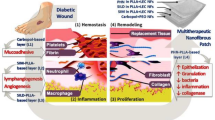
Abstract
Diabetic wounds remain a major clinical challenge due to delayed healing, persistent inflammation, and limited efficacy of existing therapies. Resveratrol possesses antioxidant, anti-inflammatory, and antibacterial properties but is restricted by poor solubility and rapid degradation. This study developed silk fibroin-coated resveratrol solid lipid nanoparticles (SF-RSLNs) to enhance resveratrol stability, provide controlled release, and improve therapeutic performance in diabetic wound healing. SF-RSLNs were fabricated using a microemulsion technique and optimized through a Box–Behnken design. The optimized nanoparticles exhibited a mean particle size of 222.1 nm, zeta potential of − 32 mV, entrapment efficiency of 85.7%, and drug loading of 10.2%. Characterization was performed using FTIR, SEM, and DLS analyses. In-vitro assessments included cytotoxicity (MTT), antioxidant activity (DPPH and ROS scavenging), antibacterial activity, scratch-wound healing, anti-inflammatory assays, and hemocompatibility evaluation. SF-RSLNs demonstrated sustained drug release (∼75% over 48 h), enhanced antioxidant activity (85% DPPH scavenging at 40 µg/mL), and significant wound closure (90% at 48 h). Antibacterial activity was improved, with inhibition zones of 18 mm against Staphylococcus aureus and 20 mm against Escherichia coli. SF-RSLNs also reduced TNF-α levels by 65% and increased IL-10 levels by 50%, while exhibiting excellent hemocompatibility. These findings indicate that SF-RSLNs serve as a multifunctional nanocarrier capable of enhancing resveratrol delivery and accelerating diabetic wound repair.
Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Deng, P. et al. Worldwide research trends on diabetic foot ulcers (2004–2020): Suggestions for researchers. J. Diabetes Res. 2022, 7592321. https://doi.org/10.1155/2022/7592321 (2022).
Matijević, T. et al. Understanding the multifaceted etiopathogenesis of foot complications in individuals with diabetes. World J. Clin. Cases 11, 1669–1682. https://doi.org/10.12998/wjcc.v11.i8.1669 (2023).
Jais, S. Various types of wounds that diabetic patients can develop: A narrative review. Clin. Pathol. 16, 2632010X231205366. https://doi.org/10.1177/2632010X231205366 (2023).
Kolimi, P., Narala, S., Nyavanandi, D., Youssef, A. A. & Dudhipala, N. Innovative treatment strategies to accelerate wound healing: Trajectory and recent advancements. Cells 11, 2439. https://doi.org/10.3390/cells11152439 (2022).
Moreira, T. D. et al. New insights into biomaterials for wound dressings and care: Challenges and trends. Prog. Org. Coat. 187, 108118. https://doi.org/10.1016/j.porgcoat.2024.108118 (2024).
Khullar, L., Harjai, K. & Chhibber, S. Therapeutic and pro-healing potential of advanced wound dressings loaded with bioactive agents. Future Microbiol. 18, 43–63. https://doi.org/10.2217/fmb-2022-0175 (2023).
Nguyen, T., Tran, H. & Pham, D. Formulation of resveratrol solid lipid nanoparticles: Application of central composite design for optimization. Pharmaceutics 15, 1234. https://doi.org/10.3390/pharmaceutics15071234 (2023).
Liu, Y., Zheng, B., Zheng, H., Xu, G. & Jiang, H. Resveratrol promotes diabetic wound healing by inhibiting the Notch pathway. J. Surg. Res. 297, 63–70. https://doi.org/10.1016/j.jss.2024.01.018 (2024).
Zhou, X. et al. Resveratrol accelerates wound healing by attenuating oxidative stress-induced impairment of cell proliferation and migration. Burns 47, 133–139. https://doi.org/10.1016/j.burns.2020.09.011 (2021).
Deng, S. et al. Chitosan/silk fibroin nanofiber-based hierarchical sponges accelerate infected diabetic wound healing via a HClO self-producing cascade catalytic reaction. Carbohydr. Polym. 321, 121340. https://doi.org/10.1016/j.carbpol.2023.121340 (2023).
