloumi, M. & Shirini, F. Nano rice husk Ash modified with acidic ionic liquid bridge: an efficient promoter for the synthesis of 1,2,4-Triazolo Quinazolinones. Poly Arom Comp. 43, 8171–8185 (2023).
Mazloumi, M., Shirini, F., Goli-Jolodar, O. & Seddighi, M. Nanoporous TiO2 containing an ionic liquid Bridge as an efficient and reusable catalyst for the synthesis of N, N′-diarylformamidines, benzoxazoles, benzothiazoles and benzimidazoles. New. J. Chem. 42, 5742–5752 (2018).
Vesoloski, J. F. et al. Immobilization of lipase from Candida Antarctica B (CALB) by Sol-Gel technique using rice husk Ash as silica source and ionic liquid as additive. Appl. Biochem. Biotechnol. 194, 6270–6286 (2022).
Nasir, I. et al. A review of rice husk silica as a heterogeneous catalyst support. J. Met. Mater. Min. 31, 1–12 (2021).
Zhao, Z., Jin, X., Liang, Y., Wang, L. M. & Liu, Y. D. TiO2 nanoparticles Dual-Modified with ionic liquid and acetic acid for use as electrorheological materials to achieve ultrahigh and stable electroresponsive performances. ACS Appl. Nano Mater. 5, 17928–17938 (2022).
Balasubramanian, S. et al. An overview of solid acid catalysts in lignocellulose biorefineries. Catalysts 15, 432 (2025).
Ansari, M. et al. Heterogeneous solid acid catalysts for sustainable biodiesel production from wastewater-derived sludge: A systematic and critical review. Chem. Eng. J. Adv. 22, 100718 (2025).
Shirini, F., Akbari-Dadamahaleh, S. & Mohammad-Khah, A. Rice husk Ash supported FeCl2·2H2O: A mild and highly efficient heterogeneous catalyst for the synthesis of polysubstituted Quinolines by Friedländer heteroannulation. Chin. J. Catal. 34, 2200–2208 (2013).
An, D., Guo, Y., Zhu, Y. & Wang, Z. A green route to Preparation of silica powders with rice husk Ash and waste gas. Chem. Eng. J. 162, 509–514 (2010).
Shirini, F., Akbari-Dadamahaleh, S., Mohammad-Khah, A. & Aliakbar, A. R. Rice husk: A mild, efficient, green and recyclable catalyst for the synthesis of 12-Aryl-8, 9, 10, 12-tetrahydro [a] xanthene-11-ones and Quinoxaline derivatives. C R Chim. 16, 207–216 (2013).
Seddighi, M., Shirini, F. & Mamaghani, M. Brønsted acidic ionic liquid supported on rice husk Ash (RHA-[pmim]HSO4): A highly efficient and reusable catalyst for the synthesis of 1-(benzothiazolylamino)phenylmethyl-2-naphthols. C R Chim. 18, 573–580 (2015).
Seddighi, M., Shirini, F. & Goli-Jolodar, O. Preparation, characterization and application of RHA/TiO2 nanocomposites in the acetylation of alcohols, phenols and amines. C R Chim. 19, 1003–1010 (2016).
Abdolmohammad-Zadeh, H., Tavarid, K. & Talleb, Z. Determination of Iodate in Food, Environmental, and Biological Samples after Solid-Phase Extraction with Ni-Al-Zr Ternary Layered Double Hydroxide as a Nanosorbent. Sci. World J. 145482 (2012). (2012).
Joao, K. Thermo-Gravimetric analysis in the investigation of catalysts: insights and innovations. J. Chromatogr. Sep. Tech. .15, 1–2 (2024).
Moseson, D. E. et al. Application and limitations of thermogravimetric analysis to delineate the hot melt extrusion chemical stability processing window. Int. J. Pharm. 590, 119916 (2020).
Xie, W., Li, R. & Xu, Q. Enhanced photocatalytic activity of Se-doped TiO2 under visible light irradiation. Sci. Rep. 8, 8752 (2018).
Nawaz, R. et al. Synthesis of Black-TiO2 and manganese-doped TiO2 nanoparticles and their comparative performance evaluation for photocatalytic removal of phenolic compounds from agro-industrial effluent. J. Nanopart. Res. 23, 263 (2021).
Ferreira-Neto, P. Thermally stable SiO2@TiO2 core@shell nanoparticles for application in photocatalytic self-cleaning ceramic tiles. Mater. Adv. 2, 2085–2096 (2021).
Kaur, A., Chahal, P. & Hogan, T. Selective fabrication of SiC/Si diodes by excimer laser under ambient conditions. IEEE Electron. Device Lett. 37, 142–145 (2016).
Lara, G. G. et al. Protection of normal cells from irradiation bystander effects by silica-flufenamic acid nanoparticles. J. Mater. Sci. : Mater. Med. 29, 130 (2018).
Stevens, J. S. et al. Proton transfer and hydrogen bonding in the organic solid state: a combined XRD/XPS/ssNMR study of 17 organic acid–base complexes. Phys. Chem. Chem. Phys. 16, 1150–1160 (2013).
Gholinejad, M., Aloueian, F. & Sansano, J. M. Nickel–Cobalt nanoprism comprising Ppm level of palladium as an efficient catalyst for Sonogashira reaction. App Org. Chem. 39, e70180 (2025).
Guo, J. et al. Atomically thin SiC nanoparticles obtained via ultrasonic treatment to realize enhanced catalytic activity for the oxygen reduction reaction in both alkaline and acidic media. RSC Adv. 7, 22875–22881 (2017).
Neto, B. A. D., Rocha, R. O. & Rodrigues, M. O. Catalytic approaches to multicomponent reactions: A critical review and perspectives on the roles of catalysis. Molecules 27, 132 (2022).
Borah, B. et al. Brønsted acid catalyzed mechanochemical domino multicomponent reactions by employing liquid assisted grindstone chemistry. Sci. Rep. 13, 1386 (2023).
Mohammad, F., Azizi, N., Mirjafari, Z. & Mokhtari, J. Catalytic acidic deep eutectic mixture for efficient and promising synthesis of Quinazolinone and Quinoxaline derivatives. RSC Adv. 15, 25971–25984 (2025).
Ghosh, T., Mandal, I., Basak, S. J. & Dash, J. Potassium tert-Butoxide promoted synthesis of Dihydroquinazolinones. J. Org. Chem. 86, 14695–14704 (2021).
Kohli, S., Rathee, G., Hooda, S. & Chandra, R. An efficient approach for the green synthesis of biologically active 2,3-dihydroquinazolin-4(1H)-ones using a magnetic EDTA coated copper based nanocomposite. RSC Adv. 13, 1923–1932 (2023).
Venugopala, K. N. et al. Larvicidal activities of 2-Aryl-2,3-Dihydroquinazolin – 4-ones against malaria vector Anopheles arabiensis, in Silico ADMET prediction and molecular target investigation. Molecules 25, 1316 (2020).
Safari, J. & Gandomi-Ravandi, S. Efficient synthesis of 2-aryl-2,3-dihydroquinazolin-4(1H)-ones in the presence of nanocomposites under microwave irradiation. J. Mol. Catal. A: Chem. 390, 1–6 (2014).
Nasirmahale, L. N., Shirini, F. & Jolodar, O. G. Poly(4-vinylpyridinum) trinitromethanide: a useful and efficient heterogeneous catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives under green conditions. Res. Chem. Intermed. 49, 4383–4403 (2023).
Li, Z. A novel magnetic heterojunction NiO@Fe3O4 photocatalyst in the synthesis of cardiovascular quinazolin-4(1H)-one drugs. Chem. Pap. 79, 2191–2200 (2025).
Jeevananthan, V., Senadi, G. C., Muthu, K., Arumugam, A. & Shanmugan, S. Construction of Indium(III)–Organic framework based on a flexible Cyclotriphosphazene-Derived hexacarboxylate as a reusable green catalyst for the synthesis of bioactive Aza-Heterocycles. Inorg. Chem. 63, 5446–5463 (2024).