Lin, D. et al. Multifunctional hydrogel based on silk fibroin promotes tissue repair and regeneration. Adv. Funct. Mater. 34, 2405255. https://doi.org/10.1002/adfm.202405255 (2024).
Gugleva, V. & Andonova, V. Recent progress of solid lipid nanoparticles and nanostructured lipid carriers as ocular drug delivery platforms. Pharmaceutics 16, 474. https://doi.org/10.3390/pharmaceutics16030474 (2023).
Elkarray, S. M., Farid, R. M., Abd-Alhaseeb, M. M., Omran, G. A. & Habib, D. A. Intranasal repaglinide-solid lipid nanoparticles integrated in situ gel outperform the conventional oral route in hypoglycemic activity. J. Drug Deliv. Sci. Technol. 68, 103086. https://doi.org/10.1016/j.jddst.2022.103086 (2022).
Rockwood, D. N. et al. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 6, 1612–1631. https://doi.org/10.1038/nprot.2011.379 (2011).
Gaviria, A., Jaramillo-Quiceno, N., Motta, A. & Restrepo-Osorio, A. Silk wastes and autoclaved degumming as an alternative for a sustainable silk process. Sci. Rep. 13, 15296. https://doi.org/10.1038/s41598-023-42367-x (2023).
Deng, M. et al. Utilization of deep eutectic solvent as a degumming protocol for raw silk: Towards performance and mechanism elucidation. Int. J. Biol. Macromol. 132, 132770. https://doi.org/10.1016/j.ijbiomac.2024.05.102 (2024).
Krishnasailaja, A. & Gazi, A. S. Formulation of methotrexate-loaded solid lipid nanoparticles by microemulsion technique. Curr. Nanomater. 8, 153–161. https://doi.org/10.2174/2452076408666221101010458 (2023).
Munir, M., Zaman, M., Waqar, M. A., Khan, M. A. & Alvi, M. N. Solid lipid nanoparticles: A versatile approach for controlled release and targeted drug delivery. J. Liposome Res. 34, 335–348. https://doi.org/10.1080/08982104.2024.1904325 (2024).
Ramadan, S. E., El-Gizawy, S. A., Osman, M. A. & Arafa, M. F. Application of design of experiment in the optimization of apixaban-loaded solid lipid nanoparticles: In vitro and in vivo evaluation. AAPS PharmSciTech 24, 167. https://doi.org/10.1208/s12249-023-02243-0 (2023).
Chen, X., Sun, M., Zhang, Y., Wang, Q. & Wang, L. Formulation and optimization of lipid nanoparticles for controlled release of poorly soluble drugs. Pharmaceutics 16, 121–135. https://doi.org/10.3390/pharmaceutics16010121 (2024).
Kim, Y., Park, J. & Lee, S. Development and optimization of a curcumin-loaded liposome using Box-Behnken design for improved bioavailability. Pharmaceutics 16, 1001. https://doi.org/10.3390/pharmaceutics16011001 (2024).
Singh, A., Sharma, M. & Kapoor, R. Optimization of controlled-release tablet formulation using design of experiments: Case study with metformin. Pharmaceutics 15, 1029. https://doi.org/10.3390/pharmaceutics15061029 (2023).
Sharma, A. N., Upadhyay, P. K. & Dewangan, H. K. Development, evaluation, pharmacokinetics, and biodistribution estimation of resveratrol-loaded solid lipid nanoparticles for prostate cancer targeting. J. Microencapsulation 39, 563–574. https://doi.org/10.1080/02652048.2022.2123148 (2022).
Bandiwadekar, A. et al. Transdermal delivery of resveratrol loaded solid lipid nanoparticle as a microneedle patch: A novel approach for the treatment of Parkinson’s disease. Drug Deliv. Transl. Res. https://doi.org/10.1007/s13346-024-00949-7 (2024).
Gokce, E. H. et al. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomedicine 7, 1841–1850. https://doi.org/10.2147/IJN.S34304 (2012).