Dutta, A., Damarla, K., Bordoloi, A., Kumar, A. & Sarma, D. KOH/DMSO: A basic suspension for transition metal-free tandem synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Tetrahedron Lett. 60, 1614–1619 (2019).
Rahimi, S. et al. Synthesis of antioxidant and antibacterial active Quinazolinones by carboxymethyl cellulose@MnFe2O4 biocatalyst. Inorg. Chem. Commun. 158, 111556 (2023).
Kumar, G. et al. An eco-friendly, sustainable, and greener approach to the synthesis of Dihydroquinazolin-4(1H)-ones using a deep eutectic solvent. J. Heterocycl. Chem. 61, 347–357 (2024).
Phulwale, S. P., Waghmare, S. D., Gurav, A. P., Gunturu, K. C. & Hangirgekar, S. P. Biosynthesis of BiFeO3 for BiFeO3@Ag-S-CH2-COOH as the nanocatalyst for one-pot synthesis of 2, 3-dihydroquinazolin-4(1H)-ones and their anti-blood cancer activity. J. Mol. Struct. 1338, 142288 (2025).
O’Brien, N. S., Gilbert, J., McCluskey, A. & Sakoff, J. A. 2,3-Dihydroquinazolin-4(1H)-ones and quinazolin-4(3H)-ones as broad-spectrum cytotoxic agents and their impact on tubulin polymerisation. RSC Med. Chem. 15, 1686–1708 (2024).
Hammett, L. P. The effect of structure upon the reactions of organic Compounds. Benzene derivatives. ACS Publications. https://doi.org/10.1021/ja01280a022 (2002). https://pubs.acs.org/doi/abs/10.1021/ja01280a022
Maghsoodlou, M. T. et al. Chloroacetic acid-promoted heterocyclic reactions: efficient Preparation of tetrahydropyridines and 2,3-dihydroquinazolin-4(1H)-ones. Iran J. Catal 5, (2015).
Sahu, A., Mishra, S., Sahu, P., Gajbhiye, A. & Agrawal, R. K. Indium(III) chloride: an efficient catalyst for One-Pot multicomponent synthesis of 2,3-dihydroquinazoline-4(1H)-ones. Curr. Organocatal. 5, 137–144 (2018).
Dutta, A. et al. Sustainable parts-per-million level catalysis with feiii: One-pot cascade synthesis of 2,3-dihydroquinazolin-4(1H)-ones in water. App Org. Chem. 35, e6116 (2021).
Bodaghifard, M. A. & Safari, S. Cu(II) complex-decorated hybrid nanomaterial: a retrievable catalyst for green synthesis of 2,3-dihydroquinazolin-4(1H)-ones. J. Coord. Chem. 74, 1613–1627 (2021).
Wu, S. J., Zhao, Z. Q., Gao, J. S., Chen, B. H. & Chen, G. F. Efficient one-pot synthesis of 2,3-dihydroquinazoline-4(1H)-ones promoted by FeCl3/neutral Al2O3. Res. Chem. Intermed. 45, 2327–2339 (2019).
Zhaleh, S., Hazeri, N. & Maghsoodlou, M. T. Green protocol for synthesis of 2,3-dihydroquinazolin-4(1H)-ones: lactic acid as catalyst under solvent-free condition. Res. Chem. Intermed. 42, 6381–6390 (2016).
Karami, Z. & Khodaei, M. M. Preparation, characterization, and application of supported phosphate acid on the UiO-66-NH2 as an efficient and bifunctional catalyst for the synthesis of acridines. Res. Chem. Intermed. 49, 1545–1561 (2023).
Amiri, Z., Malmir, M., Hosseinnejad, T., Kafshdarzadeh, K. & Heravi, M. M. Combined experimental and computational study on Ag-NPs immobilized on rod-like hydroxyapatite for promoting Hantzsch reaction. J. Mol. Catal. 524, 112319 (2022).