Cassano, R., Serini, S., Curcio, F., Trombino, S. & Calviello, G. Preparation and study of solid lipid nanoparticles based on curcumin, resveratrol, and capsaicin containing linolenic acid. Pharmaceutics 14, 1593. https://doi.org/10.3390/pharmaceutics14081593 (2022).
Patel, R., Desai, T. & Mehta, P. Formulation and evaluation of solid lipid nanoparticles for the treatment of skin diseases. Pharmaceutics 15, 1357. https://doi.org/10.3390/pharmaceutics15081357 (2023).
Lalchandani, D. S. et al. Optimization of atorvastatin and quercetin-loaded solid lipid nanoparticles using Box-Behnken design. Nanomedicine 19, 1541–1555. https://doi.org/10.2217/nnm-2023-0007 (2024).
Angellotti, G. et al. Multi-component antibiofilm lipid nanoparticles as a novel platform to ameliorate resveratrol properties: Preliminary outcomes on fibroblast proliferation and migration. Int. J. Mol. Sci. 24, 8382. https://doi.org/10.3390/ijms24098382 (2023).
Neves, A. R., Lúcio, M., Martins, S., Lima, J. L. & Reis, S. Novel resveratrol nano delivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomedicine 7, 177–187. https://doi.org/10.2147/IJN.S37807 (2013).
Abdelmalek, D. A., Abd-Elhamid, B. N., Abd Allah, N. H. & Ismail, S. Resveratrol lipid-based nanoparticles show a promising outcome on induced nonalcoholic fatty liver disease. Bull. Pharm. Sci. Assiut Univ. 46, 715–727. https://doi.org/10.21608/bpsasu.2023.141482 (2023).
Rosa, A. et al. Impact of solid lipid nanoparticles on 3T3 fibroblasts viability and lipid profile: The effect of curcumin and resveratrol loading. J. Appl. Toxicol. 43, 272–286. https://doi.org/10.1002/jat.4345 (2023).
Nazar, M. F., Myakonkaya, O., Shah, S. S. & Eastoe, J. Separating nanoparticles from microemulsions. J. Colloid Interface Sci. 354, 624–629. https://doi.org/10.1016/j.jcis.2010.11.028 (2011).
Singh, A. et al. Designing and evaluation of dermal targeted combinatorial nanostructured lipid carrier gel loaded with curcumin and resveratrol for accelerating cutaneous wound healing. Part. Sci. Technol. 42, 88–106. https://doi.org/10.1080/15422119.2023.2202929 (2024).
Liu, C. et al. Research progress of polyphenols in nanoformulations for antibacterial application. Mater. Today Bio https://doi.org/10.1016/j.mtbio.2023.100729 (2023).
Hernández, Á. -P. et al. Castro, Improving properties of podophyllic aldehyde-derived cyclolignans: Design, synthesis and evaluation of novel lignohydroquinones, dual-selective hybrids against colorectal cancer cells. Pharmaceutics 15, 886. https://doi.org/10.3390/pharmaceutics15030886 (2023).
Li, X. et al. Recent advances in the preparation and application of solid lipid nanoparticles. Int. J. Pharm. 665, 124483. https://doi.org/10.1016/j.ijpharm.2023.124483 (2024).
Rashid, A. et al. Effectiveness of lipid-based nanocarriers in enhancing the stability and bioavailability of curcumin: An overview, Drug Deliv. Transl. Res. 13, 1563–1582. https://doi.org/10.1007/s13346-023-01090-0 (2023).
Liu, Q. et al. Lipid-based nanoformulations for the treatment of cancer: Focus on controlled drug release and passive-targeting strategies. Pharmaceutics 15, 66. https://doi.org/10.3390/pharmaceutics15010066 (2023).
Abdelghany, T. M. et al. Targeting efficacy of curcumin-loaded solid lipid nanoparticles for anticancer treatment: Formulation, characterization and in vivo evaluation. Pharmaceutics 16, 641. https://doi.org/10.3390/pharmaceutics16040641 (2024).