Heravi, M. M., Hosseinnejad, T. & Nazari, N. Computational investigations on structural and electronic properties of CuI nanoparticles immobilized on modified Poly (styrene-co-maleic anhydride), leading to an unexpected but efficient catalyzed synthesis of 1,4-dihydropyridine via Hantzsch pyridine synthesis. Can. J. Chem. 95, 530–536 (2017).
Li, W., Ma, R., Wang, Z. & Lü, C. Insights for photochemical mechanisms of acridine-1,8-diones: an experimental and theoretical analysis and first application for fluorescence detection of 2,4,6-trinitrophenol. J. Mol. Liq. 415, 126401 (2024).
Zhu, A., Liu, R., Du, C. & Li, L. Betainium-based ionic liquids catalyzed multicomponent Hantzsch reactions for the efficient synthesis of acridinediones. RSC Adv. 7, 6679–6684 (2017).
Alponti, L. H. R., Picinini, M., Urquieta-Gonzalez, E. A. & Corrêa, A. G. USY-zeolite catalyzed synthesis of 1,4-dihydropyridines under microwave irradiation: structure and recycling of the catalyst. J. Mol. Struct. 1227, 129430 (2021).
Sheikhveisi, M., Hazeri, N., Lashkari, M., Niya, F., Fatahpour, M. & H., & Application of Fe3O4@THAM–CH2CH2–SCH2CO2H magnetic nanoparticles as an Acidic, recyclable and green catalyst for the synthesis of Hexahydroacridine-1,8-diones, Hexahydroquinolines, and 2-Amino-3-cyanopyridines. Org. Prep Proced. Int. 57, 322–334 (2025).
Wang, F. M., Zhou, L., Li, J. F., Bao, D. & Chen, L. Z. Synthesis, Structure, and biological activities of 10-Substituted 3,3,6,6-Tetramethyl-9-Aryl-3,4, 6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione derivatives. J. Heterocyc Chem. 54, 3120–3125 (2017).
Zolfigol, M. A., Karimi, F., Yarie, M. & Torabi, M. Catalytic application of sulfonic acid-functionalized titana-coated magnetic nanoparticles for the Preparation of 1,8-dioxodecahydroacridines and 2,4,6-triarylpyridines via anomeric-based oxidation. App Org. Chem. 32, e4063 (2018).
Amiri, Z., Malmir, M., Hosseinnejad, T., Kafshdarzadeh, K. & Heravi, M. M. Combined experimental and computational study on Ag-NPs immobilized on rod-like hydroxyapatite for promoting Hantzsch reaction. Mol. Catal. 524, 112319 (2022).
Fan, X., Li, Y., Zhang, X., Qu, G. & Wang, J. An efficient and green Preparation of 9-arylacridine-1,8-dione derivatives. Heteroat. Chem. 18, 786–790 (2007).
Dhane, N. S. et al. Synthesis of 1, 8-dioxodecahydroacridines via Hantzsch condensation using Theophylline in an aqueous medium: an eco-friendly and bio-based approach. Res. Chem. Intermed. 50, 1147–1160 (2024).
Koteeswari, R., Ashokkumar, P., Malar, E. J. P., Ramakrishnan, V. T. & Ramamurthy, P. Highly selective, sensitive and quantitative detection of Hg2+ in aqueous medium under broad pH range. Chem. Commun. 47, 7695–7697 (2011).
Chavan, P. N., Pansare, D. N. & Shelke, R. N. Eco-friendly, ultrasound-assisted, and facile synthesis of one-pot multicomponent reaction of acridine-1,8(2H,5H)-diones in an aqueous solvent. J. Chin. Chem. Soc. 66, 822–828 (2019).
Sehout, I., Boulcina, R., Boumoud, B., Boumoud, T. & Debache, A. Solvent-free synthesis of polyhydroquinoline and 1,8-dioxodecahydroacridine derivatives through the Hantzsch reaction catalyzed by a natural organic acid: A green method. Synth. Commun. 47, 1185–1191 (2017).