Hashem, A. A. et al. Advances in preparation, characterization, and pharmaceutical applications of resveratrol-loaded lipid nanoparticles. Drug Dev. Ind. Pharm. 49, 1301–1322. https://doi.org/10.1080/03639045.2023.2243224 (2023).
Mahran, M. A. et al. Formulation, characterization, and in vivo evaluation of glibenclamide-loaded solid lipid nanoparticles for the treatment of diabetes. J. Liposome Res. 34, 310–324. https://doi.org/10.1080/08982104.2023.2294392 (2024).
Ali, J. et al. Resveratrol-loaded solid lipid nanoparticles for enhanced oral bioavailability: Preparation, optimization, and pharmacokinetics evaluation. J. Drug Deliv. Sci. Technol. 79, 103010. https://doi.org/10.1016/j.jddst.2023.103010 (2023).
Singh, R., Patel, S., Soni, T. & Pathak, K. Enhanced delivery of curcumin through solid lipid nanoparticles for the treatment of Alzheimer’s disease: In vitro and in vivo studies. Nanomedicine 19, 1327–1338. https://doi.org/10.2217/nnm-2023-0040 (2024).
Raza, S. et al. Smart lipid nanoparticles for effective drug delivery: A comprehensive review on the role of lipid-core in nanocarriers. Nanomaterials 14, 501. https://doi.org/10.3390/nano14030501 (2024).
Gokce, E. H. et al. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomedicine 7, 1841–1850. https://doi.org/10.2147/IJN.S29871 (2012).
Sharma, V., Aggarwal, G., Moolchandani, S. & Joshi, A. Optimization of SLNs for enhanced bioavailability of poorly soluble drugs using Box-Behnken design: In vitro and in vivo studies. Int. J. Mol. Sci. 24, 7911–7925. https://doi.org/10.3390/ijms24157911 (2023).
Dandagi, P. M., Gadad, A. P., Dasankoppa, F. S., Khan, M. S. & Bhat, K. Development of curcumin-loaded SLNs for oral delivery using Box-Behnken design: Characterization and optimization. Int. J. Mol. Sci. 25, 502–515. https://doi.org/10.3390/ijms25030502 (2024).
Mathews, P. D. et al. Flavonoid-labeled biopolymer in the structure of lipid membranes to improve the applicability of antioxidant nanovesicles. Pharmaceutics 16, 141. https://doi.org/10.3390/pharmaceutics16010141 (2024).
Hernández, Á. -P. et al. Castro, Evaluation of lignohydroquinone hybrids as dual-selective cytotoxic agents against cancer cell lines using the MTT assay. Pharmaceutics 15, 886. https://doi.org/10.3390/pharmaceutics15030886 (2023).
Zhao, C. C. et al. Resveratrol-loaded peptide-hydrogels inhibit scar formation in wound healing through suppressing inflammation. Regen. Biomater. 7, 99–107. https://doi.org/10.1093/rb/rbz041 (2020).
Gomes Daré, R., Chieco Costa, A. B., Martins, T. S. & Biagini Lopes, L. Simvastatin and adenosine co-loaded nanostructured lipid carriers for wound healing: Development, characterization and cell-based investigation. Eur. J. Pharm. Biopharm. 205, 114533. https://doi.org/10.1016/j.ejpb.2024.114533 (2024).
Salah, N. M., Elbedaiwy, H. M., Helmy, M. W. & El-Salamouni, N. S. Topical amlodipine-loaded solid lipid nanoparticles for enhanced burn wound healing: A repurposed approach. Int. J. Pharm. 662, 124484. https://doi.org/10.1016/j.ijpharm.2023.124484 (2024).
A.A. Alghamdi, A.A. WalyEldeen, S.A. Ibrahim, Nanoparticles as a therapeutic approach for tumor angiogenesis, in: Innovative Approaches for Nanobiotechnology in Healthcare Systems, IGI Global, 2022 https://doi.org/10.4018/978-1-7998-8251-0.ch003.
Song, Y., Huang, Y., Zhou, F., Ding, J. & Zhou, W. Macrophage-targeted nanomedicine for chronic diseases immunotherapy. Chin. Chem. Lett. 33(2), 597–612. https://doi.org/10.1016/j.cclet.2021.08.090 (2022).
Xin-Yu, W. A. N. G. et al. Preparation of resveratrol-nanostructured lipid carrier and evaluation of in vitro antioxidant activity. Bull. Bot. Res. 40(4), 623–628 (2020).
Zhang, Y. et al. Polydopamine modification of silk fibroin membranes significantly promotes their wound healing effect. Biomater. Sci. 7, 5232–5237 (2019).
Koh, L. D. et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 46, 86–110. https://doi.org/10.1016/j.progpolymsci.2015.02.001 (2015).
Zemse, S. M., Chiao, C. W., Hilgers, R. H. & Webb, R. C. Interleukin-10 inhibits the in vivo and in vitro adverse effects of TNF-alpha on the endothelium of murine aorta. Am. J. Physiol. Heart Circ. Physiol. 299(4), H1160–H1167 (2010).
Wani, T. U. et al. Silk fibroin-based scaffolds in wound healing and tissue engineering. Front. Mater. 9, 852366. https://doi.org/10.3389/fmats.2022.852366 (2022).
Kundu, B., Rajkhowa, R., Kundu, S. C. & Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 65, 457–470. https://doi.org/10.1016/j.addr.2013.01.011 (2013).
Vepari, C. & Kaplan, D. L. Silk as a biomaterial. Prog. Polym. Sci. 32, 991–1007. https://doi.org/10.1016/j.progpolymsci.2007.05.013 (2007).
Farokhi, M. et al. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 36, 68–91. https://doi.org/10.1016/j.biotechadv.2017.10.001 (2018).
Mei, L. et al. Co-delivery of resveratrol and curcumin by silk fibroin nanoparticles for enhanced antibacterial and anti-inflammatory therapy. Colloids Surf. B Biointerfaces 190, 110925. https://doi.org/10.1016/j.colsurfb.2020.110925 (2020).
Zhao, M. et al. Engineered aggregation-induced emission luminogens-based framework with CO-releasing property for accelerating diabetic wound healing. Chem. Eng. J. 524, 168981 (2025).
Tai, Q. D. et al. Glucose-responsive nanozyme hydrogel for glycemic control and catalytic anti-infective therapy in diabetic wound healing. Mater. Today. Bio 35, 102405 (2025).
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research and Graduate Studies at King Khalid University for funding this work through the Large Research Program under grant number RGP2/233/46. The authors would like to thank the Department of Science and Technology—Fund for Improvement of Science and Technology Infrastructure (DST-FIST) and Promotion of University Research and Scientific Excellence (DST-PURSE) `for the facilities provided for conducting the research.
Funding
The authors, Ms. Bogadi Subhasri, wish to express their gratitude to the Department of Science and Technology (DST-INSPIRE) Fellowship (ID IF200201) application reference no DST/INSPIRE/03/20 21/001920. New Delhi and technically approved by ICMR (Indian Council of Medical Research) with Fellowship ID 2021–8531. Authors also thank the deanship of Scientific Research at King Khalid University, Saudi Arabia, for funding through the Large Program (grant number RGP-2/233/46)”.
Author information
Authors and Affiliations
Contributions
Subhasri Bogadi: Methodology; Formal Analysis; Investigation, Mohamed Rahamathulla : Conceptualization; Validation; Writing—Review and Editing; Funding Acquisition Gowthamarajan Kuppusamy: Writing—Original Draft Preparation; Project Administration Ali Alamri and Yahya Alhamhoom: Writing—Review and Editing; Visualization Veera Venkata Satyanarayana Reddy Karri: Conceptualization; methodology; Writing—Original Draft Preparation; Supervision, Ismail Pasha and Syeda Ayesha Farhana: Software; Data Curation Mohammed Muqtader Ahmed: Resources; Data Curation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bogadi, S., Rahamathulla, M., Karri, V.V.S.R. et al. Silk fibroin-coated resveratrol solid lipid nanoparticles for diabetic wound healing. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39254-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39254-4