Kiani, M. & Mohammadipour, M. Fe3O4@SiO2–MoO3H nanoparticles: a magnetically recyclable nanocatalyst system for the synthesis of 1,8-dioxo-decahydroacridine derivatives. RSC Adv. 7, 997–1007 (2017).
Aute, D., Parhad, A., Vikhe, V., Uphad, B. & Gadhave, A. Aluminized polyborate catalyzed efficient solvent-free synthesis of 1,8-dioxo-decahydroacridines via Hantzsch condensation. Curr. Chem. Lett. 13, 417–424 (2024).
Green protocol for the synthesis. Of 1,8-dioxo-decahydroacridines by Hantzsch condensation using citric acid as organocatalyst on JSTOR. Curr. Sci. 116, 936–942 (2019).
Magyar, Á. & Hell, Z. An efficient One-Pot Four-Component synthesis of 9-Aryl-Hexahydroacridine-1,8-Dione derivatives in the presence of a molecular sieves supported iron catalyst. Catal. Lett. 149, 2528–2534 (2019).
Lavanya, G. et al. The first Recyclable, nanocrystalline cds thin film mediated Eco-benign synthesis of Hantzsch 1, 4 Dihyropyridines, 1, 8-Dioxodecahydroacridine and polyhydroquinolines derivatives. App Org. Chem. 33, e5026 (2019).
Karhale, S., Patil, M., Rashinkar, G. & Helavi, V. Green and cost effective protocol for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines by using sawdust Sulphonic acid. Res. Chem. Intermed. 43, 7073–7086 (2017).
Hassanzadeh, N., Dekamin, G., Valiey, E. & M., & A supramolecular magnetic and multifunctional Titriplex V-grafted Chitosan organocatalyst for the synthesis of acridine-1,8-diones and 2-amino-3-cyano-4 H -pyran derivatives. Nanoscale Adv. 7, 99–123 (2025).
Pourdasht, S., Mousapour, M., Shirini, F. & Tajik, H. Introduction of a tropine-based dication ionic liquid catalyst for the synthesis of polyhydroquinoline and 1,8-dioxodecahydroacridine derivatives. Res. Chem. Intermed. 48, 4403–4418 (2022).
Mazloumi, M. & Shirini, F. Introduction of a new catalyst containing an ionic liquid Bridge on nanoporous Na+- montmorillonite for the synthesis of hexahydroquinolines and 1,8-dioxo-decahydroacridines via Hantzsch condensation. J. Mol. Struct. 1217, 128326 (2020).
Nasirmahale, L. N., Shirini, F., Bayat, Y. & Mazloumi, M. Introduction of TiO2-[bip]-NH2+ C(NO2)3– as an effective nanocatalyst for the Hantzsch reactions. New. J. Chem. 46, 23129–23138 (2022).
Mousapour, M. & Shirini, F. Piperazinium nano silica sulfonate: an efficient catalyst for the Hantzsch. Reaction ChemistrySelect. 6, 4247–4255 (2021).
Asareh, R., Safaiee, M., Moeinimehr, M. & Yaghoobi, F. Design and synthesis, and experimental-computational analysis of an acetic acid-functionalized zinc Tetrapyridinoporphyrazine catalyst for synthesizing acridine and Quinoline derivatives. Sci. Rep. 15, 1–22 (2025).
Acknowledgements
The authors thank the Research Council of the University of Guilan, IASBS, and the University of Alicante for helping to do this work.
Author information
Authors and Affiliations
Contributions
F. A. wrote the main manuscript text and F. S., M. G. and J. M. S corrected it. All authors reviewed the manuscript.”
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aloueian, F., Shirini, F., Gholinejad, M. et al. RHA/TiO2-[bip]-NH2+NO3− as an efficient catalyst for the solvent-free synthesis of 1,8-dioxo-decahydroacridine and 2,3-dihydroquinazolin-4(1H)-one derivatives. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38867-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38867-z